Abstract
The application of chemical-specific toxicokinetic or toxicodynamic data to address interspecies differences and human variability in the quantification of hazard has potential to reduce uncertainty and better characterize variability compared with the use of traditional default or categorically-based uncertainty factors. The present review summarizes the state-of-the-science since the introduction of the World Health Organization/International Programme on Chemical Safety (WHO/IPCS) guidance on chemical-specific adjustment factors (CSAF) in 2005 and the availability of recent applicable guidance including the WHO/IPCS guidance on physiologically-based pharmacokinetic (PBPK) modeling in 2010 as well as the U.S. EPA guidance on data-derived extrapolation factors in 2014. A summary of lessons learned from an analysis of more than 100 case studies from global regulators or published literature illustrates the utility and evolution of CSAF in regulatory decisions. Challenges in CSAF development related to the adequacy of, or confidence in, the supporting data, including verification or validation of PBPK models. The analysis also identified issues related to adequacy of CSAF documentation, such as inconsistent terminology and often limited and/or inconsistent reporting, of both supporting data and/or risk assessment context. Based on this analysis, recommendations for standardized terminology, documentation and relevant interdisciplinary research and engagement are included to facilitate the continuing evolution of CSAF development and guidance.
Introduction
This review, undertaken by experts from participating institutions in the World Health Organization (WHO) Chemical Risk Assessment Network, summarizes the state-of-the-science since the introduction of the WHO/International Programme on Chemical Safety (IPCS) guidance on chemical-specific adjustment factors (CSAF) in 2005. The objective of the review was to identify strengths, limitations, and future research needs for increasing utility and facilitating regulatory acceptance of CSAF.
The complexity of hazard characterization in toxicological risk assessment varies as a function of the precision required for the range of decision contexts. The necessary precision (i.e. the degree of uncertainty acceptable) is preferably established in the problem formulation phase and takes into consideration numerous factors, such as the extent of available data, urgency of the impending decision, and available resources. This relationship between problem formulation and the tiers of increasing complexity of hazard characterization options has been previously described (Meek et al. Citation2011, Citation2014a), including its role relevant to uncertainty analysis ().
Figure 1. Conceptual representation of WHO/IPCS’ problem formulation and its relationship with various tiers of increasing complexity of hazard characterization.
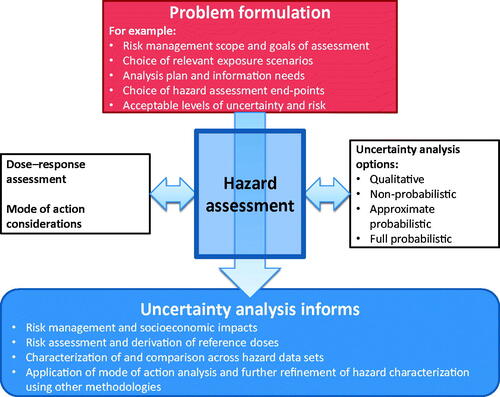
(WHO/IPCS Citation2014) further illustrates how the tiered approach to uncertainty analysis is aided by a range of increasingly data-derived approaches to characterizing exposure (see and left side of ), hazard (see right side of ), dose–response and associated uncertainty. These range from semi-quantitative to more data-derived and quantitative approaches based on increasing information on mode of action (MOA) and species concordance, increasing extent of quantitation, and probabilistic versus deterministic analyzes (Meek et al. Citation2014a). Potential tiers are described in more detail in the WHO/IPCS combined exposures framework (Meek et al. Citation2011), with lower-level tiers dealing with default (conservative) assumptions for both exposure and hazard.
Figure 2. Relationship of the consideration of uncertainty relevant to problem formulation and tiered assessment (amended from WHO/IPCS Citation2014).
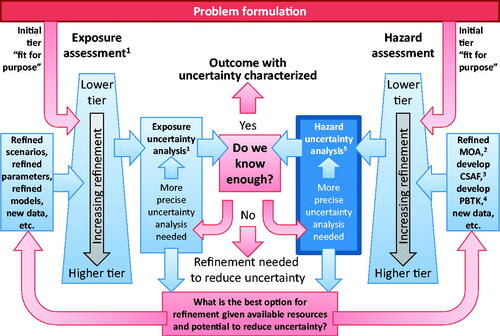
As shown in the right side of , uncertainty is taken into consideration at all tiers of hazard assessment. At lower tiers, default uncertainty factors are often applied. At higher tiers, computational modeling, such as PBPK-based deterministic and probabilistic CSAF for toxicokinetics (TK) and/or toxicodynamics (TD) can be applied. As such, precision is lower and uncertainty is higher at the lower tiers, which could be useful in priority-setting exercises, compared to higher-tiered exercises such as setting health-based guidance values.
For decades, developing "safe doses" such as the oral reference dose (RfD), the inhaled reference concentration (RfC) or the acceptable or tolerable daily intake (ADI or TDI) involved incorporation of default 10-fold uncertainty factors (UFs) to address variations between species (i.e. animal-to-human extrapolation) and between humans (i.e. interindividual or intraspecies variability) to ensure protection of average healthy adults as well as susceptible individuals within the entire population (US EPA Citation2002; WHO/IPCS Citation2005), when characterizing dose-response through identification of a point of departure (POD) such as a No Observed Adverse Effect Level (NOAEL) or the benchmark dose (BMD). Other UFs that are sometimes applied relate to database deficiencies and/or uncertainties in the key study or critical effect on which the risk assessment is based, such as when the POD is based on a LOAEL rather than a NOAEL, or a sub-chronic study to protect for lifetime exposure (see, for example, WHO/IPCS Citation1994).
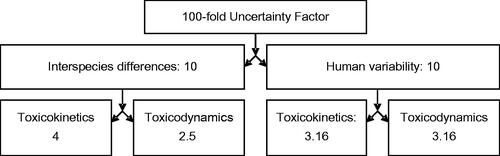
Both the original default 10-fold uncertainty factors for interspecies differences and for human variability encompass TK and TD considerations. These default factors have been additionally subdivided into two 3.16-fold (normally rounded to 3.2) subfactors for human variability and a combination of a 2.5-fold TD subfactor with a four-fold TK subfactor for interspecies differences (). The TK/TD splits for interspecies differences were originally proposed based on underlying data in rodents and humans related to basic physiological parameters, cardiac output, renal and liver blood flows (major determinants of clearance/elimination). These data were consistent with an approximately four-fold difference (according to the three-quarter power of the ratio of the body weight between rats, the most commonly used test species, and humans). For human variability, kinetic parameters and PBPK/PD modeling for a range of toxicological or therapeutic responses to pharmaceutical agents (Renwick Citation1991, Citation1993; Renwick & Lazarus Citation1998) were the basis of the kinetic and dynamic splits. The subfactors have been adopted by various national and international organizations, such as WHO/IPCS (Citation2005), the European Food Safety Authority (EFSA Citation2006), and Health Canada (Meek et al. Citation1999, Citation2002). US EPA also adopted a subfactor approach for quantitative valuation of TK and TD during derivation of inhalation RfC (US EPA Citation1994) and oral RfD (US EPA Citation2011).
Figure 3. Subdivision of default uncertainty factors into toxicokinetic and toxicodynamic subfactors for the derivation of CSAF.
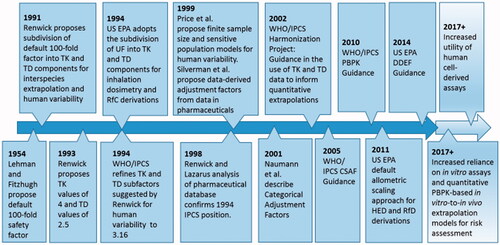
An additional notable development in this area was the 2005 publication by the WHO/IPCS entitled ”Chemical-Specific Adjustment Factors for Interspecies Differences and Human Variability: Guidance Document for Use of Data in Dose/Concentration-response Assessment“ (CSAF; WHO/IPCS Citation2005), which is referred to hereafter as the Guidance. illustrates the evolutionary timeline of the major developments surrounding publication of the Guidance. The Guidance was developed through an extended consultative process initiated in the early 2000s within the WHO/IPCS initiative on “Harmonization of Approaches to the Assessment of Risk from Exposure to Chemicals” to address the following objectives:
Figure 4. Evolutionary timeline of the major developments surrounding the WHO/IPCS (Citation2005) CSAF Guidance.
1) to increase common understanding and to encourage the incorporation of relevant quantitative data in a context consistent with traditional approaches to development of measures of dose/concentration–response and 2) to more fully delineate appropriate avenues of research to enable more predictive estimates of risk. (WHO/IPCS Citation2005).
The Guidance provided advice on how to incorporate quantitative data on TK and TD to depart from default UFs for interspecies differences and human variability in TK or TD during derivation of acceptable exposure levels, such as TDI, ADI, RfC, or RfD. It was also noted that some of the methodology was applicable to the other approaches to exposure–response analyses, such as margins of exposure or linear extrapolation from estimates of carcinogenic potency.
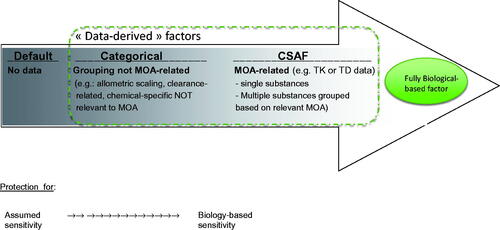
The Guidance also sought to increase understanding of the type of mechanistic data that can meaningfully inform departure from the default factors. illustrates how this process may ultimately lead to the determination of CSAF, which are part of the broader continuum of data-derived approaches to account for interspecies differences and human variability ranging from default (presumed protective) to more biologically based and predictive. The approach adopted for a given substance depends on the objectives of the assessment (i.e. problem formulation) and the availability of relevant data (Meek et al. Citation2002; Haber Citation2007; Meek et al. Citation2014a).
Figure 5. Continuum between default uncertainty factors and CSAF and relationship with fully biological-based adjustment factors, as a function of the available data. The directional arrow and intensity of the gray shade corresponds to increased robustness and decreased uncertainty.
The Guidance and associated training materials indicated that data relevant to CSAF development include the presumed or determined MOA, including the toxic moiety of interest (e.g. parent compound, reactive or circulating metabolites) and modeled (e.g. by PBPK or dynamic models) or measured data on the relevant dose metric for the active entity (e.g. area under the blood concentration versus time curve (AUC), maximum concentration (Cmax) or clearance) and effective doses for key events or the adverse outcome in the target organ. CSAF for interspecies differences are calculated as the ratio of estimated or modeled mean parameter values in animals and humans, whereas for human variability, the values are derived from the consideration of population distributions of parameters. CSAF may be calculated as the ratio between the values for an upper percentile for the relevant parameter (e.g. 95th) to its central tendency (e.g. median) in the population (Meek et al. Citation2002; Silverman et al. Citation1999), such as illustrated for benzene, chloroform, and other solvents by Valcke and Krishnan (Citation2014). Alternatively, they are calculated as the ratio between the values for an upper percentile in a presumed susceptible subpopulation and the central tendency in the general healthy population. These approaches reflect the theoretical “finite sample size” or “sensitive populations” models referred to by Price et al. (Citation1999) for estimating the human variability TK subfactor, respectively. Where the sensitive subpopulation constitutes a significant proportion of the total population, the CSAF for human variability can also be calculated as the ratio of the upper percentile to median value for this subgroup (Meek et al. Citation2002; WHO/IPCS Citation2005). If the population is sufficiently representative, some authorities have used the POD in the presumed sensitive subjects without further adjustment for variability in TD (i.e. assumed the UF for TD is 1).
The Guidance focused on the identification of relevant data and the assessment of their adequacy required to depart from the default UFs. Guidance was also provided to evaluate the relevance of the exposed population, route and dose of administration, and sample size. Case studies were included to illustrate practical application of the Guidance for the TK and TD components of interspecies differences (AKAF and ADAF, respectively) and for the TK and TD components of human variability (HKAF and HDAF, respectively).
Since the 2005 publication, it is not clear to what extent the Guidance has been implemented internationally. While the Guidance and framework have been adopted internationally by many regulatory and advisory agencies or groups worldwide (JMPR Citation2001; Meek et al. Citation2002; WHO/IPCS Citation2005; UK COT Citation2007; ECHA Citation2012; US EPA Citation2014; EFSA Citation2015a), difficulties associated with practical application in the development of CSAF have been reported. These include, but are not limited to, the scarcity of sufficient and reliable (particularly TD) data (KemI Citation2003; HPA Citation2007; UK EA Citation2009; ECHA Citation2012; OECD Citation2012), ethical issues related to obtaining or using human data (KemI Citation2003), and remaining uncertainties that preclude development and adoption of robust CSAF (e.g. EFSA Citation2006). Potential limitations of the Guidance, possibly precluding its broader application, have also not been formally considered. The objective of the present research was to review experience gained during the >10-year period since publication of the Guidance to identify strengths, limitations, and future research needs for increasing utility and facilitating regulatory acceptance of CSAF. The focus of presentation and discussion here is restricted to aspects relevant to evolution of the Guidance content. It does not represent an update of the original Guidance, nor does it replace its contents, since there are many aspects related to, for example, consideration of the adequacy of the supporting data in the original Guidance that should continue to be consulted in CSAF derivation.
Chemical-specific or chemical-related approaches addressed in this manuscript relate to knowledge of the TK or TD aspects of the specific or related chemicals, such as for individual chemicals or groups of chemicals considered to have similar TK and/or metabolic and TD (MOA) profiles – e.g. grouping of compounds based on specific knowledge that they have similar toxicological action (e.g. Silverman et al. Citation1999) or common elimination pathways (e.g. Dorne et al. Citation2005; Dorne Citation2007).
Terminology/definitions
The Guidance adopted the term “chemical-specific adjustment factors” to avoid confusion with more generic, “categorical” factors that do not relate to MOA or involve chemical-specific data, such as species-specific allometric scaling factors for TK or categorical assumptions for gases and particles based on US EPA (Citation1994) reference concentration methodology. These “categorical factors” (see, for example, Silverman et al. Citation1999; Naumann et al. Citation2001) are intermediates in the continuum of data-derived approaches (). Categorical approaches illustrate the welcomed evolution away from “10-fold default” uncertainty factors toward more refined “default” approaches, but they do not necessarily apply empirical, chemical-specific TK or TD data for the compound of interest, possibly due to the lack thereof.
While not explicitly addressed in the Guidance, associated training materials clarified that CSAF include factors developed based on an understanding of TK or TD aspects relevant to the MOA. Based on the analysis here, the Guidance may have lacked adequate clarity on terminology and definitions. This likely contributed to the diverse and inconsistent terminology applied thereafter by researchers and which affected the ability to comprehensively identify relevant case studies for inclusion in the present evaluation. Formal definitions of CSAF or related terms were not identified, based on the current analysis, although this may improve future CSAF development and regulatory acceptance. Thus, key terms and Guidance concepts are additionally defined here to clarify focus of the present analyses and for consideration in any update of the Guidance. Based on experience to date, the following distinction is suggested:
Chemical-specific adjustment factors
Adjustments for interspecies differences or human variability in TK and/or TD based on adequate quantitative chemical-specific or chemical-related data on MOA and its quantitative impact as a basis to depart from the default factors in the development of presumed “safe” (subthreshold) values, such as tolerable, acceptable, or reference intakes or concentrations. These factors can be derived for a single agent or chemical, or for a group of compounds with similar MOA. These values are designated herein and in Supplemental materials as the AKAF (interspecies TK CSAF), ADAF (interspecies TD CSAF), HKAF (human variability TK CSAF), or HDAF (human variability TD CSAF).
Both CSAF and categorical adjustment factors are consistent with the broader focus of the more recently released US EPA Guidance on “Data-Derived Extrapolation Factors” (DDEF – US EPA Citation2014). While the objectives and principles of DEEF development are similar to those for CSAF, US EPA (Citation2014) indicated that DDEFs can be derived “for a single agent or chemical, for a class of chemicals with shared chemical or toxicological properties, or for a group of chemicals that share a mode or mechanism of action or TK characteristics". Simply stated, all CSAF are DDEF, but not all DDEF are CSAF.
The approach adopted by expert committees, such as the Joint FAO/WHO Meeting on Pesticide Residues and Joint FAO/WHO Expert Committee on Food Additives (JMPR Citation2006, Citation2007, Citation2008; JECFA Citation2011b) for some pesticide residues in food, such as the picolinic acid derivative, aminopyralid, the N-methyl carbamate, carbofuran, some pyrethroids (cyfluthrin, cyhalothrin, and deltamethrin), and the mycotoxin, deoxynivalenol, illustrate principles relevant to both CSAF and categorical approaches. Adjustment factors of two-fold instead of the default TK subfactors were applied for both interspecies differences and human variability in TK, based principally on data from 75 pharmaceutical compounds (Fujita et al. Citation2006) in which there was less variation in Cmax (which reflects less variable oral absorption) than in AUC (which reflects more variable clearance). Thus, the two-fold adjustment for these specific pesticides and food contaminants was related to class-specific (i.e. chemical-related) information on MOA (i.e. that the critical effects are a function principally of the Cmax for these substances), but not on Cmax data for these specific compounds.
Variability
Variability as defined in the WHO/IPCS Guidance in Uncertainty in Hazard Characterization (WHO/IPCS Citation2014) is the “intrinsic heterogeneity about a central tendency, usually between individuals in the “target” population.” For example, individuals may exhibit differential sensitivity to the same exposure due to sources of variability, such as genetics, lifestyle, or health status. Additional data or analysis can make an estimate of human variability more precise, but the variability itself cannot be eliminated. This definition corresponds to the human variability uncertainty factor described in the present analysis, but does not explicitly address variability in interspecies differences, which has led to occasional misunderstanding between the statistical and toxicological communities, since the interspecies extrapolation uncertainty factor addresses both uncertainty and variability.
Composite factor
The Composite Factor is the composite of CSAF applied for any components for which quantitative chemical-specific or compound-related data on MOA were sufficient with remaining default subfactors for which inadequate relevant data were available (e.g. methylmercury as evaluated by JECFA Citation2004, Citation2007; EFSA Citation2012).
Methods
The WHO Chemical Risk Assessment Network (http://www.who.int/ipcs/network/en/), which held the first Network meeting in June 2014, is a voluntary collaborative initiative whose overall goal is to improve chemical risk assessment globally through facilitating sustainable interaction between institutions on chemical risk assessment issues and activities. A network working group (WG) was established to identify, review and summarize CSAF or Data-Derived Extrapolation Factors (DDEFs) with emphasis on those adopted by regulatory agencies since the CSAF concept was introduced and elaborated in the Guidance in 2005. Also included in the review were CSAF that have been proposed or evaluated by regulatory agencies, but not adopted (and the underlying reasons) as well as those proposed CSAF not originating from a regulatory agency. More conceptual investigations based on the principles of CSAF development, but not necessarily explicitly within a quantitative risk assessment context that included specification of the critical effect, MOA or POD were also considered.
Identifying pertinent CSAF
Potentially relevant CSAF were identified by searching the published literature for primary, peer-reviewed articles pertaining to CSAF or DDEFs, using the following databases:
The following search terms were used:
Search terms noted in the Guidance: “chemical-specific adjustment factor”, “CSAF”, “data-derived” and “assessment factor”, “human kinetic adjustment factor”, “toxicokinetic (or toxicokinetics) factor” “toxicodynamic (or toxicodynamics) factor” and “physiologically-based pharmacokinetic” and “risk assessment” and “uncertainty factor”.
Additional search terms: “compound-specific adjustment factor”, “compound-specific” and “assessment factor”, “data-derived evaluation factor”, “data-derived extrapolation factor”, “toxicokinetic (or toxicokinetics) adjustment factor”, “toxicodynamic (or toxicodynamics) adjustment factor”.
Although not formally “systematic”, the literature search was extensively examined to identify representative CSAF examples, such as those not originating from a regulatory agency.
A Call-for-Data was also circulated in Spring 2015 to approximately 60 participant institutions in the WHO Chemical Risk Assessment Network, principally to identify CSAF developed or adopted in regulatory contexts and in public agencies which may not be available in the published scientific literature. A similar Call-for-Data was also circulated to approximately 70 government institutions, industry associations, and other stakeholders with an interest in chemical risk assessment via the OECD Task Force on Hazard Assessment. WG members also supplied CSAF examples known to them individually. Thus, a significant proportion of relevant regulatory agencies were represented. The following questions were also posed within the Call-for-Data:
Are you aware of unsuccessful attempts to develop or adopt CSAF’s (or Data Derived Extrapolation Factors, DDEF’s) within your organization? If so, what factors were important (e.g. lack of sufficient data; perceived complexity of communication; issues of science policy related to degree of conservatism for health protection)? Attach supporting information, if available.
Do you know of any CSAF (or attempted CSAF) or DDEFs (or attempted DDEFs) outside of your organization? If so, please provide citation or focal point for contact information.
The entire text of the Call-for-Data is provided in the Appendix.
CSAF/DDEFs derived in risk assessment documents that were supplied to the WG or identified in the public domain or published literature were reviewed by the WG. The principal focus for inclusion in the current analysis was chemical-specific adjustment factors. However, some examples of categorical adjustment factors were also included in the tabulated summaries, when encountered, as a basis to consider their extent and nature and as examples, to clarify their distinction with CSAF (see section on Definitions). While as indicated above, the principles of development of CSAF may apply to any approaches to dose–response characterization, margins of exposure or development of quantitative estimates of carcinogenic potency were not considered in this analysis. Criteria for inclusion and exclusion were, then, as follows:
Included:
Assessments proposing a specific alternative to the default interspecies and/or human variability UF based on chemical-specific or compound-related information on MOA
whether or not it addressed separately TK or TD factors or both
whether or not a key study/critical effect/MOA/dose metric for quantitative risk assessment was proposed
Excluded:
CSAF published prior to calendar year 2000
Adjustments addressing other UFs, such as exposure duration extrapolation
Chemical-specific PBPK models not designed to address interspecies differences or human variability (e.g. for route-to-route extrapolation)
Approaches to dose-response estimation which do not directly incorporate UFs (e.g. cancer slope factor derivations, margins of exposure)
Data analysis
Studies meeting the inclusion criteria were tabulated (as Supplemental material) and summarized with respect to the chemical compound, the regulatory agency and context (e.g. tolerable weekly intake, reference concentration), if applicable, the type(s) of factor applied (interspecies differences and/or human variability), the CSAF value(s), and the dose metric, approach, and/or rationale of the authors.
The PBPK-derived regulatory and non-regulatory values were additionally considered to determine whether or how transparently they addressed the following considerations recommended by recent WHO/IPCS Guidance (Citation2010), Meek et al. (Citation2013):
the biological basis of the model structure and parameters;
comparison of model simulations with experimental data; and
reliability of model predictions of dose metrics relevant to risk assessment
Peer consultation meeting
Following compilation of the identified CSAF by the WG, a peer consultation meeting with invited international experts in toxicology, chemical risk assessment, and PBPK modeling was convened by WHO to review the data compilation and to reflect on the derivation and application of CSAF. The meeting occurred on 8–9 June 2016 at the WHO Collaborating Centre on Water and Indoor Air Quality and Food Safety at NSF International, Ann Arbor, MI, USA. Attendees are listed in the Acknowledgements. Potential barriers to use of the Guidance and/or adoption of the methods and potential research priorities were also discussed along with sections in the Guidance that may need clarification. The comments from the meeting were subsequently addressed by the manuscript authors.
Results
Literature searches
Early in compilation, it was recognized that a conventional literature search strategy involving the specific search terms or combinations thereof described in the Guidance would not capture some relevant CSAF. Broader search terms than included in the Guidance (specified in the Methods under “Additional Search Terms”) identified additional relevant CSAF. provides some examples of varying terminology and relevant publications that were not retrieved with the literature search terms and strategy based on inclusion of the term CSAF only.
Table 1. Some CSAF examples not retrieved by the original literature search terms.
Response to the call-for-data
Eight organizations from six countries submitted formal responses to the targeted Call-for-Data and WG members also submitted CSAF based on institutional knowledge. The formal responses informed the analysis described below. Six organizations mentioned unsuccessful attempts in general to develop or adopt CSAF within their organizations and did not note any successful attempts.
The Call-for-Data identified four CSAF for perfluorinated compounds in development by Health Canada for which documentation is not yet publicly available and as such, these assessments were not available for consideration in the present review. The Call-for-Data also identified a case where proposed CSAF published in the general literature (e.g. Hasegawa et al. Citation2013) were considered but not adopted by relevant regulatory agencies (National Institute of Health Sciences, Japan).
Given the limited nature of the information retrieved from the Call-for-Data and the low proportion of responses, however, analysis of the results contributed less to the identification of potential barriers to the development and uptake of CSAF which might be meaningfully addressed in future work. Impressions in this context from the responses are briefly summarized here.
Multiple respondents noted a need for conservatism by public health agencies. However, since default approaches have greater uncertainty associated with them, there is less confidence in reference values developed using default approaches versus those which are increasingly data-derived, such as CSAF. The fact that respondents associated default approaches with conservatism and reassurance suggests there is a need in evolved Guidance and/or training materials to emphasize the greater uncertainties and lower confidence inherent in default approaches.
Overview of reviewed CSAF
Our analysis suggests significant progress in both the development and regulatory uptake of CSAF. Indeed, more than 100 CSAF and potential CSAF were identified with multiple potential overlaps (e.g. between published literature and some of the submissions in response to the Call-for-Data). Similarly, there were many instances where the same or different organizations had evaluated the same chemical, although not necessarily for the same subfactor(s), or had considered more recent data or updated or refined PBPK model parameters. Thus, recognizing some areas of overlap, the identified CSAF were grouped as follows:
To further clarify, the regulatory (Supplemental Tables A1 and A2) and non-regulatory-derived risk assessments (Supplemental Table B) differ from the HKAF investigations (Supplemental Table C) largely by providing a clear indication of the risk assessment context, such as the dose metric, the POD/critical effect, and in most instances, the hypothesized MOA. Although not originating from a regulatory agency or serving as the basis of a public health regulation, CSAF identified in the published literature in Supplemental Table B were developed based on principles and/or objectives similar to those of regulatory agencies. Peer-reviewed publications investigating individual subfactors, most commonly HKAF, without explicitly noting the critical effect or POD (i.e. risk assessment context), were also frequently identified and thus reviewed (Supplemental Table C). Some examples of Categorical Adjustment Factors, as defined previously based on, for example, common physical/chemical characteristics are provided in Supplemental Table E. Supplemental Table F illustrates examples of the approach adopted by joint FAO/WHO expert committees for some pesticide or mycotoxin residues in food with elements related to both chemical-specific and categorical approaches.
provides a summary of identified CSAF according to geographic region, methodology (TK or TD ratio versus PBPK model) and problem formulation/context (i.e. used in regulatory decisions, non-regulatory-derived assessments, investigations of human variability in TK, or regulatory agency-investigated but not adopted CSAF).
Table 2. Geographic distribution of CSAF used in regulatory decision-making compared with non-regulatory or investigative CSAFTable Footnotea.
Regulatory-derived CSAF/DDEFs
Of the compiled CSAF, 34 originated from regulatory agencies developed for use in risk assessments to establish priorities for risk management/decision-making purposes (Supplemental Tables A1 and A2). In approximately half of these assessments, CSAF were developed based on measured or modeled TK or TD ratios for interspecies differences or human variability (Supplemental Table A1) and approximately half used a PBPK model to account for interspecies TK differences based on a TK-based adjustment of the animal POD to account for interspecies differences in internal dose, requiring no further adjustment for interspecies TK (Supplemental Table A2).
Dose metric ratios for TK or TD
Dose metric ratios for CSAF in regulatory examples commonly involved the AKAF as determined on the basis of AUC. Examples include bisphenol A (EFSA Citation2015b; MDH Citation2015) and 2-butoxyethanol (Health Canada Citation2001a). For bisphenol A, a dosimetric adjustment factor based on AUC was applied to the POD and thus the inverse of the factor can be considered the CSAF (i.e. AKAF). An example for the AKAF developed on the basis of clearance includes boron (US EPA Citation2004; Supplemental Table A1). In this single identified regulatory-derived CSAF explicitly for HKAF (as a dose-metric ratio), glomerular filtration rates were used as a surrogate for the clearance of boron (US EPA Citation2004). In three regulatory examples, ADAF were based on erythrocyte hemolysis in vitro (2-butoxyethanol by US EPA (Citation2010) and by Health Canada Citation2001a) or erythrocyte cholinesterase inhibition in vivo (N-methylcarbamates by US EPA Citation2007). In three regulatory assessments, DDEFs were derived based on semi-quantitative chemical-specific data for interspecies TD for urothelial cytotoxicity in vitro (dimethylarsinic acid by US EPA Citation2006) or nicotinic acetylcholinesterase receptor activity in vitro (sulfoxaflor by Health Canada Citation2016). In both regulatory assessments for cadmium, the default 10-fold factor for human variability was adjusted, though the TK and TD components were not distinguished and thus the relative contribution of the TK/TD adjustment is unclear (EFSA Citation2009, Citation2011; JECFA Citation2011a, Citation2013).
With respect to the magnitude of the CSAF/semi-quantitative adjustments based on TK or TD-based ratios for regulatory-agency derived CSAF, the interspecies TK factors ranged from 0.3 (1,3-butadiene; TCEQ Citation2015) to 6.4 (methyl mercury; JECFA Citation2004; EFSA Citation2012) among the five identified CSAF included in Supplemental Table A1. Interspecies TD factors ranged from 0.1 (2-butoxyethanol; Health Canada Citation2001a) to 5 (the N-methyl carbamate, methomyl) among these five regulatory CSAF values, with three being for N-methylcarbamates (US EPA Citation2007). The one identified regulatory example in which a HKAF was applied was for boron and this factor was 2.0 (US EPA Citation2004). The composite human TK subfactor of 6.4 for methylmercury was comprised of a two-fold factor for hair:blood mercury concentrations in addition to the default 3.2 value for this subfactor (JECFA Citation2004, Citation2007; EFSA Citation2012). No examples from regulatory agencies explicitly deriving a HDAF were identified. This is in line with the conclusions of the WHO/IPCS Guidance in 2005, that available TD data will rarely be sufficient as a basis for developing HDAF. Regardless of whether based on TK or TD parameter ratios or PBPK models for interspecies factors, the species on which the extrapolations were most commonly based was the rat (in approximately 75% of the cases) followed by the mouse.
PBPK models
Rather than a TK parameter ratio, in approximately one-half of the regulatory assessments, interspecies TK differences were taken into account with PBPK modeling to estimate toxicokinetically-equivalent internal doses in animals and humans at the animal POD and the corresponding human equivalent external dose (HED). Thus, the default four-fold subfactor for interspecies differences in TK is replaced with a “CSAF” of 1 (Supplemental Table A2). Most of the values for these cases were based on a classical, state-of-the-art PBPK-based extrapolation, i.e. chloroform (Health Canada Citation2001b), chromium (Health Canada Citation2015), ethylbenzene (Health Canada Citation2014a), N-methyl-2-pyrrolidone (US EPA Citation2015), trichloroethylene (US EPA Citation2014), tetrachlorethylene (Health Canada Citation2014b), vinyl chloride (Health Canada Citation2013), and xylenes (Health Canada Citation2014a). That is, the POD determined in animals was converted to a corresponding internal dose metric in humans by an animal PBPK model, followed by back-calculation to the HED using a human PBPK model. Since interspecies differences in TK are taken into account by species-specific physiological parameters in the PBPK model, no additional TK adjustment factor is needed. In two other cases, a variant of this classical approach was applied. In the case of 2-butoxyethanol, the selected dose metric (hemosiderin staining in the liver) was measured directly in experimental animals, thus necessitating only a human PBPK model to estimate the external inhaled concentration (HEC) consistent with the dose metric (US EPA Citation2010). For vinyl chloride (US EPA Citation2000), a “total liver metabolites-to-air concentration” ratio of 1.18 mg/L of liver per mg/m3 was calculated with a human PBPK model by simulating a range of exposure concentrations (1 μg/m3 to 100 mg/m3) followed by a HEC of 2.5 mg/m3 determined using an animal PBPK model based on reactive metabolite concentration in the liver as the dose metric (e.g. total amount of vinyl chloride metabolism/volume of the liver) at the animal POD of 0.13 mg/kg/day (US EPA Citation2000).
When interspecies TK differences were taken into account with comparative TK values predicted by animal and human PBPK models, where possible, to facilitate comparison for the purposes of this manuscript, the conversion was expressed in Supplemental Table A2 as a CSAF (i.e. as the ratio of the external doses in humans and animals associated with comparable internal doses of the active metabolite at the target site). This conversion was precluded in approximately half (7/15) of the relevant regulatory examples, due to the use of an inhalation-to-oral route-extrapolation-based PBPK model. With the exception of perfluorooctanoic acid (PFOA CSAF for interspecies TK =189; US EPA Citation2016a) and perfluorooctanesulfonic acid (PFOS CSAF =196; US EPA Citation2016b), regulatory assessments for which corresponding CSAF could be calculated ranged from 0.44 (chloroform) to 4.2 (EGBE).
In the case of chloroform, a PBPK-based animal-to-human extrapolation was applied by Health Canada (Citation2001b) in the assessment of Priority Substances under the Canadian Environmental Protection Act, while for the development of a maximum acceptable concentration (MAC) for trihalomethanes (THMs, for which chloroform is the principal surrogate) in drinking water (Health Canada Citation2006), the default four-fold subfactor was applied. The latter was justified on the basis of the need for conservatism, given that reliance on the PBPK model “would lead to a considerable raising of the MAC” (i.e. aspects of science policy) and in view of “uncertainties surrounding the health effects of THMs in drinking water in humans” (for which chloroform is a surrogate) for THMs. The case is an interesting example to illustrate the importance of decision context or problem formulation in regulatory application and the need for transparency in reporting of policy versus technical considerations.
For the PBPK model-based regulatory CSAF, the three considerations outlined in the WHO/IPCS PBPK Guidance (Citation2010) of biological basis, performance and reliability were addressed to a greater or lesser extent in all of the relevant regulatory CSAF (). In this context, the recent update to the US EPA (Citation2015) N-methylpyrrolidone assessment and associated PBPK model (Poet et al. Citation2016) provides a particularly transparent example. For example, model structure (Poet et al. Citation2010), refinements and assumptions were all well described based on consideration of the state of biological knowledge; model sets used for optimization (calibration) and model verification (validation) were identified; correspondence between model simulations and experimental data were reasonable (within a factor of two according to WHO/IPCS Citation2010); confidence in the model(s) for each specific dose metric prediction was characterized qualitatively (e.g. medium to high confidence) and the nature of the remaining uncertainties was articulated. Finally, the derived risk values were compared with one or more alternatives (e.g. value based on default UFs).
Analysis here indicates that those elements relevant to consideration of the adequacy and relevance of PBPK models to specific risk assessment application were generally well addressed in the regulatory documents reviewed. However, documentation was sometimes limited, ranging from summary narratives for which details were not provided, to more comprehensive model descriptions and characterization of the quality of predictions. Only approximately half of the identified regulatory examples employing PBPK models referenced the terms “verification” or “validation,” and fewer still quantitatively characterized the level of agreement between the model simulations/predictions and measured concentration data, as a basis to consider their purpose-specific performance in the context of risk assessment. The reliability of predicted dose metrics was in some cases evaluated by uncertainty and/or sensitivity analysis, but often characterization of the confidence in the model (e.g. moderate) for specific end-uses was lacking. In a substantial number of studies reviewed, models were well described or referenced; however, it was difficult or impossible to ascertain the extent of model verification from the publication and Supplementary materials.
Multiple regulatory evaluations for the same chemical
Interpretation of qualitative or quantitative variations in CSAF developed for the same compound by more than one Agency (Supplemental Table B), is complicated by many different factors, such as different critical effect and/or POD, potentially (sometimes undocumented) varying objectives or problem formulations, variations in timeframes of their development and associated supporting databases. For example, CSAF for 2-butoxyethanol were developed for interspecies TK by both Health Canada (Citation2001a) and US EPA (Citation2010) based on the AUC for hematotoxicity, but the critical effect (decreased mean cell hemoglobin concentration versus liver hemosiderin deposition) and thus PODs differed. Further, Health Canada (Citation2001a, Citation2001b) derived a Tolerable Concentration for inhalation exposure and US EPA (Citation2010) used inhalation-to-oral route-extrapolation to derive a RfD. Health Canada (Citation2001a) developed a CSAF of 0.5 based on the ratio of the measured rat to human AUC adjusted for varying durations of exposure based on a PBPK model, while the US EPA (Citation2010) used a PBPK model to account for differences between rats and humans, subsequently applying a “CSAF” of 1 for interspecies TK. When expressed as a ratio of the external dose in rats (5.83 mg/kg day) over the human equivalent dose (HED of 1.4 mg/kg day) estimated to result in the same AUC (133 μmol/L h), the AKAF is 4.2 (Supplemental Table A2).
Vinyl chloride is an example in which the same POD (i.e. rat NOAEL of 0.13 mg/kg-day) and dose metric of reactive metabolite concentration in the liver (i.e. total amount of vinyl chloride metabolism/volume of the liver) was used (Health Canada Citation2013; US EPA Citation2000). US EPA (Citation2000) pooled dose metric data in males and females and assumed 2 L of water consumed/day (i.e. 3 mg/L resulted in a HED of 0.09 mg/kg day). Whereas Health Canada (Citation2013) used dose metric data in females, as the more sensitive sex based on higher internal doses, and assumed 1.5 L of water consumed/day (i.e. 2.85 mg/L resulted in a HED of 0.224 mg/kg day). While the internal doses are similar, expressed as a ratio of rat/human external doses, the AKAF are 1.4 (0.13/0.09) and 0.6 (0.13/0.224), respectively.
In another example, EFSA (Citation2009, Citation2011) and JECFA (Citation2011a, Citation2013) both applied a CSAF for human variability, with neither value being explicitly subdivided into TK or TD components, when evaluating the safety of cadmium exposure through food. In both cases, the POD was based on urinary beta-2-microglobulin (B2M) as a biomarker of renal toxicity. The EFSA factor of 3.9 was the ratio of the 95th percentile to the median BMDL05 based on modeling of the urinary concentration-effect relationship (beta-2-microglobulin) (B2M) for a meta-analysis of epidemiological studies. The group-based BMDL05 was further adjusted to account for limitations of the summary based measures – i.e. geometric means and standard deviations rather than the raw data.
JECFA largely adopted the same approach as that of EFSA. However, there were some differences as noted in EFSA (Citation2011) with the variation in statistical approaches having the greatest impact:
The parameter used as the reference point to derive the Provisional Tolerable Monthly Intake.
The statistical approach to account for the variability and uncertainty of the marker of exposure (urinary cadmium concentration) and the marker of response (B2M) in the concentration-effect model.
The methodology for transforming urinary cadmium concentrations into dietary intake values.
Recent assessments for PFOA provide an interesting example of seeming variations in considering the available data to be adequately supportive of CSAF derivation, particularly when subfactors are increased rather than decreased based on chemical-specific data. EFSA (Citation2008) applied a default UF of 100 for interspecies differences and human variability to the POD based on an external dose in rats; an additional factor of 2 was applied to compensate for uncertainties relating to the internal dose kinetics. For interspecies differences, this can be interpreted as corresponding to application of an eight-fold factor for TK, akin to a composite factor (i.e. 2 × 4, the latter being the default subfactor for interspecies TK and the former addressing uncertainties in available TK data).
Considering the EFSA (Citation2008) evaluation and a provisional US EPA (Citation2009) assessment that applied a large TK subfactor for PFOA based on species differences in clearance, using T½ as an indirect surrogate (due to inadequate AUC or clearance data), the UK COT (Citation2009), adopted the value recommended by EFSA on the basis that the two-fold factor, in addition to the default value of 4 was appropriate for application, in view of the large but uncertain difference in half-life and clearance of PFOA between humans and mice. They considered that the value should remain provisional and be reviewed as new information became available. The provisional US EPA value was not adopted by the UK COT, based on the rationale that “it makes too many assumptions that cannot be supported robustly by the available data” and that “In addition, ongoing work in the US has estimated a shorter half-life for PFOA……, which introduces additional uncertainty.” In a recent, final assessment for PFOA, US EPA (Citation2016a) used a PBPK model to estimate serum concentrations and determine the HED and thus applied an interspecies TK “CSAF” of 1.
The human variability TK subfactor for methylmercury has also been examined by multiple regulatory agencies. EFSA (Citation2012) and JECFA (Citation2004, Citation2007) applied a composite TK subfactor of 6.4 comprised of the default 3.2 subfactor for uncertainty related to the rate of elimination and a two-fold factor to account for human variability in the hair:blood ratio. US EPA (Citation2001) used a PBPK model relating maternal blood concentrations to ingested dose to demonstrate that external doses producing the same maternal blood concentrations of 1 ppm varied up to three-fold, and thus applied a HKAF of 3.
In summary, some observations from multiple regulatory evaluations for the same chemical included:
Depending on decision context, different organizations may have different critical effects/PODs for the same MOA and dose metric thus impacting the magnitude of the derived CSAF (e.g. 2-butoxyethanol).
Differences in PBPK model inputs or statistical considerations for the same dose metric could impact the magnitude of derived CSAF (e.g. vinyl chloride or cadmium).
Different dose metrics can impact the derived CSAF (e.g. methylmercury).
Depending on decision context, different organizations could have differing opinions on the adequacy of the available data (e.g. PFOA).
Regulatory assessments considering but not adopting CSAF
Some regulatory authorities have considered but rejected CSAF derivations (Supplemental Table D), for reasons such as the lack of adequate supporting TK data or verification or “validation” of the PBPK model for the intended purpose of underlying the CSAF. For example, at the 2010 expert meeting of the Japanese Food Safety Commission (NIHS, personal communication), a CSAF approach was proposed for deriving the Tolerable Daily Intake for boron (Hasegawa et al. Citation2013), but the default UFs were adopted due largely to expert judgment that the TK data considered were insufficient based in part on their representing non-Asian demographics. The UK COT (Citation2006, Citation2009) assessment for PFOA is another example in which the default interspecies factor was applied due to inadequate confidence in the use of an internal dose metric given that available data indicated a sex-related difference in half-life in rats and since the active renal clearance in female rats is specific to this species. The draft US EPA (Citation2012b) N-methyl-2-pyrrolidone assessment and draft Health Canada (Citation2015) bromate assessment provide examples of where insufficient documentation, verification, “validation,” and/or confidence in underlying PBPK models for interspecies extrapolation precluded their adoption in the development of CSAF. While not accepted when first submitted, a model with further documentation and improvements (though collaborative effort by both the model developer and US EPA) was subsequently adopted for use by US EPA (Citation2015) in the final N-methyl-2-pyrrolidone assessment. This assessment illustrates the importance of iterative development and refinement of PBPK models through continuing consultation of risk assessors/the regulatory community and modelers to ensure their suitability for intended application as recommended in the WHO/IPCS (Citation2010) PBPK Guidance. In the draft Health Canada (Citation2015) risk assessment for bromate in drinking water, a classical PBPK approach was considered, but the default 10-fold factor was adopted for interspecies extrapolation due primarily to the lack of human data to “validate” the model.
Specified uncertainties
The Guidance recommends that attendant uncertainties in the CSAF derivation be specified. This is a favorable best practice in risk assessment; such information is critical to interpretation for purpose-specific application (i.e. based on acceptable degree of uncertainty specified in problem formulation). However, such uncertainties are often inconsistently documented and may vary depending on their capacity to impact the magnitude of the derived CSAF(s). Explicit delineation of uncertainties and their impact on derived CSAF does not imply greater confidence (or vice versa). Likewise, specifying numerous uncertainties (as opposed to one or two) also does not necessarily imply that the overall magnitude of uncertainty is greater. Rather, what is important is consideration of the relative impact of different sources of uncertainty (e.g. a sensitivity analysis of factors likely to have greatest impact on the outcome). The majority of regulatory assessments that derived the AKAF (Supplemental Table A1) include qualitative statements related to sources of uncertainty, although some do not address uncertainty in the CSAF derivation specifically and rarely include an indication of their impact. For example, at least two assessments mention potential analytical limitations in measurement of the CSAF dose metric (i.e. serum levels of bisphenol A or glomerular filtration rate for boron) and one assessment mentioned that data on the dose metric were available for only a limited number of time points and sample sizes (AUC for 2-butoxyethanol). The draft Health Canada (Citation2015) bromate assessment and Japanese Food Safety Commission (NIHS Japan Citation2015) boron assessments are examples where the specified uncertainty (i.e. lack of PBPK model validation for bromate and lack of TK data in Asian demographics for boron) precluded use of derived CSAF altogether (Supplemental Table D).
Non-regulatory agency-derived CSAF
Interspecies TK subfactor
For the assessments identified in Supplemental Table B, the majority of CSAF developed by (principally) research groups external to regulatory agencies were based on PBPK models and addressed interspecies differences by the commonly adopted convention to adjust the POD for external exposure by the ratio of PBPK estimated internal doses in animals and humans, with no further TK adjustment necessary. Thus, the TK subfactor is commonly reported as 1. Similar to the regulatory PBPK-based interspecies TK CSAF, the desired PBPK model assessment elements recommended in the WHO/IPCS guidance (Citation2010) (i.e. biological basis, performance, and reliability) were addressed to a greater or lesser extent by these non-regulatory assessments (Supplemental Table B). Among these examples, those noted to address most, if not all, of the recommended elements included acrylamide (Sweeney et al. Citation2010), methylphenidate (Yang et al. Citation2014), 2-phenoxyethanol (Troutman et al. Citation2015), and 1,1,1-trichoroethane (Lu et al. Citation2008), although terminology varied (e.g. “validation” versus “verification").
Similar to the regulatory assessments, the proportion of PBPK derived health-based values for which corresponding CSAF could be calculated was approximately 50% (11/22) for the non-regulatory assessments. With the exception of 1,3-butadiene in mice (Kirman & Grant Citation2012) and methylphenidate in monkeys (Yang et al. Citation2014), CSAF estimated from PBPK-based extrapolations were all based on data in rats and ranged from 0.6 for acrylonitrile ingestion to 2.5 for acrylonitrile inhalation (Kirman et al. Citation2008) (Supplemental Table B). For 1,3-butadiene (Kirman & Grant Citation2012), the calculated CSAF of 0.03, thereby increasing the HED by ∼30-fold relative to the POD in mice, was based on a model incorporating TK data from both rats and mice; that for methylphenidate was based on juvenile or adult rhesus monkeys (where the calculated CSAF ranged from 0.03 to 0.1 depending on AUC or Cmax and age (juvenile or adult).
For the remaining half of the non-regulatory assessments, corresponding CSAF could not be calculated for various reasons, most commonly related to application of the subfactor for remaining TD uncertainty to the internal dose prior to (rather than after) estimation of the HED. A CSAF was not estimated for two examples in which the human PBPK model input was based on the arithmetic (Kirman et al. Citation2005) or geometric mean (Poet et al. Citation2016) AUC of AUC estimates obtained from more than one key animal study. In other less frequent cases, it was not possible to express a CSAF as a human/animal dose metric ratio due to lack of clear distinction of TK from TD elements within the model, inhalation-to-oral-route extrapolation, or inadequate reporting of critical data (Supplemental Table B).
Human variability TK subfactor (HKAF)
In contrast to the non-regulatory agency-derived quantitative risk assessments that used PBPK modeling for the AKAF (Supplemental Table B), there was a greater tendency for investigations of individual subfactors by non-regulatory agencies to use PBPK models to derive a HKAF (Supplemental Table C). These investigations also tended to include multiple dose metrics without specifying which may be most relevant. Some authors, such as Clewell et al. (Citation2004), Valcke and Krishnan (Citation2014) and Valcke and Krishnan (Citation2011) computed HKAF as a function of the exposure route and duration by simulating exposure to concentrations that were determined based on route-specific chronic and acute guidelines of interest. Similarly, Mörk et al. (Citation2014) simulated exposure concentrations corresponding to the RfC for styrene, toluene, and methyl chloride, and Mörk and Johanson (Citation2010) simulated exposures to an acetone concentration (29 ppm) proposed previously in the literature for the purposes of RfC and RfD determination (Gentry et al. Citation2003). However, not all the examples in Supplement Table C explicitly related the simulated exposures to a potential or existing guideline value. For instance, Pelekis et al. (Citation2001), Sarangapani et al. (Citation2003) and Clewell et al. (Citation2004) simulated exposure concentrations consistent with expected lifetime human exposure levels, but their approach relied strictly on non-stochastic, median, child-to-adult comparisons. Only some of the derived HKAF included reference an existing regulatory risk assessment and/or probable critical effect or POD. This rather critical context for interpretation might have been apparent from a literature review, but was outside of the scope of the present review.
Despite the numerous examples in the published literature, few CSAF/semi-quantitative factors for human variability in TK have been adopted in regulatory assessments in the period since the Guidance was released (e.g. boron, methylmercury, and trichloroethylene, by US EPA and methylmercury by JECFA and EFSA). Of these, methylmercury and trichloroethylene by US EPA were based on a PBPK model [with the others being based on TK ratios (or semi-quantitative ratios)]. Also, as was reported above, a CSAF for human variability without explicit subdivision into TK and TD components was derived for cadmium (EFSA Citation2009, Citation2011; JECFA Citation2011a, Citation2013).
Discussion and recommendations
The objective of the present research was to review experience gained during the >10-year period since publication of the Guidance. This analysis identified critical aspects, that if clarified, could facilitate future CSAF development and regulatory uptake. The subsections hereafter relate to improving common understanding of the focus and communication of the Guidance and its updating to reflect increasing experience, such as the need for standardized reporting formats (that include specified uncertainties and associated sensitivity considerations), evolution towards increasing reliance on in vitro data, and the need for more effective communication of the value of increased precision and predictive power and decreased uncertainty inherent in the application of more data- derived, chemical-specific approaches, such as CSAF, compared with default approaches.
Focus and communication of the guidance
The content of the 2005 Guidance was, perhaps not surprisingly, limited by the extent of experience acquired in their development at that time. Acquired experience and analysis herein identified inconsistencies with some early principles for CSAF development. Further, limited documentation of critical aspects underlying CSAF development in many case examples made it difficult to discern whether inconsistencies relate to a lack of familiarity with, or a misunderstanding of, the Guidance. Regardless of the basis, our analysis suggests that additional guidance might promote consistency in CSAF development and application. This could be in the form of evolved training materials which were originally prepared after release of the Guidance and presented and modified based on continuing feedback at various specialty courses at scientific conferences.
Important aspects addressed in the original Guidance and documented more explicitly as “rules of thumb” in associated training materials, which could be reiterated to increase consistency in CSAF development include the following.
Explicit relationship of CSAF with the point of departure (POD)
CSAF are context dependent and specific to the relevant species/strain and dose range for the critical study from which the POD for the risk assessment is derived. CSAF derivations for interspecies TK should define the internal dose at the POD and the corresponding HED associated with that internal dose. This concept of equivalency is equally relevant to CSAF derivations for interspecies TD (i.e. toxicodynamic equivalency), which should define equipotent exposures in animals and humans that are associated with the same critical effect and MOA, such as derived by US EPA (Citation2007) for erythrocyte cholinesterase inhibition by N-methyl carbamates in rats and humans.
Relationship of CSAF with allometric scaling
While allometric scaling for interspecies differences reflects a generic, categorical approach, it is noteworthy that the default TK subfactor (i.e. 4×) for interspecies differences is historically based on allometric scaling for rats. As a result, if the risk assessment key study and POD is based on another species, this should be accounted for in a comparison of the derived CSAF to the species-specific default value for interspecies TK (e.g. it is greater for mice than rats).
Minimum information preferable for CSAF development
Sufficient information on MOA to establish the active moiety (i.e. parent compound or metabolite),
For interspecies variation, sufficient quantitative information to characterize the ratio between mean or median values for the relevant TK parameter or effective dose (TD) in the experimental animal species in which the critical effect was observed and that in humans; sample sizes of 5 are normally sufficient for this purpose, and
For human variability, sufficient supporting data to adequately characterize population distributions of the relevant kinetic parameter or effective dose in the general and sensitive subpopulations.
Implications of the default “splits” between TK and TD
The Guidance splits the continuum of TK and TD processes leading to toxicity at the level of delivery of the parent compound or a circulating active metabolite to the target tissue/organ. Events up to this point were considered as TK, and events within the target tissue/organ were considered as TD (i.e. the dose in the target tissue reflects TK and a portion of TD). Thus, a comparison of blood-borne concentrations of the active entity as a basis for deriving an adjustment factor addresses solely TK, whereas CSAF based on target tissue concentrations take into account a portion of TD in the framework construct and this should be borne in mind when considering a remaining adjustment for the TD subfactor. Similarly, for CSAF based on PBPK models which incorporate bioactivation and/or detoxication processes within the target tissue/organ or effects at the site of contact, the resulting PBPK-based adjustment replaces the default for TK and a portion of the default for TD.
Updating and revision of the guidance
Along with the acquired experience in CSAF development documented herein, other notable developments since release of the Guidance could contribute significantly to its evolution, such as recent advances in both risk assessment and toxicity testing. The WHO/IPCS MOA/species concordance framework has been updated recently to reflect increasing experience in MOA development and assessment and continuing developments in toxicity testing and non-testing methods (Meek et al. Citation2014a). The modified framework, incorporated within an iterative roadmap, encourages continuous refinement of problem formulation, MOA-based testing strategies and risk assessment. It includes templates to promote consistent consideration of the weight of evidence for hypothesized MOA and extension to dose–response analysis (e.g. as a basis for CSAF development). The framework can be used as originally intended, where the outcome of chemical exposure is known, or in hypothesizing potential effects resulting from exposure, based on information on putative key events in established MOA from appropriate in vitro or in silico systems and other evidence. Considerations related to weight of evidence for MOA have been additionally articulated in a subsequent publication (Meek et al. Citation2014b) and evolved in international collaborative efforts on adverse outcome pathways (AOPs) (OECD Handbook Citation2016; Becker et al. Citation2015; Edwards et al. Citation2016). The US EPA (Citation2014) guidance on Data-Derived Extrapolation Factors also contributes significantly to CSAF evolution by encouraging the identification and use of TK or TD data to inform the magnitude of interspecies differences and human variability (e.g. rather than immediately applying default or categorical subfactors). Thus, developments reflected in the DDEF Guidance should be carefully considered if updating or revising the Guidance.
Analysis here indicates that the majority of CSAF developed since the Guidance was released address interspecies differences in TK based on incorporation of predicted values from PBPK models, consistent with their anticipated increasing and often preferable contribution to measured data, based on incorporation of additional biological information. Although the potential of PBPK models to contribute significantly was acknowledged in the Guidance, considerations of their adequacy as a basis for CSAF development were not articulated. This has been addressed more recently in WHO/IPCS guidance to inform the purpose-specific characterization, documentation, evaluation, and communication of PBPK models for application in health risk assessment (WHO/IPCS Citation2010; Meek et al. Citation2013) which incorporates the output of earlier initiatives within the USA, Canada and Europe (Gentry et al. Citation2004; US EPA Citation2006; Barton et al. Citation2007; Loizou et al. Citation2008). The Guidance includes a checklist to assist risk assessors in considering the adequacy of PBPK models for specified purposes (e.g. exposure, hazard, and/or dose–response analysis). As described previously, CSAF derived on the basis of PBPK models were reviewed here, taking into consideration aspects of confidence identified in the PBPK Guidance:
the biological basis of the model structure and parameters
performance of the model (comparison of model simulations with experimental data) and
reliability of model predictions of dose metrics relevant to risk assessment (i.e. model testing, uncertainty and sensitivity analyses).
Analysis here indicates that these elements were often well addressed in the development of PBPK-based-CSAF for regulatory application, reflecting likely increasing consensus on good practice in assessment and reporting. Thus, consideration and incorporation of recent PBPK guidance is likely to inform a decision tree or application guide for CSAF derivation while also facilitating more standardized and transparent reporting of PBPK adjustments to PODs and/or UFs, based on delineated rationales.
Standardized CSAF reporting format, including problem formulation
The variable and often non-transparent basis for the CSAF reviewed here suggest the need for a standardized reporting format to facilitate CSAF development and meaningful analyses. As mentioned, the critical context for CSAF development and regulatory acceptance includes the direct relevancy of the CSAF species/strain and dose range in relation to the MOA, critical effect, and POD. These aspects should be systematically assessed and reported as background information during CSAF development.
Consideration of the extent and adequacy of supporting data for CSAF development is a function of the hazard assessment objectives as delineated in problem formulation. This can vary from, for example, preliminary consideration of quantitative data in a priority setting or screening contexts, to detailed characterization of hazard with considerable implications for risk management. To facilitate use, the scope of developed CSAF should be delineated with associated limitations for a range of potentially relevant risk assessment contexts. This was not articulated in the 2005 Guidance, with the domain of applicability being specified only as dose/concentration–response analyses for approaches that led to the estimation of presumed “safe” (subthreshold) values, such as tolerable, acceptable, or reference intakes or concentrations. The concepts in CSAF development have, however, broader applicability to other approaches to dose–response characterization, such as in interpreting margins of exposure.
As mentioned, experience from the recent international initiative to inform the characterization, documentation, evaluation, and communication of PBPK models for health risk assessment (WHO/IPCS Citation2010; Meek et al. Citation2013) can also facilitate CSAF development and regulatory uptake. A PBPK template was developed based on dialog between technical scientists and risk assessors to ensure standardization and sufficiency of documentation of model descriptions and supporting data to permit independent evaluation. This template was viewed as an important tool to facilitate communication between the modeling and risk assessment communities to increase understanding and regulatory acceptance of purpose oriented models. The template addresses four primary areas:
background on the chemical, its TK and MOA
characterization and evaluation of the PBPK model
modeling and evaluation of the model-derived dose metrics
PBPK modeling and comparison with default approaches
The envisaged content of such a reporting format for CSAF development and review should include reference to the following aspects:
Decision context/problem formulation: Description of the objectives of the hazard characterization/dose–response analysis (e.g. development of a RfD for daily intake or guidance values for various media, as part of a screening assessment to establish priorities, or in-depth assessment of risk as a basis to determine the need for risk management, etc.)
Interspecies differences and/or human variability: Specification of the proposed critical effect and its MOA; identification of the active (i.e. toxic) moiety and its most relevant animal or human TK and/or TD parameter(s) with supporting rationales and key studies, including species, life-stage, dose, route (vehicle), group size (n), dose metric, central tendency metric (e.g. simple arithmetic mean, geometric mean, median); transformation (e.g. lognormal distribution), applicable TK normalizations [normalized to dose (AUC, Cmax, clearance), or body weight (for clearance) if applicable], SD, SE, and adequacy of data assessed for each of the elements (including sample size) identified in the CSAF Guidance (WHO/IPCS Citation2005).
Dose metric considerations: Rationale for the choice of dose metric (i.e. AUC, Cmax, clearance) should be included as the choice can impact the magnitude of the CSAF. Even for the same dose metric, slight differences in PBPK model inputs can impact the magnitude of the CSAF as seen with vinyl chloride (US EPA Citation2000; Health Canada Citation2013). Knowledge of the interrelationships among dose metric options may help inform whether additional normalizations are necessary, such as elimination half-lives should be normalized for clearance or volume of distribution, if used to derive CSAF. Adjustments for varying durations of exposure may be necessary as with 2-butoxyethanol evaluated by Health Canada (Citation2001a, Citation2001b).
Derivation of the CSAF: Type (e.g. AUCanimal/AUChuman, Cmaxanimal/Cmaxhuman, CLanimal/CLhuman, PBPK based, other), basis for any required normalizations or adjustments (e.g. varying durations of exposure in animals and humans), additional questions, or remaining uncertainties. If a dosimetric adjustment factor is applied to the POD to derive a HED and the AKAF is reduced to 1 (e.g. bisphenol A by EFSA Citation2015b; MDH Citation2015), it is useful to also represent the factor as a CSAF according to the inverse of the factor for comparison with the default TK subfactor.
Comparison of derived CSAF with categorical or default approaches: Compare and contrast of the CSAF to various options to characterizing uncertainty, including those based on species-specific and non-specific defaults. This should include representing the PBPK adjustments for interspecies TK differences as a corresponding CSAF (e.g. ratio of rat and human external does such as in Supplemental Tables A2 and B).
Assessment of the adequacy of available data: CSAF derivations (including unsuccessful attempts) should include a statement of confidence in relation to the availability or adequacy of relevant data for CSAF derivation and remaining specific data or research needs (e.g. 2,4-dichlorophenoxyacetic acid in Supplemental Table D).
Evolution of the guidance to address additional types of in vitro data
The Guidance recognized the utility of in vitro data to inform TK (e.g. enzyme kinetics) or TD (e.g. MOA) subfactor derivations. However, considerable efforts now being devoted to developing in silico and in vitro methods for identifying and quantifying molecular initiating and intermediate key events may have implications when establishing health-based guidance values. The application of uncertainty and adjustment factors to precursor biologic responses will not necessarily involve the same factors as currently used (NRC Citation2007). To date, CSAF have been associated most commonly with clinical, phenotypical, or in vitro measures of apical adverse outcomes. For example, in vitro data for erythrocyte hemolysis and for inhibition of acetyl cholinesterase activity have been used to derive CSAF for interspecies TD for 2-butoxyethanol and N-methylcarbamates, respectively. Methods to describe the quantitative relationship between the in silico/in vitro determination of earlier key events and the adverse outcomes are needed, since the quantitative relationship to interspecies differences in TD may be less readily apparent.
Many of the wide range of in silico and in vitro methods emerging are based on human-derived systems, which negate the need for interspecies extrapolation, but will require quantitative in vitro-to-in vivo extrapolation (see, for example, Bhattacharya et al. Citation2011; Yoon et al. Citation2012). There is currently limited experience and no consensus framework for the application of such methods to developing health-based guidance values and implications of these evolving methods should be considered if the Guidance is updated.
Communication of predictivity/uncertainty of CSAF relative to default
A common finding in this analysis was the tendency to rely on default UFs when chemical-specific information led to a decrease in TK or TD subfactor(s) and resulting increase in health-based guidance values, possibly due to a misperception that CSAF are not adequately protective. In part, this reflects a failure to recognize that a composite factor less than default is an inevitable consequence of current risk analysis policies meeting desired objectives that default assumptions be conservative by design, such as intake estimates based on high percentiles of population distributions for consumption (see for example Codex Alimentarius Commission Citation2013). Hence, while there is uncertainty about health based guidance values, this is not symmetrical, i.e. it is not evenly distributed on either side of the value.
This conservatism extends to the choice of default uncertainty factors. The typical value of 100 is based on a limited historical analysis of the variation between and within species in the biological processes relevant to the adverse effects for a range of chemicals which had information available in both experimental animals and humans at the time (Lehman & Fitzhugh Citation1954). This analysis led to wide-spread and long-standing adoption of the default 100-fold safety factor as being conservative, particularly when extrapolating from effects observed in rodents.
Our analysis here indicates that TK or TD subfactors based on chemical-specific information tended to be less than the default subfactors resulting in guidance values greater than those based on defaults. This reflects the adequately protective, but less precise or informative nature and thus lower confidence associated with default approaches. A notable exception is when there are marked species differences in clearance of the (active) chemical, such as for some perfluorinated compounds when elimination is much slower in humans than in laboratory species. For CSAF greater than the default subfactor, the 2005 Guidance emphasized that the derived CSAF value should be applied since the default may not be adequately protective, but in fact, equal emphasis should have been noted for the preference to use derived CSAF, whether greater or less than the default, due to their more precise, chemical-specific and quantitative nature relative to the critical effect and MOA (compared to more generic, less data-derived, default approaches) and thus higher degree of confidence attained in the derivation of the health-based guidance value.
Knowledge of the chemical structures or classes for which there are likely to be marked species differences has increased over the last few decades such that in many cases, species differences can be anticipated and investigated further, if necessary. Similarly, there have been appreciable advances in knowledge of MOAs and AOPs over the last few years to provide insight into how likely humans will be appreciably more sensitive than experimental animals to adverse effects of a chemical, resulting in the infrequent instances, such as PFOA/PFOS, in which derived CSAF are greater than default (and hence the guidance value is less).
Using chemical-specific information to depart from the default factor inevitably reduces uncertainty, yet this fundamental concept is not generally commonly understood and/or accepted. Alternatively, there is a view amongst many that it will be difficult to defend a higher reference value (i.e. due to a lower uncertainty factor) to risk managers and the public. Communication of the general principles reiterated here, such as the nonspecific and conservative basis of default values, the consequence that CSAF are generally less than default but more scientifically precise, and the underlying basis of anticipated exceptions are likely to increase general and regulatory utility of CSAF. Society benefits, regardless of whether resultant factors are greater or less than default, since CSAF more accurately reflect the TK, TD, and MOA knowledge and increase reliance on science-based evidence in decision making and subsequent confidence in those decisions.
Standardized toxicology study designs are not particularly well suited for MOA elucidation and thus CSAF derivation. Consideration could be given, for example by OECD, on how study designs could be improved to better address MOA and thus facilitate use in risk assessment, including CSAF derivation. As novel, non-animal testing methods are introduced, such considerations could facilitate their role and applicability in regulatory risk assessment. Based on the analyses here, critical aspects related to the availability or adequacy of relevant data for CSAF derivation were also often not reported and/or explicitly evaluated, though this was emphasized in the Guidance and associated case examples. As a basis to facilitate practical application and regulatory acceptance, our analysis underscored the need for more explicit CSAF reporting formats to effectively convey relevant supporting data, limitations, and explicit comparison with species-specific and nonspecific default approaches.
Conclusions
Analysis of over 100 CSAF indicates significant progress in both the development and the regulatory uptake of CSAF since publication of the 2005 Guidance. Opportunities for improvement when developing CSAF include more transparent and standardized reporting that includes critical background context (e.g. critical effect, POD, MOA, PBPK model parameters), comparative calculations based on alternative approaches (e.g. dose metrics), including categorical or default approaches, and more explicit specification of uncertainties related to the derived CSAF(s) and their impact (i.e. sensitivity analysis) on the health-based guidance value. The analysis also underscores the need for more effective communication of the value of increased precision, confidence, and predictive power and decreased uncertainty inherent in the application of more data-derived, chemical-specific approaches compared with default approaches.
Declaration of interest
The authors are all affiliated with public institutions. The review was undertaken by a Working Group of experts from public institutions who participate in the World Health Organization’s (WHO) Chemical Risk Assessment Network, a voluntary collaborative initiative whose overall goal is to improve chemical risk assessment globally through facilitating sustainable interaction between institutions on chemical risk assessment issues and activities. Preparation of the manuscript was facilitated by an expert peer consultation meeting held 8–9 June 2016 hosted by NSF International, Ann Arbor, MI. NSF International is a global not-for-profit, non-government organization focused on improving public health and safety, including the development of consensus public health standards and is a WHO Collaborating Centre on Water and Indoor Air Quality and Food Safety. The expert peer consultation meeting and preparation of the review were produced with financial assistance from the European Union. None of the authors received financial support or an honorarium for preparation of the review. None of the authors has recently or is currently involved as an expert witness in litigation or in formal rule-making on the subject matter of this paper. The authors alone are responsible for the views and conclusions stated in this paper and the views and conclusions do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated or the European Union. The draft manuscript was reviewed during preparation by an expert peer consultation meeting convened by the World Health Organization. The participants in that meeting are listed in the “Acknowledgements” section of the manuscript. This article was produced with the financial assistance of the European Union (travel support for attendees of the expert peer consultation meeting and article publishing charge for open access publication). The authors declare they have no conflicts of interest.
CSAF_supplemental_tables_corrected.pdf
Download PDF (1.3 MB)Acknowledgements
This review was undertaken by a Working Group of experts from public institutions who participate in the WHO Chemical Risk Assessment Network, a voluntary collaborative initiative whose overall goal is to improve chemical risk assessment globally through facilitating sustainable interaction between institutions on chemical risk assessment issues and activities. The authors gratefully acknowledge the comments and suggestions provided during an expert peer consultation meeting held 8–9 June 2016 at the WHO Collaborating Centre on Water and Indoor Air Quality and Food Safety at NSF International, Ann Arbor, MI, USA. The authors gratefully acknowledge the comments of reviewers selected by the Editor and anonymous to the authors. These comments were helpful in revising the manuscript. In addition to the members of the Working Group (the authors of the present paper), attendees were Laurent Bodin (ANSES, France), Julia Dady (Minnesota Department of Health, USA), Sandrine Fraze-Frontier (ANSES, France), Abderrazzek Hedhili (Unité de Recherche du Laboratoire de Toxicologie et Environnement, Tunis, Tunisia), Matthias Herzler (BfR, Germany), Debabrata Kanungo (Food Safety and Standard Authority, India), George Kowalczyk (Public Health England, UK), Kannan Krishnan (Université de Montréal, Canada), Kumiko Ogawa (National Institute of Health Sciences, Japan), and Edward Ohanian (Environmental Protection Agency, USA). In addition, Christoph Klein (European Commission) and Jean-Lou Dorne (European Food Safety Authority) provided written comments on the draft findings.
Funding
This article was produced with the financial assistance of the European Union (travel support for attendees of the expert peer consultation meeting and article publishing charge for open access publication).
Supplemental material
Supplemental data for this article can be accessed here.
References
- Barton HA, Chiu WA, Setzer RW, Andersen ME, Bailer AJ, Bois FY, DeWoskin RS, Hays S, Johanson G, Jones N, Loizou G. 2007. Characterizing uncertainty and variability in physiologically based pharmacokinetic models: state of the science and needs for research and implementation. Toxicol Sci. 99:395–402.
- Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, Watanabe H. 2015. Increasing scientific confidence in adverse outcome pathways: application of tailored Bradford-Hill considerations for evaluating weight of evidence. Reg Toxicol Pharmacol. 72:514–537.
- Bhattacharya S, Zhang Q, Carmichael PL, Boekelheide K, Andersen ME. 2011. Toxicity testing in the 21 st century: defining new risk assessment approaches based on perturbation of intracellular toxicity pathways. PLoS One 6:e20887.
- Codex Alimentarius Commission. 2013. Codex Alimentarius Commission Procedural Manual. Twenty-first edition. World Health Organization, Food and Agriculture Organization of the United Nations. Rome, 2013. Available from: http://www.fao.org/3/a-i3243e.pdf
- Clewell HJ, Gentry PR, Covington TR, Sarangapani R, Teeguarden JG. 2004. Evaluation of the potential impact of age- and gender specific pharmacokinetic differences on tissue dosimetry. Toxicol Sci. 79:381–393.
- Dorne JL. 2007. Human variability in hepatic and renal elimination: implications for risk assessment. J Appl Toxicol. 27:411–420.
- Dorne JL, Walton K, Renwick AG. 2005. Human variability in xenobiotic metabolism and pathway-related uncertainty factors for chemical risk assessment: a review. Food Chem. Toxicol. 43:203–216.
- ECHA (European Chemical Agency). 2012. Guidance on information requirements and chemical safety assessment – Chapter R.8: Characterisation of dose [concentration]–response for human health. Version 2.1. Helisnki, Finland. [cited 2016 Jan 7]. Available from: https://echa.europa.eu/documents/10162/13632/information_requirements_r8_en.pdf
- Edwards SW, Tan YM, Villeneuve DL, Meek ME, McQueen CA. 2016. Adverse outcome pathways—organizing toxicological information to improve decision making. J Pharmacol Exper Ther. 356:170–181.
- EFSA (European Food Safety Authority). 2006. Opinion of the scientific panel on plant protection products and their residues (PPR Panel) on a request from the Commission on the Safety of Aldicarb MRLs. EFSA J. 409:1–23.
- EFSA (European Food Safety Authority). 2008. Scientific Opinion on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. Panel on Contaminants in the Food Chain (CONTAM). Question Number: EFSA-Q-2004-163. [cited 2016 Jan 6]. Available from: http://www.efsa.europa.eu/en/efsajournal/pub/653
- EFSA (European Food Safety Authority). 2009. Scientific opinion. Cadmium in food. Panel on Contaminants in the Food Chain (CONTAM). Question No EFSA-Q-2007-138. EFSA J. 980:1–139. Available from: http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/contam_op_ej980_cadmium_en_rev.1%2C2.pdf
- EFSA (European Food Safety Authority). 2011. Scientific Report: Comparison of the Approaches Taken by EFSA and JECFA to Establish a HBGV for Cadmium. EFSA, Parma, Italy. Available from: http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/2006.pdf
- EFSA (European Food Safety Authority). 2012. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. Panel on Contaminants in the Food Chain (CONTAM). Question Number: EFSA-Q-2011-00923. EFSA J. 10:2985.
- EFSA (European Food Safety Authority). 2015a. Guidance on Uncertainty in EFSA Scientific Assessment EFSA Scientific Committee (draft). [cited 2016 Jan 6]. Available from: http://www.efsa.europa.eu/sites/default/files/consultation/150618.pdf
- EFSA (European Food Safety Authority). 2015b. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: PART II – Toxicological assessment and risk characterisation. EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) European Food Safety Authority (EFSA), Parma, Italy. Available from: http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/3978part2.pdf
- Fujita T, Matsumoto Y, Ozaki M, Majima M, Kumagai Y, Ohtani Y. 2006. Comparison of maximum drug concentration and area under the time–concentration curve between humans and animals for oral and intravenous investigational drugs. J Clin Pharmacol. 46:674–692.
- Gentry PR, Covington TR, Andersen ME, Clewell HJ. 2002. Application of a physiologically based pharmacokinetic model for isopropanol in the derivation of a reference dose and reference concentration. Regul Toxicol Pharmacol. 36:51–68.
- Gentry PR, Covington T, Clewell H, Anderson M. 2003. Application of a physiologically based pharmacokinetic model for reference dose and reference concentration estimation for acetone. J Toxicol Environ Health Part A. 66:2209–2225.
- Gentry PR, Haber LT, McDonald TB, Zhao Q, Covington T, Nance P, Clewell III HJ, Lipscomb JC, Barton HC. 2004. Data for physiologically based pharmacokinetic modeling in neonatal animals: physiological parameters in mice and Sprague-Dawley rats. J Children’s Health. 2:363–411.
- Haber L. 2007. Overview of approach to noncancer risk assessment. In: Lipscomb JC, Ohanian E, editor. Toxicokinetics and risk assessment. New York, NY: Informa Healthcare; p. 1–25.
- Hasegawa R, Hirata-Koizumi M, Dourson ML, Parker A, Ono A, Hirose A. 2013. Safety assessment of boron by application of new uncertainty factors and their subdivision. Regul Toxicol Pharmacol. 65:108–114.
- Health Canada 2001a. Priority Substances List Assessment Report for 2-Buthoxyethanol. Environment Canada, Health Canada. Available from: http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/psl2-lsp2/2_butoxyethanol/index-eng.php
- Health Canada. 2001b. Environment Canada, Health Canada. Priority substances list assessment report for chloroform. Available from: http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/psl2-lsp2/chloroform/index-eng.php
- Health Canada 2006. Guidelines for Canadian drinking water quality: guideline technical document. Trihalomethanes. Federal-Provincial-Territorial Committee on Drinking Water of the Federal-Provincial-Territorial Committee on Health and the Environment, Health Canada, Ottawa, Ontario, May 2006 (with April 2009 addendum). Available from: http://www.hc-sc.gc.ca/ewh-semt/alt_formats/pdf/pubs/water-eau/trihalomethanes/trihalomethanes-eng.pdf
- Health Canada. 2013. Guideline technical document. Guidelines for Canadian drinking water quality: guideline technical document – vinyl chloride. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Cat.: H144-13/6-2013E-PDF). Available from: http://www.hc-sc.gc.ca/ewh-semt/alt_formats/pdf/pubs/water-eau/vinyl_chloride/vinyl_chloride-eng.pdf
- Health Canada. 2014a. Guideline technical document. Guidelines for Canadian drinking water quality: guideline technical document — toluene, ethylbenzene and xylenes. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No H144-20/2015E-PDF). Available from: https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/publications/healthy-living-vie-saine/water-toluene-eau/alt/water-toluene-eau-eng.pdf
- Health Canada. 2014b. Guideline technical document (draft for public comment). Tetrachloroethylene in drinking water. Released June 2014. Federal-Provincial-Territorial Committee on Drinking Water. Available from: http://www.hc-sc.gc.ca/ewh-semt/alt_formats/pdf/consult/_2014/tetrachloroethylene/consult-eng.pdf
- Health Canada. 2015. Draft, public consultation version. Bromate in drinking water. Released on November 27, 2015. Available from: http://www.healthycanadians.gc.ca/health-system-systeme-sante/consultations/bromate/document-eng.php
- Health Canada 2016. Sulfoxaflor Registration Decision RD2016-12, Sulfoxaflor, 1 April 2016. Available from: http://www.hc-sc.gc.ca/cps-spc/pubs/pest/_decisions/rd2016-12/index-eng.php [further details noted in 2015 draft compared to 2016 final].
- HPA (Health Protection Agency). 2007. Comparison of processes and procedures for deriving exposure criteria for the protection of human health: chemicals, ionising radiation and non-ionising radiation. Health Protection Agency, RCE-3, 2007. [cited 2016 Jan 6]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/335159/RCE-3_protectionHH_July07_full.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). 2004. Evaluation of certain food additives and contaminants. Methylmercury. TRS 922. Available from: http://whqlibdoc.who.int/trs/WHO_TRS_922.pdf?ua=1
- JECFA (Joint FAO/WHO Joint Expert Committee on Food Additives). 2007. Evaluation of certain food additives and contaminants. Methylmercury. TRS 940. JECFA 67. Available from: http://apps.who.int/iris/bitstream/10665/43592/1/WHO_TRS_940_eng.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). 2009. A risk-based decision tree approach for the safety evaluation of residues of veterinary drugs, WHO, Geneva. [cited 2016 Jan 6]. Available from: http://www.who.int/foodsafety/chem/jecfa/decision_tree_mar_2009_final_for_web.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). 2011a. Evaluation of certain food additives and contaminants. Cadmium. WHO TRS 960. Available from: http://apps.who.int/iris/bitstream/10665/44515/1/WHO_TRS_960_eng.pdf
- JECFA (Joint FAO/WHO Joint Expert Committee on Food Additives). 2011b. Evaluation of certain contaminants in food. Deoxynivalenol. TRS 959. JECFA 72. Available from: http://whqlibdoc.who.int/trs/WHO_TRS_959_eng.pdf?ua=1
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). 2013. Evaluation of certain food additives and contaminants. Cadmium. WHO TRS 983. JECFA 77. Available from: http://apps.who.int/iris/bitstream/10665/98388/1/9789241209830_eng.pdf?ua=1
- JMPR (Joint Meeting on Pesticide Residues). 2001. Pesticide residues in food – 2000: Report of the Joint FAO/WHO Meeting on Pesticide Residues, Geneva, Switzerland, 20–29 September 2000. ISBN 92-5-104547-X. Plant Production and Protections Papers 163, FAO, Rome.
- JMPR (Joint FAO/WHO Expert Meeting on Pesticide Residues). 2006. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 3–12 October 2006. Available from: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/JMPRrepor2006.pdf
- JMPR (Joint FAO/WHO Expert Meeting on Pesticide Residues). 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 18–27 September 2007. Available from: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/report2007jmpr.pdf
- JMPR (Joint FAO/WHO Expert Meeting on Pesticide Residues). 2008. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 9–18 September 2008. Available from: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/JMPRReport08.pdf
- Kirman CR, Sweeney LM, Corley R, Gargas ML. 2005. Using physiologically‐based pharmacokinetic modeling to address nonlinear kinetics and changes in rodent physiology and metabolism due to aging and adaptation in deriving reference values for propylene glycol methyl ether and propylene glycol methyl ether acetate. Risk Anal. 25:271–284.
- Kirman CR, Sweeney LM, Gargas ML, Strother DE, Collins JJ, Deskin R. 2008. Derivation of noncancer reference values for acrylonitrile. Risk Anal. 28:1375–1394.
- Kirman CR, Grant RL. 2012. Quantitative human health risk assessment for 1, 3-butadiene based upon ovarian effects in rodents. Regul Toxicol Pharmacol. 62:371–384.
- KemI (The Swedish National Chemicals Inspectorate). 2003. Proposals for the use of assessment (uncertainty) factors: Application to risk assessment for plant protection products, industrial chemicals and biocidal products within the European Union. Stockholm, Sweden.
- Lehman AJ, Fitzhugh OG. 1954. Ten-fold safety factor studies. Assoc. Food Drug Off US Q Bull XVIII. 1:33–35.
- Liao KH, Tan YM, Conolly RB, Borghoff SJ, Gargas ML, Andersen ME, Clewell HJ. 2007. Bayesian estimation of pharmacokinetic and pharmacodynamic parameters in a mode‐of‐action‐based cancer risk assessment for chloroform. Risk Anal. 27:1535–1551.
- Loizou G, Spendiff M, Barton HA, Bessems J, Bois FY, d’Yvoire MB, Buist H, Clewell HJ, Meek B, Gundert-Remy U, Goerlitz G. 2008. Development of good modelling practice for physiologically based pharmacokinetic models for use in risk assessment: the first steps. Regul Toxicol Pharmacol. 50:400–411.
- Lu Y, Rieth S, Lohitnavy M, Dennison J, El-Masri H, Barton HA, Bruckner J, Yang RS. 2008. Application of PBPK modeling in support of the derivation of toxicity reference values for 1,1,1-trichloroethane. Regul Toxicol Pharmacol. 50:249–260.
- Meek ME, Ohanian E, Renwick A, Naumann B, Lake B, Vu V, Dourson M. 1999. Guidelines for application of data-derived uncertainty factors in risk assessment. Report of a Meeting by Toxicology Excellence for Risk Assessment for US EPA/Health Canada, Washington, March 25th–26th.
- Meek ME, Renwick A, Ohanian E, Dourson M, Lake B, Naumann BD, Vu V. 2002. Guidelines for application of chemical-specific adjustment factors in dose/concentration-response assessment. Toxicology. 181-182:115–120.
- Meek ME, Boobis AR, Crofton KM, Heinemeyer G, Van Raaij M, Vickers C. 2011. Risk assessment of combined exposure to multiple chemicals: a WHO/IPCS framework. Regul Toxicol Pharmacol. 60:S1–S14.
- Meek ME, Barton HG, Bessems J, G, Lipcomb J, C, Krishnan K. 2013. Case study illustrating the WHO/IPCS guidance on characterization and application of physiologically based pharmacokinetic models in risk assessment. Regul. Toxicol. Pharmacol. 66:116–129.
- Meek ME, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S, Seed J, Vickers C. 2014a. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol. 34:1–18.
- Meek ME, Palermo CM, Bachman AN, North CM, Jeffrey Lewis R. 2014b. Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol. 34:595–606.
- MDH (Minnesota Department of Health). 2015. Bisphenol A. CAS: 80-05-7. Health Risk Limits for Groundwater. Health Risk Assessment Unit, Environmental Health Division. Available from: http://www.health.state.mn.us/divs/eh/risk/guidance/gw/bpatoxsumm.pdf
- Mörk AK, Jonsson F, Johanson G. 2014. Adjustment factors for toluene, styrene and methyl chloride by population modeling of toxicokinetic variability. Reg Toxicol Pharmacol. 69:78–90.
- Mörk AK, Johanson G. 2010. Chemical specific adjustment factors for intra-species variability of acetone toxicokinetics using a probabilistic approach. Toxicol Sci. 116:336–348.
- National Research Council (NRC). (2007). Toxicity testing in the 21st century: a vision and a strategy. Washington, DC: National Academy Press.
- Naumann BD, Silverman KC, Dixit R, Faria EC, Sargent EV. 2001. Case studies for catergorical data-derived adjustment factors. Human Ecol. Risk Assess. 7:61–105.
- NIHS Japan. Personal communication from Akihiko Hirose to WHO in response to Call-for-Data. 3 September, 2015. CSAF approach for boron assessment.
- OECD (Organisation of Economic Co-operation and Development). 2012. Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology – Important Issues on Risk Assessment of Manufactured Nanomaterials ENV/JM/MONO(2012)8. [cited 2016 Jan 6]. Available from: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2012)8&doclanguage=en
- OECD (Organization for Economic Cooperation and Development). 2016. Users' Handbook supplement to the Guidance Document for developing and accessing Adverse Outcome Pathways. OECD Series on Adverse Outcome Pathways. Available from: http://dx.doi.org/10.1787/2415170X. July
- Pelekis M, Gephart LA, Lerman SE. 2001. Physiological-model-based derivation of the adult and child pharmacokinetic Human Variability uncertainty factors for volatile organic compounds. Regul Toxicol Pharmacol. 33:12–20.
- Poet TS, Kirman CR, Bader M, van Thriel C, Gargas ML, Hinderliter PM. 2010. Quantitative risk analysis for N-methyl pyrrolidone using physiologically based pharmacokinetic and benchmark dose modeling. Toxicol Sci. 113:468–482.
- Poet TS, Schlosser PM, Rodriguez CE, Parod RJ, Rodwell DE, Kirman CR. 2016. Using physiologically based pharmacokinetic modeling and benchmark dose methods to derive an occupational exposure limit for N-methylpyrrolidone. Regul. Toxicol. Pharmacol. 76:102–122.
- Price PS, Keenan RS, Schwab B. 1999. Defining the interindividual (intraspecies) uncertainty factor. Human Ecol. Risk Assess. 5:1023–1033.
- Renwick AG. 1991. Safety factors and establishment of acceptable daily intakes. Food Addit Contam. 8:135–149.
- Renwick AG. 1993. Data-derived safety factors for the evaluation of food additives and environmental contaminants. Food Addit Contam. 10:275–305.
- Renwick AG, Lazarus NR. 1998. Human variability and noncancer risk assessment–an analysis of the default uncertainty factor. Regul. Toxicol. Pharmacol. 27:3–20.
- Sarangapani R, Gentry PR, Covington TR, Teeguarden JG, Clewell HJ, III. 2003. Evaluation of the potential impact of age- and gender-specific lung morphology and ventilation rate on the dosimetry of vapors. Inhal. Toxicol. 15:987–1016.
- Silverman KC, Naumann BD, Holder DJ, Dixit R, Faria EC, Sargent EV, Gallo MA. 1999. Establishing data-derived adjustment factors from published pharmaceutical clinical trial data. Human Ecol. Risk Assess. 5:1059–1089.
- Sweeney LM, Kirman CR, Gargas ML, Carson ML, Tardiff RG. 2010. Development of a physiologically-based toxicokinetic model of acrylamide and glycidamide in rats and humans. Food Chem Toxicol. 48:668–685.
- TCEQ (Texas Commission on Environmental Quality). 2015. 1,3-butadiene. CAS Registry Number: 106-99-0. Development Support Document. Available from: https://www.tceq.texas.gov/assets/public/implementation/tox/dsd/final/butadiene,%201,3-.pdf
- Troutman JA, Rick DL, Stuard SB, Fisher J, Bartels MJ. 2015. Development of a physiologically-based pharmacokinetic model of 2-phenoxyethanol and its metabolite phenoxyacetic acid in rats and humans to address toxicokinetic uncertainty in risk assessment. Regul Toxicol Pharmacol. 73:530–543.
- Thompson CM, Kirman CR, Proctor DM, Haws LC, Suh M, Hays SM, Hixon JG, Harris MA. 2014. A chronic oral reference dose for hexavalent chromium‐induced intestinal cancer. J Appl Toxicol. 34:525–536.
- UK COT (UK Committee on Toxicity). 2006. Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. COT Statement on the Tolerable Daily Intake for Perfluorooctanoic Acid. Available from: http://cot.food.gov.uk/sites/default/files/cot/cotstatementpfoa200610.pdf
- UK COT (United Kingdom Committee on Toxicity). 2007. Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Variability and Uncertainty in Toxicology of Chemicals in Food, Consumer Products and the Environment. [cited 2016 Jan 6]. Available from: http://cot.food.gov.uk/sites/default/files/cot/vutreportmarch2007.pdf
- UK COT (UK Committee on Toxicity). 2009. Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Update statement on the Tolerable Daily Intake for Perfluorooctanoic Acid. Available from: http://cot.food.gov.uk/sites/default/files/cot/cotstatementpfoa200902.pdf
- UK EA (United Kingdom Environmental Agency). 2009. Human health toxicological assessment of contaminants in soil. Science Report – Final SC050021/SR2. [cited 2016 Jan 6]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/291011/scho0508bnqy-e-e.pdf
- US EPA (US Environmental Protection Agency). 1994. Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. Office of Research and Development. EPA/600/8-90/066F. Research Triangle Park, NC.
- US EPA (US Environmental Protection Agency). 2000. Toxicological review of vinyl chloride. CAS No. 75-01-4. In Support of Summary Information on the Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency, Washington, DC Available from: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/1001tr.pdf
- US EPA (US Environmental Protection Agency). 2001. Water quality criterion for the protection of human health: methylmercury. EPA/823/R 01/001. Washington, DC: U.S. Environmental Protection Agency, Office of Water. Available from: http://www.waterboards.ca.gov/water_issues/programs/tmdl/records/state_board/2008/ref2664.pdf
- US EPA (US Environmental Protection Agency). 2002. A review of the reference dose and reference concentration process. U.S. Environmental Protection Agency. Risk Assessment Forum. EPA/630/P-02/00F. Washington DC 20460.
- US EPA (Environmental Protection Agency). 2004. IRIS toxicological review of boron and compounds. Available from: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0410tr.pdf
- US EPA (Environmental Protection Agency). 2006. Revised Reregistration Eligibility Decision for MSMA, DSMA, CAMA, and Cacodylic Acid. Office of Pesticide Programs and Toxic Substances (OPPTS), Washington, DC. Available from: https://archive.epa.gov/pesticides/reregistration/web/pdf/organic_arsenicals_red.pdf
- US EPA (US Environmental Protection Agency). 2007. N-methyl carbamates revised cumulative risk assessment. Office of Pesticide Programs. Washington DC. Available from: http://www.regulations.gov/#!docketDetail;D=EPA-HQ-OPP-2007-0935
- US EPA (US Environmental Protection Agency). 2009. Provisional Health Advisories for Perfluorooctanoic Acid (PFOA) and Perfluorooctyl Sulfonate (PFOS). Available from: https://www.epa.gov/sites/production/files/2015-09/documents/pfoa-pfos-provisional.pdf
- US EPA (US Environmental Protection Agency). 2010. Toxicological review of ethylene glycol monobutyl ether (EGBE). (CAS No. 111-76-2. In Support of Summary Information on the Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency, Washington, DC. Available from: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0500tr.pdf
- US EPA (US Environmental Protection Agency). 2011. Recommended Use of Body Weight ¾ as the Default Method in Derivation of the Oral Reference Dose. U.S. Environmental Protection Agency, Risk Assessment Forum. EPA/100/R11/0001.
- US EPA (U.S. Environmental Protection Agency). 2012a. Advances in Inhalation Gas Dosimetry for Derivation of a Reference Concentration (RfC) and Use in Risk Assessment. National Center for Environmental Assessment. EPA/600/R-12/044. Washington, DC 20460.
- US EPA (Environmental Protection Agency). 2012b (draft). TSCA Work Plan Chemical Risk Assessment. N-methylpyrrolidone: paint stripper use. CAS#872-50-4. External Review Draft, December 2012. Office of Chemical Safety and Pollution Prevention, Washington, DC. Available from: http://www.scgcorp.com/dcm-nmp2013/PDFs/NMP%20Draft%20Risk%20Assessment%20(External%20Review%20Draft).pdf
- US EPA (U.S. Environmental Protection Agency). 2014. Guidance for applying quantitative data to develop data-derived extrapolation factors for interspecies and intraspecies extrapolation. Risk Assessment Forum. EPA/100/R-14/002F. Washington (DC) 20460.
- US EPA (US Environmental Protection Agency). 2015. TSCA Work Plan Chemical Risk Assessment. N-methylpyrrolidone: paint stripper use. CAS#872-50-4. EPA/740/R1/5002, March 2015. Available from: http://www.epa.gov/sites/production/files/2015-11/documents/nmp_ra_3_23_15_final.pdf
- US EPA (Environmental Protection Agency). 2016a. PFOA. Health effects support document for perfluorooctanoic acid (PFOA). Health and ecological criteria division, Washington, DC. EPA Document Number: 822-R-16-003. May 2016. Available from: https://www.epa.gov/sites/production/files/2016-05/documents/pfoa_hesd_final-plain.pdf
- US EPA (Environmental Protection Agency). 2016b. Health effects support document for perfluorooctane sulfonate (PFOS). Health and ecological criteria division, Washington, DC. EPA Document Number: 822-R-16-002. May 2016. Available from: https://www.epa.gov/sites/production/files/2016-05/documents/hesd_pfos_final-plain.pdf
- Valcke M, Krishnan K. 2014. Characterization of the human kinetic adjustment factor for the health risk assessment of environmental contaminants. J Appl Toxicol. 34:227–240.
- Valcke M, Krishnan K. 2011. Assessing the impact of the duration and intensity of inhalation exposure on the magnitude of the variability of internal dose metrics in children and adults. Inhal Toxicol. 23:863–877.
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety). 1994. Environmental Health Criteria 170: Assessing human health risks of chemicals: Derivation of guidance values for health-based exposure limits. WHO/IPCS. Geneva, Switzerland. Available from: http://www.inchem.org/documents/ehc/ehc/ehc170.htm
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety). 2005. Chemical-Specific Adjustment Factors (CSAF) for interspecies differences and human variability: guidance document for the use of data in dose/concentration-response assessment. (IPCS harmonization project document no. 2). WHO/IPCS/01.4, 1-96. Geneva, Switzerland. Available from: http://www.inchem.org/documents/harmproj/harmproj/harmproj2.pdf
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety). 2010. Characterization and application of physiologically based pharmacokinetic models in risk assessment. (IPCS harmonization project document no. 9). Geneva, Switzerland. Available from: http://www.who.int/ipcs/methods/harmonization/areas/pbpk/en/
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety). 2014. Guidance document on evaluating and expressing uncertainty in hazard characterization. (IPCS harmonization project document no. 11). Geneva, Switzerland. Available from: http://www.who.int/ipcs/methods/harmonization/uncertainty_in_hazard_characterization.pdf
- Yang X, Morris SM, Gearhart JM, Ruark CD, Paule MG, Slikker Jr W, Mattison DR, Vitiello B, Twaddle NC, Doerge DR, et al. 2014. Development of a physiologically based model to describe the pharmacokinetics of methylphenidate in juvenile and adult humans and nonhuman primates. PloS One. 9:e106101.
- Yoon M, Campbell JL, Andersen ME, Clewell HJ. 2012. Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit Rev Toxicol. 42:633–652.
Appendix
Call-for Data
Call to OECD member countries for data to inform the WHO Chemical Risk Assessment Network project entitled “Review of the application of chemical-specific adjustment factors (CSAF) in quantitative risk assessment; strengths, limitations and research needs"
The World Health Organization (WHO) Chemical Risk Assessment Network is requesting the assistance of OECD member countries (via the Task Force on Hazard Assessment) with a project called “Review of the application of Chemical-Specific Adjustment Factors (CSAF) in quantitative risk assessment; strengths, limitations and research needs." The objective of this project is to consider the use of CSAF in chemical risk assessment as a basis to further facilitate uptake.
The WHO/IPCS Guidance on CSAF (published in 2005) is available at http://whqlibdoc.who.int/publications/2005/9241546786_eng.pdf.
The project is being led by WHO Collaborating Centre on Water and Indoor Air Quality and Food Safety, at NSF International, USA.
A working group formed by members of the WHO Chemical Risk Assessment Network is requesting the cooperation of OECD member countries to supply data to this project. OECD member countries and stakeholders are requested to provide chemical risk assessments that may have used (or tried unsuccessfully to use) CSAF to the working group. The formal status of the assessment (e.g. published, submitted to a regulatory scheme, incomplete, internal prioritization only) is not important since relevant information on CSAF could be obtained from many types of document. However, completed (and if possible externally-reviewed) assessments are preferred.
It would also be helpful to the project if the questions listed below could be completed.
Are you aware of unsuccessful attempts to develop or adopt CSAF’s (or Data Derived Extrapolation Factors, DDEF’s) within your organization? If so, what factors were important (e.g., lack of sufficient data; perceived complexity of communication; issues of science policy related to degree of conservatism for health protection)? Attach supporting information, if available.
Do you know of any CSAF (or attempted CSAF) or DDEFs (or attempted DDEFs) outside of your organization? If so, please provide citation or focal point for contact information.

