Abstract
Keflin (kefl) interacts with Co(II), Cu(II), Ni(II) and Zn(II) metal ions leading to complexes of the type M(kefl)2Cl2 and M(kefl)Cl2, which have been characterized by physicochemical and spectroscopic methods. Magnetic moment, IR, electronic spectral and elemental analyses data suggest that keflin behaves tridentately forming octahedral or trigonal bipyramidal complexes with the metal ions mentioned above. The new compounds have been screened in-vitro for antibacterial and cytotoxic activity against Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, Shigella dysentriae, Bacillus cereus, Corynebacterium diphtheriae, Staphylococcus aureus and Streptococcus pyogenes bacterial strains. Compounds, 4 and 8 showed promising activity (90%) against seven, compound 6 showed significant activity (52%) against four and, compounds 1 and 5 showed activity (40%) against three test bacterial strains at concentration of 10 μM.
Introduction
Metal ions play a key role in the actions of antibiotic drugs. They are involved in specific interactions with antibiotics, proteins, membrane components, nucleic acids, and other biomolecule [Citation1]. DNA can also bind many different biomolecule and synthetic compounds, including proteins, antibiotics, polyamines, metal complexes and organometallic compounds [Citation2]. In such specific interactions, transcription is regulated to turn on and/or off a specific biological process. DNA is also a target for therapeutic treatment of various disorders and diseases, such as cancers via direct ligand binding to it or binding to DNA-regulating biomolecule. Several clinically used anti-cancer antibiotics such as bleomycin, streptonigrin and albomycin are DNA binding agents [Citation3]. A better understanding of the structure of these antibiotics and their DNA complexes, and their relationship [Citation4] between structure, function, and toxicity can provide information for the design of more effective and less toxic drugs. Such investigations of the interaction between DNA and synthetic compounds or metal complexes can improve our understanding of DNA-ligand binding, which may provide clues for rational DNA-specific drug design. This demand is driven by an emerging medical problem, i.e., the bacterial drug resistance to presently available antibiotics, with an accelerating rate at which bacteria develop resistance [Citation5]. This resistance is alarmingly spreading among Gram-positive organisms. The release of relatively large amounts of β-lactamase into the surrounding media actually destroy the β-lactam antibiotics by hydrolysis of the β-lactam ring, this being the most prevalent mechanism of bacterial drug resistance [Citation6,Citation7].
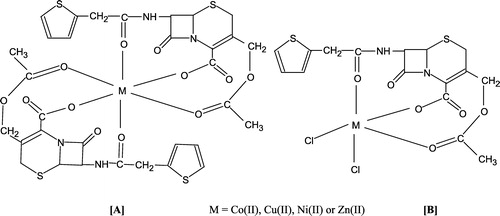
In order to address this problem, we have commenced a program Citation8-15 for synthesizing novel classes of bactericidal and fungicidal complexes of transition metals which could potentially reduce (interfere with) the mechanism of bacterial resistance due to coordination of the cation(s) Citation16-20. In continuation of this research, the present paper describes the synthesis, characterization and in-vitro evaluation of the antibacterial and cytotoxic activities of newly synthesized Co (II), Cu (II), Ni (II) and Zn (II) complexes with the antibiotic drug, keflin () against Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, Shigella dysentriae, Bacillus cereus, Corynebacterium diphtheriae, Staphylococcus aureus and Streptococcus pyogenes bacterial strains. The in-vitro antibacterial activity results were found to be generally stronger than those of the uncoordinated keflin. We found that the solubility of these reported metal complexes is better than that of keflin itself, both in water and ethanol. On the basis of IR, UV, molar conductivity together with elemental analysis data, reasonable structures for these newly synthesized complexes have been proposed by using two different molar ratios of metal:keflin (1:2) and (1:1) ( & B). The in-vitro antibacterial results indicated the complexes having molar ratio metal: keflins of 1:1 () were found to be more antibacterial than the complexes with molar ratio 1:2 ().
Material and methods
Keflin sodium salt was obtained from Pharmagen Beximco Ltd, Pakistan. Solvents used were analar grade. All metal (II) salts were used as chlorides. IR spectra (KBr pellets) were recorded on a Philips Analytical PU 9800 FTIR spectrophotometer. NMR spectra were recorded on a Perkin–Elmer 283B spectrometer. UV–Visible spectra were obtained in DMF on a Hitachi U-2000 double-beam spectrophotometer. C, H and N analyses were carried out by Butterworth Laboratories Ltd. Conductances of the metal complexes were determined in DMF on a Hitachi YSI-32 model conductometer. Magnetic measurements were done on solid complexes using the Gouy's’ method. Melting points were recorded on a Gallenkamp apparatus and are not corrected.
Preparation of metal (II) complexes
The complexes, M(kefl)2Cl2 and M(kefl)Cl2 were prepared by mixing keflin (2 mmol) and (1 mmol) respectively with the metal (II) as chloride (1 mmol) in methanol (50 ml). The pH of the solution was adjusted to 8.0 with 5.0 M NaOH. Then the mixture was refluxed for 1 h and then cooled at room temperature. On cooling, a solid product was precipitated which was filtered off, washed with methanol, then with ether and dried. Crystallization from aqueous-methanol (30:70) gave the desired metal complex ().
Table I. Physical and spectral data of the metal (II) complexes.
Biological activity
Antibacterial bioassay (in-vitro)
All the synthesized metal (II) complexes were screened in-vitro for their antibacterial activity against E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, S. typhi, S. dysentriae, B. cereus, C. diphtheriae, S. aureous and S. pyogenes bacterial strains using the agar well diffusion method [Citation21]. Two to eight hours old bacterial inoculums containing approximately 104–106 colony forming units (CFU)/ml were used in these experiments. The wells were dug in the agar media using a sterile metallic borer with centers at least 24 mm. Recommended concentration (100 μl) of the test sample (1 mg/ml in DMSO) was introduced in the respective wells. Other wells supplemented with DMSO and reference antibacterial drug, keflin served as negative and positive controls respectively. The plates were incubated immediately at 37oC for 20 h. Activity was determined by measuring the diameter of zones showing complete inhibition (mm). Growth inhibition was compared with the standard drug, keflin.
Minimum inhibitory concentration (MIC)
Those compounds alone which showed promising antibacterial activity, were selected for minimum inhibitory concentration (MIC) studies. MIC was determined using the disc diffusion technique by preparing discs containing 10, 25, 50 and 100 μM of the compounds and applying the reported protocol [Citation22].
Cytotoxicity (in-vitro)
Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm), filled with artificial seawater, which was prepared with commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened while the minor compartment was opened to ordinary light. After two days nauplii were collected by a pipette from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 ml of DMF. From this stock solutions 500, 50 and 5 μg/ml were transferred to 9 vials (three for each dilutions were used for each test sample and LD50 is the mean of three values) and one vial was kept as control having 2 ml of DMF only. The solvent was allowed to evaporate overnight. After two days, when shrimp larvae were ready, 1 ml of seawater and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with seawater to 5 ml per vial. After 24 h the number of survivors were counted. Data were analyzed by a Finney computer program to determine the LD50 values [Citation22].
Results and discussion
Chemistry
The interaction of metal ions with keflin in molar ratios of metal:keflin (1:2 and 1:1) resulted in the formation of the complexes, [M(kefl)2]Cl2 and [M(kefl)Cl2] where M = cobalt (II), copper (II), nickel(II) and zinc(II) ( & B). The molar conductance values in methanol fall within the usual range 145–150 Ω− 1 cm2 mol− 1 for complexes 1–4, showing their electrolytic and nature 18–20 Ω− 1 cm2 mol− 1 for complexes 5–8 with a non-electrolytic nature [Citation23] which, suggests that the chloride ions are not coordinated with the metal ions in complexes 1–4 and remain coordinated in complexes 5–8. The complexes decomposed rather than melting above 200°C. All of the complexes are stable in air and moisture and their solubility is much better than uncoordinated keflin, both in water and methanol.
IR spectra
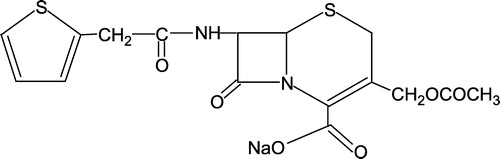
The infrared spectral data of and their assignments are given in comparing mainly IR frequencies of the metal complexes with that of keflin. The drug ligand exhibits strong absorption bands at 1765, 1730, 1715 and 1665 cm− 1 due to νC = O of β-lactam, νCOO− , νC = O of amide and νC = O of acetyl stretching vibrations [Citation24]. On comparison with the metal complexes, the band at 1765 assigned to νC = O of β-lactam remained unchanged suggesting non-involvement of this group. However, the bands at 1730, 1715 and 1665 cm− 1 completely vanished in the spectra of metal complexes and instead, strong bands positioned at 1605 and 1420 cm− 1 indicating that the νCOO− group emerged as two absorption bands νasym COO− and νsym COO− suggesting [Citation25] coordination via the carboxylate group with the metal atoms. Similarly, bands at 1715 and 1665 cm− 1 due to amide-C = O and acetyl-C = O moieties in the spectrum of the drug ligand disappeared and shifted to lower frequencies (20–30 cm− 1) at 1690 and 1645 cm− 1 indicating [Citation26] involvement of these groups in coordination. On the basis of this data we propose that the drug ligand, keflin is acting as tridentate. Other, conclusive evidence [25] of the coordination of the oxygen of these moieties with the metal atom was observed by the appearance of a new band at 435–445 cm− 1 assigned as metal-oxygen ν(M–O) in the spectra of the metal complexes which in turn, was not observable in the spectrum of the drug ligand. Also, in the far IR region a band at 315 cm− 1 was observed in the complexes 5–8 indicating coordination of metal to the chloride atoms (M–Cl).
Table II. Selected IR and UV–visible spectral data of keflin and its complexes.
UV–visible spectra and magnetic moment
The Co(II) complex exhibited well-resolved, low-energy bands at 7,425 cm− 1 and 17,170 cm− 1 and a strong high-energy band at 20,450 cm− 1 assigned [Citation27] to the transitions 4T1g(F) → 4T2g(F), 4T1g(F) → 4A2g(F) and 4T1g(F) → 4T2g(P) for a high-spin octahedral geometry [Citation28]. A high intensity band at 29,775 cm− 1 was assigned to the metal to ligand charge transfer (). The magnetic susceptibility measurements (4.1 B.M) for the solid Co (II) complex is also indicative [Citation29] of three unpaired electrons per Co (II) ion suggesting [Citation30] consistency with their octahedral environment ().
The electronic spectra of the Cu (II) complex () showed a low-energy weak band at 16,590 cm− 1 and a strong high-energy band at 30,325 cm− 1. The low-energy band in this position typically is expected for a square-planar configuration [Citation31] and may be assigned to 2B1g → 2Eg transitions, respectively. The strong high-energy band, in turn, is assigned to metal → ligand charge transfer. Also, the magnetic moment () for the Cu (II) complex (1.6 B.M) was found to be consistent with the proposed octahedral structure of the Cu (II) complex ().
The electronic spectra of the Ni (II) complex showed d–d bands in the region 10,655, 15,560 and 26,375 cm− 1. These are assigned [Citation29] to the transitions 3A2g(F) → 3T2g(F), 3A2g(F) → 3T1g(F) and 3A2g(F) → 3T2g(P), respectively, consistent with their well-defined octahedral configuration. The band at 30,145 cm− 1 was assigned to the metal → ligand charge transfer. The magnetic measurements (3.3 B.M) showed two unpaired electrons per Ni (II) ion suggesting [Citation30] also an octahedral geometry for the Ni (II) complex (). The electronic spectra of the Zn (II) complex exhibited only a high-intensity band at 29,775 cm− 1 and are assigned [Citation28] to a ligand-metal charge transfer. However, using an equimolar ratio (1:1) of metal:drug ligand, a trigonal bipyramidal structure () for the metal complexes (5–8) was proposed.
Biological activity
Antibacterial bioassay
All metal complexes were screened against E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, S. typhi, S. dysenteriae, B. cereus, C. diphtheriae, S. aureus and S. pyogenes bacterial strains () according to literature protocol [Citation21,Citation22]. The results were compared with those of the standard uncoordinated drug, keflin. It was evident that generally, the overall potency of keflin was enhanced upon coordination with the metal ions and complexes and 1–4 were found to be more antibacterial than the complexes 5–8.
Table III. In-vitro antibacterial activity data of the keflin and metal (II) complexes.
Cytotoxic bioassay
All the synthesized compounds were screened for their cytotoxicity (brine shrimp bioassay) using the reported protocol [Citation22]. It has been observed that only copper (II) complexes (2 & 6) displayed a weak cytotoxic activity (LD50 = 395 & 288 μg) against Artemia salina, while the other compounds gave values of LD50 (1000 μg in this assay, and therefore can be considered to be inactive for this assay.
Minimum inhibitory concentration
The MIC of some selected compounds, which showed significant activity against selected bacterial species, was determined using the disc diffusion method [Citation21,Citation22]. MIC of these compounds varies from 10–100 μM. The results shown in , indicated that compound 4 showed a promising activity (90%) at concentration 10 μM against seven bacterial strains (a), (b), (c), (d), (e), (f) and (k) and, a significant activity (52%) against bacterial strain (j) at concentration 25 μM. Compound 8 similarly, showed a promising activity (90%) at concentration 10 μM against seven bacterial strains (a), (b), (c), (d), (f), (j) and (k) and, a significant activity (52%) against bacterial strain (e) at concentration 25 μM. A significant activity (52%) for compound 6, was shown against (c), (d), (j) and (k) bacterial strains at concentration 25 μM. The remaining compounds showed activity (40%) at concentration 100 μM against test strains.
Table IV. Minimum inhibitory concentration (μM) of Keflin and its metal complexes against some selected bacterial species.
The antibacterial activity data of the antibiotic drug keflin generally exhibited enhancement in activity upon coordination with the metal ions. The compounds generally showed moderate antibacterial activity against two or four species and significant activity against one or two species. It was also observed that the activity of complexes 5–8 which were formed by 1:1 molar ratio of metal:keflin, was more than that of the complexes 1–4 in which metal:keflin ratio was 1:2. This enhancement in the activity may be rationalized on the basis of their chelation property in which the coordination mainly reduces the polarity [Citation32,Citation33] of the metal ion because of the partial sharing of its positive charge with the donor groups within the chelate ring system formed during coordination. This process may favor its permeation through the biological membranes of the micro-organism Citation34-39 thus destroying them more aggressively, and making metal complexes of antibiotic drugs potentially more effective than the antibiotic itself. However our in-vivo studies are in progress, which may introduce in future, the potential use of metalloantibiotics in clinical practice, provided that the presence of heavy metal ions does not induce an undesired toxicity to such compounds.
References
- Ming Li-June. Metalloantibiotics. Med Res Rev 2003; 23: 697
- Tullius TD. Metal-DNA Chemistry. ACS Symposium Series 402, The American Chemical Society, New York 1989
- Barton JK. Metal-Nucleic acid Interactions. Bioinorganic Chemistry, University Science Books, London 1994
- Hinton JF, Koeppe RE. Complexing properties of gramicidins. Met Ion Biol Syst 1985; 19: 173
- Claussen CA, Long EC. Nucleic acid recognition by metal complexes of bleomycin. Chem Rev 1999; 99: 2797
- Bravo A, Anacona JR. Synthesis and characterization of metal complexes with ampicillin. J Coord Chem 1998; 44: 173
- Anacona JR, Figueroa EM. Zinc (II), cadmium (II), mercury (II) and lead (II) semiquinone-type complexes of a new Schiff base ligand: Antibacterial studies. J Coord Chem 1999; 48: 181
- Chohan ZH, Scozzafava A, Supuran CT. Synthesis of biologically active Co(II), Cu(II), Ni(II) and Zn(II) complexes of symmetrically 1,1′-disubstituted ferrocene-derived compounds. Synth React Inorg Met-Org Chem 2003; 33: 241
- Chohan ZH, Scozzafava A, Supuran CT. Zinc complexes of benzothiazole-derived Schiff-bases with antibacterial activity. J Enz Inhib Med Chem 2003; 17: 261
- Chohan ZH. Antibacterial copper(II) complexes of 1,1-symmetric ferrocene-derived Schiff-base ligands: Studies of the effect of anions on their antibacterial properties. Appl Organomet Chem 2002; 16: 17
- Chohan ZH, Farooq MA, Scozzafava A, Supuran CT. Antibacterial Schiff bases of oxalyl-hydrazine/diamide incorporating pyrrolyl and salicylyl moieties and of their zinc(II) complexes. J Enz Inhib Med Chem 2002; 17: 1
- Chohan ZH, Rauf A, Supuran CT. Antibacterial Co(II) and Ni(II) complexes of N-(2-furanylmethylene)-2-aminothisdiazole and role of , NO3, and anions on biological properties. Metal-Based Drugs 2002; 8: 287
- Chohan ZH, Iqbal MS, Iqbal HS, Scozzafava A, Supuran CT. Transition metal acetylsalicylates and their anti-inflammatory activity. J Enz Inhib Med Chem 2002; 17: 87
- Hassan MU, Chohan ZH, Supuran CT. Antibacterial Zn(II) compounds of Schiff bases derived from some benzothiazoles. Main Group Metal Chemistry 2002; 25: 291
- Chohan ZH. Biologically active Co(II) and Ni(II) complexes of N-(2-thienylmethylene)-2-aminothiadiazole. Metal Based Drugs 2002; 8: 323
- Chohan ZH, Pervez H, Rauf A, Scozzafava A, Supuran CT. Antibacterial Co(II), Cu(II), Ni(II) and Zn(II) complexes of thiadiazole derived furanyl, thiophenyl and pyrrolyl Schiff bases. J Enz Inhib Med Chem 2002; 17: 117
- Chohan ZH, Pervez H, Kausar S, Supuran CT. Synthesis and characterization of antibacterial metal-[Co(II), Cu(II), Ni(II) and Zn(II)] acylhydrazine derived pyrrolyl compounds. Synth React Inorg Met-Org Chem 2002; 3: 529
- Chohan ZH, Rauf A, Noreen S, Scozzafava A, Supuran CT. Antibacterial cobalt(II), nickel(II) and zinc(II) complexes of nicotinic acid-derived Schiff-bases. J Enz Inhib Med Chem 2002; 17: 101
- Hassan MU, Chohan ZH, Supuran CT. Antibacterial Co(II) and Ni(II) complexes of benzothiazole-derived Schiff-bases. Synth React Inorg Met-Org Chem 2002; 32: 1649
- Chohan ZH, Pervez H, Rauf A, Supuran CT. Antibacterial role of , , and , anions on Cu(II) and Zn(II) complexes of thidiazole derived pyrrolyl Schiff base. Metal Based Drugs 2002; 8: 263
- Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR. Vogels' Text Book of Practical Organic Chemistry5th ed. Longman (U.K.) 1994
- Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay Techniques for Drug Development. Harwood Academic Publishers, The Netherlands 2001; 16
- Geary WJ. Use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord Chem Rev 1971; 7: 81
- Nakamoto K. Infrared Spectra of Inorganic and Coordination Compounds2nd ed. Wiley Interscience, New York 1970
- Bellamy LJ. The Infrared Spectra of Complex Molecules3rd ed. John Wiley, New York 1971
- Ferraro JR. Low Frequency Vibrations of Inorganic and Coordination Compounds2nd ed. John Wiley, New York 1971
- Carlin RL. Transition Metal Chemistry. Marcel Decker, New York 1965; Vol 1
- Ballhausen CJ. An Introduction to Ligand Field. McGraw-Hill, New York 1962
- Lever ABP. Inorganic Electronic Spectroscopy. Elsevier, Amsterdam 1984
- Meek DW, Drago RS, Piper TS. Spectrochemical studies of dimethyl sulfoxide, tetramethylene sulfoxide and pyridine-N-oxide as ligands with nickel (II), chromium (II) and cobalt (II). Inorg Chem 1962; 1: 285
- Drago RS. Physical Methods in Inorganic Chemistry. Reinhold, New York 1965
- Chohan ZH. Synthesis and biological properties of Cu(II) complexes with 1,1′-disubstituted ferrocenes Synth. React Inorg Met-Org Chem 2004; 34: 833
- Chohan ZH, Scozzafava A, Supuran CT. Zinc complexes of benzothiazole-derived Schiff-bases with antibacterial activity. J Enz Inhib Med Chem 2003; 18: 259
- Chohan ZH, Supuran CT, Scozzafava A. Metalloantibiotics: Synthesis and Antibacterial Activity of Cobalt(II), Copper(II), Nickel(II) and Zinc(II) Complexes of Kefzol. J Enz Inhib Med Chem 2004; 19: 79
- Chohan ZH, Praveen M. Synthesis, characterization and co-ordination properties of Cu(II), Co(II), Ni(II) and Zn(II) complexes with novel asymmetric 1,1-diacetylferrocene-derived Schiff-base ligand. Syth React Inorg Met-Org Chem 2000; 30: 175–182
- Chohan ZH, Praveen M. Synthesis, characterization and antibacterial properties of symmetric 1,1-ferrocene derived Schiff-base ligands and their Co(II), Cu(II), Ni(II) and Zn(II) Chelates. Appl Organomet Chem 2000; 14: 376–382
- Chohan ZH, Munawar A, Supuran CT. Transition metal ion complexes of Schiff bases. Synthesis, characterization and antibacterial properties. Metal Based Drugs 2001; 8: 137
- Chohan ZH, Supuran CT. Synthesis and characterization of Zn(II) complexes with some acylhydrazine Schiff bases. Main Group Metal Chem 2001; 24: 399
- Chohan ZH, Jaffery MF, Supuran CT. Antibacterial Co(II), Cu(II), Ni(II) and Zn(II) complexes of thiadiazoles Schiff bases. Metal Based Drugs 2001; 8: 95