Abstract
The tripeptide antioxidant glutathione (γ-l-glutamyl-l-cysteinyl-glycine; GSH) essentially contributes to thiol-disulphide conversions, which are involved in the control of seed development, germination, and seedling establishment. However, the relative contribution of GSH metabolism in different seed structures is not fully understood. We studied the GSH/glutathione disulphide (GSSG) redox couple and associated low-molecular-weight (LMW) thiols and disulphides related to GSH metabolism in bread wheat (Triticum aestivum L.) seeds, focussing on redox changes in the embryo and endosperm during germination. In dry seeds, GSH was the predominant LMW thiol and, 15 h after the onset of imbibition, embryos of non-germinated seeds contained 12 times more LMW thiols than the endosperm. In germinated seeds, the embryo contained 17 and 11 times more LMW thiols than the endosperm after 15 and 48 h, respectively. This resulted in the embryo having significantly more reducing half-cell reduction potentials of GSH/GSSG and cysteine (Cys)/cystine (CySS) redox couples (EGSSG/2GSH and ECySS/2Cys, respectively). Upon seed germination and early seedling growth, Cys and CySS concentrations significantly increased in both embryo and endosperm, progressively contributing to the cellular LMW thiol-disulphide redox environment (Ethiol-disulphide). The changes in ECySS/2Cys could be related to the mobilisation of storage proteins in the endosperm during early seedling growth. We suggest that EGSSG/2GSH and ECySS/2Cys can be used as markers of the physiological and developmental stage of embryo and endosperm. We also present a model of interaction between LMW thiols and disulphides with hydrogen peroxide (H2O2) in redox regulation of bread wheat germination and early seedling growth.
Introduction
There is a long-standing interest in the role of the low-molecular-weight (LMW) thiol tripeptide glutathione (γ-l-glutamyl-l-cysteinyl-glycine, GSH) in the seeds of higher plants. Glutathione has a plethora of roles in eukaryote and prokaryote cells [Citation1], and in seeds is crucial to many seed quality traits, including viability and longevity [Citation2,Citation3]. Glutathione synthesis involves two ATP-dependent reactions. First, γ-l-glutamyl-l-cysteine (γ-Glu-Cys) is formed, catalysed by γ-Glu-Cys ligase (EC 6.3.2.2), and then glycine (Gly) is added by glutathione synthase (EC 6.3.2.3). Glutathione degradation likely involves the formation of cysteinyl-glycine (Cys-Gly) and 5-oxoproline [Citation4,Citation5]. As a major water-soluble antioxidant and redox buffer, the nucleophile GSH is involved in the control of reactive oxygen species (ROS) through non-enzymatic and enzymatic reactions, including the glutathione–ascorbate cycle [Citation6]. Redox control by GSH is crucial throughout the whole seed life cycle from seed development, maturation drying, imbibition, germination, to seed ageing, and death. Furthermore, GSH is the storage and long-distance transport form of reduced sulphur during early seedling growth [Citation3,Citation7]. The majority of crop plants produce orthodox (i.e. desiccation tolerant) seeds, which gradually desiccate upon maturation drying and, when shed from the mother plant, can survive in a desiccated state for many years before germination. During seed maturation drying, GSH is progressively oxidised to glutathione disulphide (GSSG), which can bind to cysteine (Cys) residues of proteins via S-glutathionylation, resulting in a temporary increase of protein-bound glutathione (PSSG). This redox conversion protects free protein thiol groups (PSH) from auto-oxidation and oxidation to sulphonic or sulphenic acids [Citation3,Citation7–10]. Once shed, a further conversion of thiols to disulphides can accompany after-ripening of dry seeds [Citation11], and another oxidative shift accompanies seed ageing [Citation2,Citation12].
The seeds of bread wheat (Triticum aestivum L., Poaceae) have received attention regarding thiol-disulphide conversions since the discovery of GSH by Hopkins [Citation13–15]. Furthermore, the LMW thiol dipeptides Cys-Gly and γ-Glu-Cys were first isolated from wheat seeds [Citation16,Citation17]. Species of the Poaceae produce caryopses (in which the fruit coat, or “pericarp”, and the seed coat, or “testa”, are fused), hereafter called “seeds” for simplicity. Wheat is among the three most important crops used for staple food, including bread [Citation18]. Starch is the main energy reserve in wheat seeds which, on a dry weight (DW) basis, also contain 10–15% of proteins in the aleurone layer and the starchy endosperm (a seed tissue that provides nutrients to the emerging seedling), mostly prolamins [Citation19]. In wheat, prolamins are major components of gluten, a continuous matrix within the endosperm whose structural properties are governed by disulphide bonds between Cys residues [Citation19,Citation20].
Germination of orthodox seeds starts with the uptake of water, which enables metabolism to resume and energy reserves to be mobilised [Citation21–23]. By definition, germination sensu stricto is completed when the radicle protrudes through the structures surrounding the embryo [Citation24]. PSSG and GSSG are rapidly reduced early upon germination [Citation25,Citation26], and protein redox state is enzymatically controlled by thioredoxins (TRXs) and glutaredoxins (GRXs), which use NADPH and GSH for de-glutathionylation [Citation7,Citation10]. Cellular redox changes during seed imbibition also involve the production of ROS, which promote key events in germination and seedling development. Particularly, the ROS hydrogen peroxide (H2O2) is thought to be a critical player in alleviating seed dormancy (i.e. the inability to germinate under optimal conditions), stimulating reserve mobilisation, and participating in the regulation of early seedling growth and development [Citation27–32]. However, if the control of ROS levels by antioxidants fails, oxidative damage may result in seed ageing, and eventually death [Citation33].
The half-cell reduction potential (Ehc) is an accurate descriptor of the redox state of concentration-dependent redox couples, and changes in the glutathione half-cell reduction potential (EGSSG/2GSH) accompany cell development from mitoses to cell death in human cells [Citation34]. In the orchard grass, Dactylis glomerata L., more negative (i.e. more reducing) values of EGSSG/2GSH accompany proliferation, and more positive (i.e. more oxidising) values accompany differentiation upon somatic embryogenesis [Citation35]. Furthermore, EGSSG/2GSH has been proposed to be a useful marker of seed viability and ageing, as a pronounced shift towards strongly oxidising conditions accompanies programmed cell death (PCD) [Citation2,Citation11,Citation36,Citation37]. Besides the GSH/GSSG redox couple, the Cys and cystine (CySS) couple and the dipeptides γ-Glu-Cys and Cys-Gly, along with their corresponding disulphides (i.e. bis-γ-glutamyl-cystine (bis-γ-Glu-Cys), and cystinyl-bis-glycine (Cys-bis-Gly)) contribute to the cellular LMW thiol-disulphide redox environment (Ethiol-disulphide) in seeds [Citation37]. Ethiol-disulphide is calculated by the sum of the products of the individual half-cell reduction potentials (Ehcs) of all LMW thiol-disulphide redox couples and the reducing capacity of each individual couple [Citation34]. Changes in Ethiol-disulphide have been related to somatic embryogenesis and seed ageing [Citation11,Citation35,Citation37].
In summary, thiol-disulphide conversions of the GSH/GSSG redox couple play important roles during seed maturation and germination, but their relative contribution in different wheat seed structures has not been studied. Furthermore, the intermediates of GSH metabolism, namely Cys, γ-Glu-Cys, and Cys-Gly, have not been assessed in different seed tissues. In the present study, we elucidate changes in the concentrations and redox state of the GSH/GSSG redox couple, alongside those of its metabolic intermediates, in the embryo and the endosperm of wheat seeds during germination and early seedling growth. To provide a more comprehensive view of the changes in LMW thiol-disulphide redox couples that accompany the establishment of the next plant generation, their concentrations, and redox state are considered in embryos and endosperms, separately.
Materials and methods
All chemicals were purchased from Sigma Aldrich, Co. (St. Louise, MO), unless specified otherwise.
Seed material, germination assays, and water content measurements
Bread wheat (Triticum aestivum L.) cultivar Rebelde (Apsovsementi S.p.a., Voghera, Italy – CO.NA.SE. Consorzio Nazionale Sementi S.r.l., Italy) was grown in an open field in Sant’Angelo Lodigiano (Lodi, Italy) in 2013-2014. After harvest, seeds were immediately stored at 4 °C and <8% water content (WC), on a fresh weight (FW) basis, and used within 1 year. Seed samples were cleaned (i.e. damaged seeds were discarded and residual glumes were removed) and germinated in 90 mm Petri dishes between two layers of filter paper (Whatman 1, GE Healthcare, Little Chalfont, United Kingdom) moistened with 3 mL of distilled water, each containing 35 seeds (n = 7), in darkness at 20 °C. Completion of germination was defined as radicle protrusion by at least 1 mm through the coleorhiza, a protective sheath of the radicle, and the seed coat and was scored regularly until all viable seeds had germinated. After 15 h from the onset of imbibition, it was possible to cleanly excise the embryo, including the scutellum (a part of the single modified cotyledon), from the seed using a sterile scalpel. Two time points, 15 h and 48 h, were chosen for detailed studies into the redox state of different seed structures. The remaining seed parts with the endosperm, including the aleurone layer and the fused seed coat and pericarp are hereafter referred to as “endosperm”, for simplicity. All material was weighed to determine the FW, frozen in liquid nitrogen, freeze-dried for 5 days, and then weighed to record the DW. Freeze-dried seeds were finely ground in 5-mL liquid-nitrogen-cooled Teflon capsules with one 7 mm diameter agate ball, using a Mikro-Dismembrator S (B. Braun, Biotech International, Melsungen, Germany) at 3000 rpm for 4 min. Seed WC was calculated on a FW basis by the formula: WC = (FW – DW)/FW ×100.
HPLC analysis of LMW thiols and disulphides
At nine intervals up to 48 h after the onset of imbibition, whole seeds (n = 4 replicates of 35 seeds), and dissected embryos and endosperms isolated after 15 h or 48 h of imbibition (n = 4 replicates of 40 seeds) were analysed by HPLC. Freeze-dried powder from whole seeds (70 ± 6 mg), endosperms (50 ± 0.5 mg), or embryos (25 ± 5.0 mg) were extracted at 4 °C in 1 mL of 0.1 M HCl, vortexed at full speed for 1 min before centrifugation at 20,000 × g for 20 min at 4 °C. An aliquot of 120 μL of the supernatant was used for the determination of total LMW thiols and disulphides, and 400 μL for assessing LMW disulphides according to Kranner (1998) [Citation38], and described in detail by Bailly and Kranner (2011) [Citation39]. The pH of the extracts was adjusted to values between 8.0 and 8.3 with 200 mM bicine buffer. To measure both LMW thiols and disulphides, the latter were reduced by 273 μM dithiothreitol (DTT, Applichem Gmbh, Darmstadt, Germany) for 1 h at room temperature, before labelling of thiols with 857 μM monobromobimane (mBBr) for 15 min at room temperature, and stopping the reaction with 0.104% (v/v) methanesulfonic acid. To measure disulphides, thiols were blocked with 583 μM N-ethylmaleimide (NEM) for 15 min at room temperature, before excess NEM was removed five times with toluene, and disulphides were reduced with DTT, then labelled with mBBr as for measuring total LMW thiols and disulphides. Labelled LMW thiols were separated by reversed-phase HPLC using an Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA, USA) on a ChromBudget 120-5-C18 column (5.0 μm, BISCHOFF GmbH, Leonberg, Germany), and detected by a fluorescence detector (the excitation wavelength was set at 380 nm and the emission wavelength at 480 nm). Data were calculated using individual calibration curves for each LMW thiol that were linear over the range measured. The concentrations of LMW thiols were calculated by subtracting the concentrations of LMW disulphides from those of total LMW disulphides and thiols.
Calculation of the cellular LMW thiol-disulphide based redox environment
The half-cell reduction potential (Ehc) for each thiol-disulphide couple was calculated according to the Nernst equation, and mathematically combined into the LMW thiol-disulphide redox environment (Ethiol-disulphide) [Citation2,Citation37]:
(1)
(2) where R is the gas constant (8.314 J K−1 mol−1); T, temperature in K; n, number of transferred electrons (2 GSH → GSSG + 2 H+ + 2 e−); F, Faraday constant (9.6485 × 104 C mol−1); E0′, standard half-cell reduction potential of a thiol-disulphide redox couple at an assumed cellular pH of 7.3 (E0′GSSG/2GSH = −258 mV, E0′CySS/2Cys = −244 mV, E0′Cys-bis-Gly/2Cys-Gly = −244 mV, E0′bis-γ-Glu-Cys/2γ-Glu-Cys = −252 mV) [Citation37]. Ei is the half-cell reduction potential of an individual redox couple i, and [reduced species]i is the concentration of the reduced species in that redox pair. The molar concentrations of LMW thiols and disulphides for each redox couple were calculated based on seed WC calculated as g H2O g−1 DW.
Oxalate oxidase activity measurements and quantification of extracellular H2O2 production
Oxalate oxidase (EC 1.2.3.4, oxalate: oxygen oxidoreductase, OXO) activity of whole seeds was determined spectrophotometrically, using a modification of the method of Laker et al. (1980) [Citation40]. OXO catalyses the oxidative breakdown of one mole of oxalate to one mole of H2O2 and two moles of CO2. Briefly, H2O2 was indirectly quantified via the formation of indamine, catalysed by the H2O2-dependent horseradish peroxidase (HPOX), by following the increase in absorbance at 555 nm (A555). Absorbance values were calibrated using a standard curve for H2O2 within a 2.4–120 μM range. All extraction steps were conducted at 4 °C (on ice). 50 ± 0.5 mg of seed powder were homogenised in 0.4 mL of 50 mM succinate buffer (pH 3.8) containing 1 mM EDTA and 9.6 mM oxalic acid in 2 mL Eppendorf tubes with two 3 mm glass beads (Carl Roth GmbH + Co. KG, Karlsruhe, Germany), using a TissueLyser II (Qiagen, Hilden, Germany) for 1 min at 30 Hz. After centrifugation at 20,000 × g for 5 min at 4 °C, the supernatant was collected and centrifuged again at 20,000 × g for 2 min. OXO activity was measured in 96-well BRANDplates® (pureGRADE™ S-clear, Sterile R, BRAND GmbH + CO KG, Wertheim, Germany), using a Synergy-HTX plate reader (BioTek® Instruments, Inc., Winooski, VT) at 35 °C after pre-incubating 51.2 μL of protein extract and oxalic acid for 15 min. The increase in A555 was followed for 10 min after adding 185 μL of a chromogenic solution (790 μM of N,N-dimethylaniline and 110 μM of 3-methyl-2-benzothiazolinone hydrazone dissolved in 50 mM succinate buffer, pH 3.8) and 2.5 U/mL of HPOX in a total volume of 240 μL, with a final concentration of oxalic acid of 2 mM. A reaction mixture without protein extract and oxalic acid was used as a blank. Three technical replicates for each biological replicate (n = 4) were measured.
H2O2 was quantified using a “Red Hydrogen Peroxide Assay Kit”, following optimisation of instructions by the manufacturer (Enzo Life Sciences Inc., Farmingdale, NY). Whole seeds and seedlings were incubated in 1 mL of two times concentrated reaction mixture containing 10 μL of red peroxidase substrate stock solution in dimethyl sulphoxide (DMSO), 40 μL of 20 U/mL HPOX stock solution, and 950 μL of assay buffer in darkness at room temperature for 30 min, then diluted 1:2 with the reaction buffer. Fluorescence of the HPOX- and H2O2-catalysed formation of Resorufin (excitation wavelength =540 ± 35 nm, emission wavelength =590 ± 20 nm) was measured with a Synergy-HTX plate reader (BioTek® Instruments, Inc., Winooski, VT). Data were corrected for background fluorescence occurring in the presence of pure assay buffer. Three technical replicates were measured for each biological replicate (n = 4, each containing 25 seeds). The content of extracellular H2O2 produced was calculated from a standard curve for H2O2 (0–3 μM range), and data are expressed as nmol g−1 DW s−1.
Statistical analysis
Data were analysed for significance (α = .05) by one-way ANOVA in combination with Tukey's HSD (Honest Significant Difference) test for post-hoc comparisons of means (p-value ≤.05), using the IBM SPSS Statistics 21 software package. Arcsine transformation was applied to germination and WC values to simulate normal distribution of data. The assumption of normal distribution was assessed via the Shapiro–Wilk test, and further verified with QQ-plots. The assumption of homoscedasticity of variances across groups was checked through Levene's test, and, whenever not respected, the appropriate mathematical transformations were applied to the data. To compare different seed structures at the same time interval a one-sample t-test was applied (p-value ≤.05).
Results
Seed germination and water uptake
The first radicle protruded 12 h after the onset of imbibition, and 50% of total germination (TG) was reached after approximately 22 h (). Within 48 h, 96% of seeds had germinated. The radicle and two seminal roots developed (in wheat this is indicative of healthy seedlings), and the coleoptile (a protective sheath covering the emerging shoot in monocotyledon plants) could be clearly distinguished in most seedlings. After 8 h of imbibition, the water uptake slowed down (), typical for Poaceae seeds [Citation41,Citation42].
Figure 1. TG and WC during seed germination and early seedling growth of Triticum aestivum. Closed circles show TG, and open circles show WC. Data are means ± SE (n = 7 replicates of 35 seeds for TG and n = 4 replicates of 35 seeds for WC). Error bars within symbols are not shown.
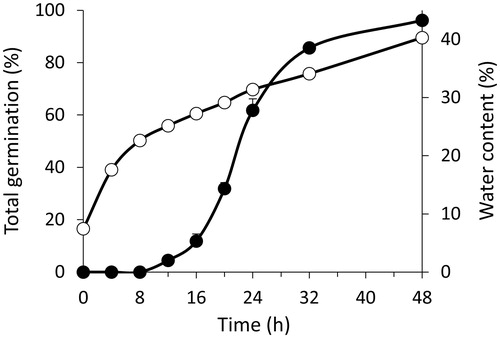
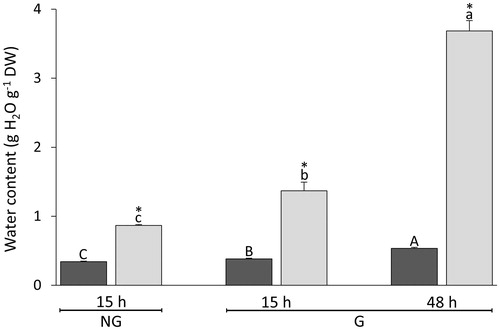
After 15 h from the onset of imbibition, it was first possible to accurately dissect the embryo from the endosperm; embryos and endosperms isolated from germinated (G) seeds and non-germinated (NG) seeds were analysed separately. After 15 and 48 h from the onset of imbibition, the embryo made up only 3.1 ± 0.2% and 4.9 ± 0.2% (means ± SE) of seed DW, respectively. Embryos and endosperms took up water at different rates, and the former always contained more water than the latter ().
Figure 2. Changes in WC during imbibition of Triticum aestivum seeds. After 15 and 48 h from the onset of imbibition, NG seeds were sorted from G seeds. Endosperms (dark grey bars) and their embryos or seedlings (light grey bars) were dissected and analysed separately. Data are means ± SE (n = 4 replicates of 40 endosperms and embryos). After the onset of imbibition, bars labelled with the same letter do not differ significantly (one-way ANOVA analyses followed by post-hoc Tukey's HSD test, p-value ≤.05). Asterisks indicate significant differences (one-sample t-test, p-value ≤.05) between seed structures at the same time interval.
Changes in LMW thiols and disulphides in whole seeds during germination and early seedling growth
GSH + GSSG [hereafter termed “total glutathione”] and Cys + CySS [hereafter referred to as “cyst(e)ine”] dominated the LMW thiol-disulphide redox pool in dry whole seeds, whereas the concentrations of Cys-Gly + Cys-bis-Gly [hereafter termed as “cyst(e)inyl-(bis)-glycine”] and γ-Glu-Cys + bis-γ-Glu-Cys [hereafter referred to as “(bis)-γ-glutamyl-cyst(e)ine”] were lower by one order of magnitude (). Dry wheat seeds contained 4.7 times higher concentrations of total glutathione than cyst(e)ine; total glutathione was 7.4 and 67.8 times more abundant than cyst(e)inyl-(bis)-glycine and (bis)-γ-glutamyl-cyst(e)ine, respectively (). Disulphide contents in dry whole seeds, expressed as a percentage of total thiols and disulphides, were 30% for bis-γ-Glu-Cys, 36% for GSSG, 40% for Cys, and 41% for Cys-bis-Gly (). Species of the Poaceae may also contain a GSH homologue termed hydroxy-methyl-glutathione (hGSH, γ-l-glutamyl-l-cysteinyl-β-serine) [Citation43], but no corresponding peak was detected in seeds or seedlings during the first 48 h after the onset of imbibition.
Figure 3. Concentrations of LMW thiols and corresponding disulphides during germination and early seedling growth of Triticum aestivum. The abscissa shows the time after the onset of imbibition, and time 0 indicates dry seeds. (A) GSH and GSSG; (B) Cys and CySS; (C) Cys-Gly and Cys-bis-Gly; (D) γ-Glu-Cys and bis-γ-Glu-Cys. Data are means ± SE for the four LMW thiols (white bars) and corresponding disulphides (black bars). Data labelled with the same letters do not differ significantly (one-way ANOVA analyses followed by post-hoc Tukey's HSD test, p-value ≤.05). For ease of comparison, germination curve is indicated by the grey line.
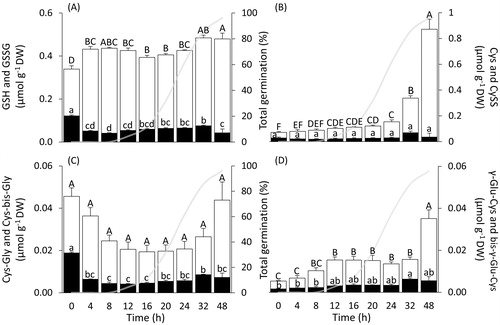
Total glutathione concentrations rose 1.3-fold within the first 8 h of imbibition, GSH increased by 43%, and GSSG decreased by 42%. A further significant increase in total glutathione, mostly due to GSH, was recorded during early seedling growth (i.e. between 24 h and 48 h after the onset of imbibition) ().
Cyst(e)ine concentrations steadily increased within 24 h, after which concentrations greatly increased (). Hence, after 48 h, cyst(e)ine was the most abundant LMW thiol-disulphide redox couple, and its concentration became 1.8 times higher than that of total glutathione (). Within the first 12 h of imbibition, cyst(e)inyl-(bis)-glycine decreased 2.3-fold, (bis)-γ-glutamyl-cyst(e)ine increased 2.8-fold, then both plateaued for the subsequent 12 h (). From 24 h of imbibition, when approximately 62% of seeds had germinated (), cyst(e)inyl-(bis)-glycine and (bis)-γ-glutamyl-cyst(e)ine both increased progressively.
Changes in LMW thiol-disulphide redox couples in endosperm and embryo on a DW and on a seed basis
After 15 and 48 h from the onset of imbibition, seeds were sorted into two lots, G and NG, and embryos and seedlings were separated from the endosperms. At these intervals, we characterised the concentrations of LMW thiols and disulphides, expressed on a DW basis (), and their corresponding Ehcs in the two seed structures (). As in whole seeds, total glutathione and cyst(e)ine were the predominating LMW thiol-disulphide redox couples in both endosperm and embryo. Fifteen hours after the onset of imbibition, the endosperms isolated from NG seeds (termed “NG15_endosperms”) contained the same concentrations of LMW thiols and disulphides as the endosperms isolated from germinated seeds (termed “G15_endosperms”), and had the same thiol-disulphide ratios (p-value >.05; ). By contrast, embryos isolated from NG seeds 15 h after the onset of imbibition (termed “NG15_embryos”) contained 3–19 times higher concentrations of all LMW thiols than whole dry seeds. Embryos with radicles protruding at least 1 mm and isolated from germinated seeds 15 h after the onset of imbibition (termed “G15_embryos”) had about one third more total glutathione, one forth more cyst(e)inyl-(bis)-glycine, and five times more (bis)-γ-glutamyl-cyst(e)ine than NG15_embryos. In particular, G15_embryos contained more disulphides than NG15_embryos (p-value ≤.05), except for CySS ().
Figure 4. Concentration, on a DW basis, and redox state of four low-molecular-weight (LMW) redox couples in structures isolated from Triticum aestivum seeds upon imbibition. After 15 h from the onset of imbibition, white bars for LMW thiols and black bars for their corresponding disulphides show dry weight-based concentrations in endosperms and embryos isolated from NG seeds or G seeds. After 48 h from the onset of imbibition, bars show thiol and disulphide DW-based concentrations for endosperms and seedlings isolated from germinated seeds (panels A--D). Bars on the right side show the half-cell reduction potentials of the four LMW thiol-disulphide redox couples in the endosperm (dark grey bars) and embryo or seedling (light grey bars), respectively (panels E--H). Data for endosperms and embryos or seedlings were tested for significance using one-way ANOVA analyses followed by post-hoc Tukey's HSD test for thiols (lower case letters) and disulphides (upper case letters). Data points labelled with the same letter do not differ significantly (p-value ≤.05; italics is used for comparing the endosperms). Data are means ± SE (n = 4 replicates of 40 endosperms and embryos or seedlings per condition). NG: non-germinated; G: germinated; 15 and 48 indicate the hours after the onset of imbibition.
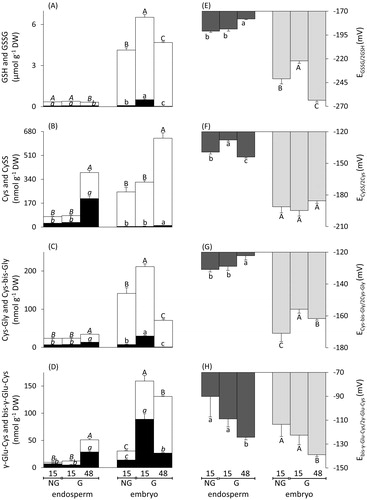
After 48 h from the-onset of imbibition, 96% of seeds had germinated (). Only germinated seeds were characterised for their concentrations of LMW thiols and disulphides in isolated seed structures 48 h after the onset of imbibition. Total glutathione in the endosperms isolated from germinated seeds 48 h after the onset of imbibition (termed “G48_endosperms”) dropped by 16% compared to G15_endosperms (p-value ≤.05). Conversely, cyst(e)ine, cyst(e)inyl-(bis)-glycine, and (bis)-γ-glutamyl-cyst(e)ine increased about five-, two-, and four-fold, with high disulphide percentages (52, 44, and 40%, here arranged from the highest to the lowest contribution to each thiol-disulphide redox pool). In seedlings with coleoptile, radicle, and two seminal roots isolated from germinated seeds 48 h after the onset of imbibition (termed “G48_embryos”), total glutathione, cyst(e)inyl-(bis)-glycine, and (bis)-γ-glutamyl-cyst(e)ine concentrations dropped two-, three-, and two-fold compared to G15_embryos, whereas cyst(e)ine almost doubled. Low disulphide percentages were found for total glutathione (0.1%), cyst(e)inyl-(bis)-glycine (1.5%), and (bis)-γ-glutamyl-cyst(e)ine (1.8%), but not for (bis)-γ-glutamyl-cyst(e)ine, which was found as bis-γ-Glu-Cys for 20.4% (). In summary, G15_embryos showed markedly higher concentrations of LMW thiols and disulphides than NG15_embryos, but this trend was not evident in NG15_endosperms and G15_endosperms. NG15_ embryos, G15_embryos and G48_embryos had far higher thiol-disulphide ratios than NG15_endosperms, G15_endosperms and G48_endosperms (except for the redox couple γ-Glu-Cys/bis-γ-Glu-CySS in G15_embryos) (). Thereafter, a phase of early seedling growth was identified by comparing G15_embryos and G48_embryos. In both G48_embryos and G48_endo-sperms, total glutathione declined, while cyst(e)ine increased. In contrast, cyst(e)inyl-(bis)-glycine and (bis)-γ-glutamyl-cyst(e)ine increased in G48_endosperms, but decreased in G48_embryos ().
When the total contents of LMW thiol-disulphide redox couples were expressed on a seed basis, their concentrations and proportions in the overall LMW thiol-disulphide redox pool changed distinctly (Figure S1). In particular, NG15_endosperms and G15_endosperms had the same concentrations and proportional distributions of the four LMW thiol-disulphide redox couples. In G48_endosperms, the total LMW thiol-disulphide redox pool increased 1.5-fold, and cyst(e)ine became the dominant LMW thiol-disulphide redox couple (Figure S1(A)). By contrast, after 15 h, the total LMW thiol-disulphide redox pool in G15_embryos was greater than in NG15_ embryos, with a larger proportion represented by (bis)-γ-glutamyl-cyst(e)ine (Figure S1(B)). The total LMW thiol-disulphide redox pool further increased in G48_embryos compared to G15_embryos, with a decline in cyst(e)inyl-(bis)-glycine, and an increase in cyst(e)ine proportions, but total glutathione remained the dominant LMW thiol-disulphide redox couple (Figure S1(B)).
Changes in LMW thiol-disulphide half-cell reduction potentials in endosperm and embryo
The tissue WCs (g H2O g−1 DW; ) were used to calculate the molar concentrations of all thiols and disulphides (Figure S2), which are required for the individual Ehcs of the four thiol-disulphide redox couples (). The EGSSG/2GSH, the half-cell reduction potential of the cysteinyl-glycine/cystinyl-bis-glycine redox couple (ECys-bis-Gly/2Cys-Gly), and the half-cell reduction potential of the γ-Glu-Cys/bis-γ-Glu-Cys redox couple (Ebis-γ-Glu-Cys/2γ-Glu-Cys) did not differ between NG15_endosperms and G15_endosperms. However, values of the half-cell reduction potential of the CySS/2Cys redox couple (ECySS/2Cys) were more oxidising in NG15_endosperms. ECySS/2Cys shifted back to more negative values in G48_endosperms compared to G15_endosperms (), and EGSSG/2GSH and ECys-bis-Gly/2Cys-Gly slightly shifted towards more positive values ().
In G15_embryos, EGSSG/2GSH and ECys-bis-Gly/2Cys-Gly values were by 19 mV and 15 mV more positive than in NG15_embryos. These values shifted back to more negative values (by 41 mV and 6 mV) in G48_embryos (). Values of ECySS/2Cys did not differ significantly between NG15_embryos and G15_embryos or G15_embryos and G48_embryos. The Ebis-γ-Glu-Cys/2γ-Glu-Cys did not differ between NG15_embryos and G15_embryos, but in G48_embryos, it shifted to a more negative value than that of NG15_embryos.
Due to their highest molar concentrations (Figure S2), EGSSG/2GSH and ECySS/2Cys were the most influential contributors to the mathematically combined Ethiol-disulphide of dry whole seeds, NG15_endosperms, G15_endosperms, G48_endosperms, and NG15_embryos, G15_embryos and G48_embryos (i.e. all measured samples), whereas ECys-bis-Gly/2Cys-Gly and Ebis-γ-Glu-Cys/2γ-Glu-Cys together contributed less than 8% (). ECySS/2Cys contributed more to Ethiol-disulphide in endosperms than in embryos or seedlings, and the contributions of ECySS/2Cys increased in both seed structures during early seedling growth.
Table 1. Relative contribution of the individual half-cell reduction potentials (Ehcs) of the four LMW thiol-disulphide redox couples to the LMW thiol-disulphide redox environment (Ethiol-disulphide) in dry whole seeds and isolated Triticum aestivum seed structures upon imbibition. Values for the individual contributions of the thiol-disulphide redox couples are expressed as percentages of Ethiol-disulphide calculated for dry whole seeds and seed structures isolated after 15 or 48 h from the onset of imbibition. Data show means ± SE (n = 4). G: germinated; NG: non-germinated.
Changes in the H2O2 production during germination and early seedling growth
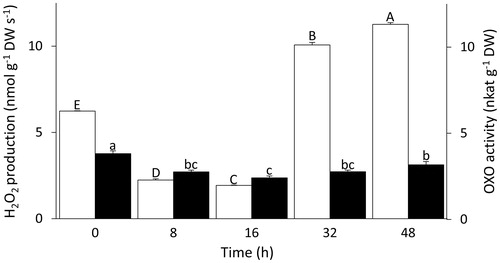
The H2O2 production rate decreased three-fold from the first imbibition interval to 16 h after the onset of imbibition (). Between 16 and 32 h after the onset of imbibition, when the majority of seeds germinated (), the rate of H2O2 production increased by five-fold and further increased after 48 h (). Similar to the pattern of H2O2 production, the activity of OXO decreased in the first 16 h of imbibition and then increased, but the trend was much less pronounced ().
Figure 5. Changes in H2O2 production and OXO activity during Triticum aestivum seed germination and early seedling growth. White bars show the rates of H2O2 production (left axis) and black bars show OXO activity (right axis). Both variables were tested for significance using one-way ANOVA analyses followed by post-hoc Tukey's HSD test. Bars labelled with the same letter do not differ significantly (p-value ≤.05). Upper case letters are used for H2O2 production rates, and lower case letters for OXO activity. Data show means ± SE (n = 3 replicates of 25 seeds for H2O2 production; n = 4 replicates of 35 seeds for OXO activity).
Discussion
Early seedling growth is accompanied by GSH synthesis and mobilisation of Cys
In this paper, we report on LMW thiol-disulphide conversions involved in the transition from a seed to a seedling, with a specific focus on the different seed structures. Embryo and endosperm have distinct physiological and developmental fates. The former develops into a new plant, whereas the latter provides nutrients to support the seedling growth. This process is orchestrated by the scutellum, which secretes gibberellins (a group of phytohormones) into the endosperm, inducing the release of amylases and proteases from the aleurone layer to mobilise reserves [Citation44,Citation45]. The resulting peptides, amino acids, and LMW carbohydrates are then absorbed by the scutellum and provided as building blocks to the germinating embryo. The aleurone layer later undergoes PCD, which in wheat starts approximately 48 h after the onset of imbibition [Citation44] when all viable seeds had germinated (). Upon post-germination seedling growth and development, tracheary elements are formed by PCD in the scutellum, and develop into the protoxylem, which together with the protophloem develops into vascular bundles that transport water and nutrients, including thiols, to the growing seedling tissues [Citation45,Citation46].
In wheat, GSH-dependent redox activity is apparently required for storage protein mobilisation, one of the key events in early seedling growth [Citation21,Citation47]. In dry seeds, 36% of total glutathione was present as GSSG (), which typically accumulates during seed maturation drying, and has been related to the protection of PSH from oxidation upon desiccation [Citation8,Citation10]. These relatively high GSSG contents, which have potential to inhibit protein synthesis [Citation8,Citation25,Citation48–50], were rapidly reduced within the first 4 h of imbibition, in agreement with earlier studies [Citation25,Citation26]. Cysteinyl-bis-glycine was the only other disulphide that degraded before radicle protrusion (), but the role of Cys-Gly in plant metabolism is far from understood. In whole seeds, GSH contents increased within the first 4 h, likely due to GSSG reduction and GSH synthesis (). Furthermore, both Cys and γ-Glu-Cys steadily increased within 12 h of imbibition, when the first radicles protruded, and Cys concentrations further rose up to 32 h. A further significant increase of Cys and γ-Glu-Cys occurred between 32 and 48 h, when all viable seeds had developed into seedlings (). Further to being a precursor of GSH, γ-Glu-Cys is a by-product from glutathione-associated catabolism via cytosolic γ-glutamyl cyclotransferases or vacuolar γ-glutamyl transpeptidases [Citation5]. Forty-eight h after the onset of imbibition, the approximately four-fold increase in γ-Glu-Cys argues for GSH synthesis. However, at this interval Cys concentrations were much higher than GSH concentrations, suggesting that sources other than GSH metabolism were implicated in Cys production. Notably, the endosperm of wheat seeds contains gluten proteins, 75% of which are Cys-rich prolamins [Citation19], and their mobilisation and degradation after 48 h are a likely source for the raised Cys, and possibly Cys-Gly, concentrations. Because of its potential toxicity [Citation51–53], plants readily incorporate Cys into GSH, which is the main transport form of reduced sulphur [Citation4]. The high Cys concentrations found in wheat seeds are unusual. Therefore, we assessed whether the LMW thiols and disulphides in whole seeds were located in the endosperm or the embryo. The embryo and endosperm took up water at different rates (). As the Ehcs () are calculated from molar concentrations [Citation34], it is helpful to show thiol and disulphide concentrations on a molar basis (Figure S2). In the Nernst equation, the molar concentrations of the thiols are squared terms (EquationEquation (1)(1) ), and therefore the Ehc of a thiol-disulphide redox couple depends on both the thiol-disulphide ratio and the molar thiol concentrations [Citation34]. Finally, considering that the embryo makes up only 3% of the DW of a seed, we also show the LMW thiol-disulphide concentrations on a seed basis (Figure S1), which is useful when considering the total pool sizes of each redox couple.
Changes in EGSSG/2GSH during the transition from seed to seedling
Changes in the cellular redox environment appear to be intricately involved in the control of the life cycle in animal and plant cells, from the first mitotic division, through differentiation, up to cell death [Citation34,Citation54,Citation55]. Before the growth of the radicle and the extension of the coleoptile, seed germination mostly occurs by cell expansion rather than cell division [Citation56]. The net production of total glutathione, supported by elevated concentrations of GSH intermediates (γ-Glu-Cys and Cys-Gly), was observed in G15_embryos compared to NG15_embryos (). These data suggest that radicle protrusion and early seedling growth could be supported by de novo synthesis of GSH. Elevated GSH contents were reported to occur in meristematic regions in rapidly growing tissues by Bielawsky and Joy (1986) [Citation57]. A glutathione-dependent pathway was shown to control the initiation and maintenance of cell division in the roots of the model plant Arabidopsis thaliana [Citation58], and specifically in the root apical meristem [Citation58–60]. However, in G15_embryos of wheat this net GSH production was accompanied by net GSSG, Cys-bis-Gly and bis-γ-Glu-Cys accumulation (). Because the molar concentrations of GSH and Cys-Gly did not differ between NG15_embryos and G15_embryos (Figure S2(A)), partly due to a higher WC in the latter, the resulting EGSSG/2GSH and ECys-bis-Gly/2Cys-Gly values were more oxidising in G15_embryos compared to NG15_embryos (). A shift towards more oxidising conditions was reported to enhance histodifferentiation and post-embryonic growth in somatic embryos of various plants species [Citation35,Citation61]. Taken together, increased GSSG levels, in conjunction with more oxidising conditions in G15_embryos, suggest that cell differentiation took place.
Upon early seedling growth, between 15 and 48 h after the onset of imbibition, the concentrations of GSH, γ-Glu-Cys and Cys-Gly decreased, and EGSSG/2GSH and ECys-bis-Gly/2Cys-Gly and Ebis-γ-Glu-Cys/2γ-Glu-Cys shifted towards more reducing conditions (). This is in agreement with the requirement of the growing seedling for conditions that support cell division, and consistent with reports on cell cultures. For example, exogenous treatment of white spruce somatic embryos with GSH led to more reducing conditions and increased mitotic activity over cellular expansion [Citation62]. Furthermore, the formation of pro-embryogenic masses, requiring cell division, before the formation of somatic orchard grass embryos correlates with reducing cellular conditions [Citation35]. Therefore, more reducing conditions in G48_embryos (significant for all LMW thiol-disulphide redox couples, except for cyst(e)ine) could be associated with progressive proliferation required for organ growth.
ECySS/2Cys and EGSSG/2GSH related to physiological processes in the endosperm
The endosperm of a mature wheat seeds consists mainly of dead tissue, except for the aleurone layer, which also undergoes PCD from approximately 48 h after the onset of imbibition [Citation44]. Therefore, the finding that in all conditions studied the Ehcs of all four thiol-disulphide redox couples in the endosperm were more oxidising than those measured in the embryos and seedlings () is consistent with the positive correlation between PCD and shifts towards more oxidising conditions [Citation2].
Already after 15 h, significant increases in the thiol-disulphide redox couples were found between NG15_embryos and G15_embryos, but no significant increases were recorded for NG15_endosperms and G15_endosperms. However, upon seedling growth (between 15 and 48 h), the two dipeptide thiols (and their corresponding disulphides) and cyst(e)ine accumulated at higher concentrations than can be explained from GSH degradation at this time period ().This trend became particularly clear when data were expressed on a seed basis (Figure S1). A possible interpretation for the accumulation of the intermediates of GSH metabolism could be the de-glutathionylation of PSSG. After 48 h, proteolytic activities are detected in wheat and are crucial for the mobilisation Cys-rich prolamins located in the endosperm [Citation63,Citation64], which are a likely source for the increase in cyst(e)ine ().
The role of ECySS/2Cys did not receive attention by plant scientists. However, in animal cells the redox state of Cys/CySS is viewed as an essential part of thiol-disulphide conversions [Citation65,Citation66]. Upon early seedling growth, the difference between EGSSG/2GSH and ECySS/2Cys in the endosperm decreased ( and ). The significant shift towards more reducing ECySS/2Cys values between G15_endosperms and G48_endosperms resulted from the increase in Cys concentrations (). By contrast, the equivalent EGSSG/2GSH values showed a significant oxidative shift (), which could be due to processes such as de-glutathionylation of storage proteins that releases GSSG, or GRXs-mediated reduction of TRX h. In wheat, TRX h is directly involved in the reduction of prolamins, an essential step to increase storage protein susceptibility to proteolytic degradation during the first 48 h after the onset of imbibition [Citation67]. GRX/GSH-mediated reduction of TRX h has been shown to occur in different plant systems [Citation68,Citation69], but a role for GRX/GSH in storage protein mobilisation in cereals has yet to be confirmed. Finally, the oxidative shift in EGSSG/2GSH could also mark the commencement of the apoptotic-like PCD programme in the aleurone layer after the release of hydrolases [Citation44].
For comparison with previous work, we also calculated Ethiol-disulphide, as suggested by Schafer and Buettner [Citation34]. Ethiol-disulphide is perhaps a crude estimate of the cellular redox environment, which would benefit from including other redox couples, such as NADP+/NADPH + H+, but Ethiol-disulphide may suffice to take a “snapshot” of the metabolic state. Ethiol-disulphide was useful for assessing seed ageing [Citation35] and the capability for somatic embryogenesis [Citation37]. The current study shows that ECySS/2Cys contributed more to Ethiol-disulphide in endosperms than in embryos or seedlings, reinforcing the role of ECySS/2Cys in the endosperm, and in both embryo and endosperm during early seedling growth.
A role for H2O2 production in altering E2GSH/GSSG?
Production of H2O2 increased more than five-fold after 16 h of imbibition, similar to elevated ROS production in imbibed pea seeds [Citation30]. Oxidation of protein cysteine thiolates by H2O2 is key to redox signalling in mammalian cells [Citation70,Citation71]. Interestingly, the only protein known to be synthetised de novo upon the first hours of cereal seed imbibition was called “germin” for several years. Later, germin was characterised as an OXO, a main source of H2O2 production during wheat seed germination and seedling growth [Citation56,Citation72]. The rates of H2O2 production between 0 and 16 h of imbibition paralleled the activity of OXO (), which is located in the cell wall [Citation73]. Therefore, H2O2 production could be related to OXO activity within the first 16 h of imbibition. Notably, the lowest H2O2 production rates at 16 h were close to the time interval when values of EGSSG/2GSH in G15_embryo were the most oxidising. The increase in H2O2 production between 16 to 48 h of imbibition was higher than could be expected from OXO activity (), indicating that alternative sources of H2O2 production, such as NADPH oxidases, were active [Citation30,Citation74]. From 15 to 48 h after the onset of imbibition, the majority of seedlings had developed a radicle, two seminal roots and a coleoptile. While H2O2 progressively increased over this period, EGSSG/2GSH values shifted to more oxidising conditions in the endosperm and more reducing conditions in the seedling ( and ), as summarised in (). Considering that H2O2 is a pro-oxidant, H2O2 levels were closer linked to the EGSSG/2GSH of the endosperm than the embryo. At around 48 h after the onset of the imbibition, the aleurone surrounding the endosperm initiates PCD [Citation44], which could be related to the elevated H2O2 concentrations. In conclusion, this study shows that EGSSG/2GSH was the most influential LMW thiol-disulphide redox couple in growing seedlings, as expected for the dominant role of GSH/GSSG in the cell cycle [Citation54]. However, the major increase of cyst(e)ine, which became the dominant LMW thiol-disulphide redox couple of the endosperm in germinated seeds, highlights the relevance of this redox couple to early seedling growth of bread wheat.
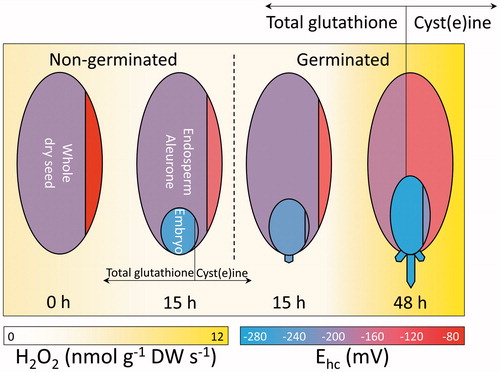
Figure 6. Overview of the changes in H2O2 and LMW thiols and disulphides during bread wheat germination and early seedling growth. From left to right, changes in LMW thiol-disulphides and H2O2 production rates in Triticum aestivum are schematically represented for a whole dry seed, seed structures isolated from non-germinated seeds after 15 h from the onset of imbibition, and seed structures isolated from germinated seeds after 15 and 48 h. Whole dry seed, endosperm including aleurone (large oval), and embryo or seedling (small oval) are divided by vertical lines. These lines delimit areas proportional to the concentrations of total glutathione (i.e. GSH (glutathione) + GSSG (glutathione disulphide), area left of line) and cyst(e)ine (i.e. Cys (cysteine)+ CySS, (cysteine) area right of line), in the respective seed structure. The redox states (Ehcs in mV) of total glutathione and cyst(e)ine are indicated by the blue-to-red (reducing-to-oxidising) shadings of each area, as shown by the bottom right scale. Yellow background shadings indicate the rates of H2O2 production (nmol g−1 DW s−1), as shown by the bottom left scale. The dashed vertical line separates seeds from seedlings.
| Abbreviations | ||
| bis-γ-Glu-Cys | = | bis-γ-glutamyl-cystine |
| Cys + CySS | = | cyst(e)ine |
| Cys | = | cysteine |
| Cys-bis-Gly | = | cystinyl-bis-glycine |
| Cys-Gly + Cys-bis-Gly | = | cyst(e)inyl-(bis)-glycine |
| Cys-Gly | = | cysteinyl-glycine |
| CySS | = | cystine |
| DMSO | = | dimethyl sulphoxide |
| DTT | = | dithiothreitol |
| DW | = | dry weight |
| Ebis-γ-Glu-Cys/2γ-Glu-Cys | = | half-cell reduction potential of the γ-glutamyl-cysteine/bis-γ-glutamyl-cystine redox couple |
| ECys-bis-Gly/2Cys-Gly | = | half-cell reduction potential of the cysteinyl-glycine/cystinyl-bis-glycine redox couple |
| ECySS/2Cys | = | half-cell reduction potential of the cysteine/cystine redox couple |
| EGSSG/2GSH | = | half-cell reduction potential of the glutathione/glutathione disulphide redox couple |
| Ehc | = | half-cell reduction potential |
| Ei | = | half-cell reduction potential of an individual low-molecular-weight thiol-disulphide redox couple |
| Ethiol-disulphide | = | LMW thiol-disulphide redox environment |
| FW | = | fresh weight |
| γ-Glu-Cys | = | γ-glutamyl-cysteine |
| γ-Glu-Cys + bis-γ-Glu-Cys | = | (bis)-γ-glutamyl-cysteine |
| G15_embryo | = | embryo with the radicle protruding by at least 1 mm, and isolated from germinated seeds 15 h after the onset of imbibition |
| G15_endosperm | = | endosperm isolated from germinated seeds 15 h after the onset of imbibition |
| G48_embryo | = | seedling with coleoptile, radicle and two seminal roots isolated from germinated seeds 48 h after the onset of imbibition |
| G48_endosperm | = | endosperm isolated from germinated seeds 48 h after the onset of imbibition |
| Gly | = | glycine |
| GRXs | = | glutaredoxins |
| GSH | = | glutathione (γ-L-glutamyl-L-cysteinyl-glycine) |
| GSH + GSSG | = | total glutathione |
| GSSG | = | glutathione disulphide |
| LMW | = | low-molecular-weight |
| H2O2 | = | hydrogen peroxide |
| hGSH | = | hydroxy-methyl-glutathione |
| HPOX | = | horseradish peroxidase |
| mBBr | = | monobromobimane |
| NEM | = | N-ethylmaleimide |
| NG | = | non-germinated |
| G | = | germinated |
| NG15_embryo | = | embryo isolated from non-germinated seeds 15 h after the onset of imbibition |
| NG15_endosperm | = | endosperm isolated from non-germinated seeds 15 h after the onset of imbibition |
| OXO | = | oxalate oxidase |
| PCD | = | programmed cell death |
| PSSG | = | protein-bound glutathione |
| PSH | = | protein thiol groups |
| ROS | = | reactive oxygen species |
| TG | = | total germination |
| TRXs | = | thioredoxins |
| WC | = | water content |
IFRA_1338344_-_SuppFile.pdf
Download PDF (467.9 KB)Acknowledgements
Comments on the manuscript by Ruben Sommaruga, Florian Steiner and Božo Frajman (University of Innsbruck) are gratefully acknowledged.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Fahey RC. Glutathione analogs in prokaryotes. Biochim Biophys Acta 2013;1830:3182–3198.
- Kranner I, Birtić S, Anderson KM, Pritchard HW. Glutathione half-cell reduction potential: a universal stress marker and modulator of programmed cell death? Free Radic Biol Med 2006;40:2155–2165.
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. What is stress? Concepts, definitions and applications in seed science. New Phytol 2010;188:655–673.
- Rennenberg H. Glutathione metabolism and possible biological roles in higher plants. Phytochemistry 1982;21:2771–2781.
- Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, et al. Glutathione in plants: an integrated overview. Plant Cell Environ 2012;35:454–484.
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 2011;155:2–18.
- Rouhier N, Cerveau D, Couturier J, Reichheld JP, Rey P. Involvement of thiol-based mechanisms in plant development. Biochim Biophys Acta 2015;1850:1479–1496.
- Kranner I, Grill D. Significance of thiol-disulfide exchange in resting stages of plant development. Bot Acta 1996;109:8–14.
- Rhazi L, Cazalis R, Lemelin E, Aussenac T. Changes in the glutathione thiol-disulfide status during wheat grain development. Plant Physiol Biochem 2003;41:895–902.
- Colville L, Kranner I. Desiccation tolerant plants as model systems to study redox regulation of protein thiols. Plant Growth Regul 2010;62:241–255.
- Morscher F, Kranner I, Arc E, Bailly C, Roach T. Glutathione redox state, tocochromanols, fatty acids, antioxidant enzymes and protein carbonylation in sunflower seed embryos associated with after-ripening and ageing. Ann Bot 2015;116:669–678.
- Nagel M, Kranner I, Neumann K, Rolletschek H, Seal CE, Colville L, et al. Genome-wide association mapping and biochemical markers reveal that seed ageing and longevity are intricately affected by genetic background and developmental and environmental conditions in barley. Plant Cell Environ 2015;38:1011–1022.
- Hopkins FG, Dixon M. On glutathione. II. A thermostable oxidation-reduction system. J Biol Chem 1922;54:527–563.
- Hopkins FG. On glutathione: a reinvestigation. J Biol Chem 1929;84:269–320.
- Hopkins FG, Morgan EJ. Appearance of glutathione during the early stages of the germination of seeds. Nature 1943;152:288–290.
- Tkachuk R. L-Cysteinylglycine: its occurrence and identification. Can J Biochem 1970;48:1029–1036.
- Tkachuk R, Mellish VJ. γ-L-Glutamyl-L-cysteine: its isolation and identification from wheat germ. Can J Biochem 1977;55:295–300.
- Shewry PR. Wheat. J Exp Bot 2009;60:1537–1553.
- Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 2002;53:947–958.
- Tatham AS, Shewry PR. The S-poor prolamins of wheat, barley and rye: revisited. J Cereal Sci 2012;55:79–99.
- Bewley JD. Seed germination and dormancy. Plant Cell 1997;9:1055–1066.
- Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D. Seed germination and vigor. Annu Rev Plant Biol 2012;63:507–533.
- Tan-Wilson AL, Wilson KA. Mobilization of seed protein reserves. Physiol Plant 2012;145:140–153.
- Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H, Seeds: physiology of development, germination and dormancy. New York: Springer; 2013.
- Fahey RC, Distefano DL, Meier GP, Bryan RN. Role of hydration state and thiol-disulfide status in the control of thermal stability and protein synthesis in wheat embryo. Plant Physiol 1980;65:1062–1066.
- Kranner I, Grill D. Content of low-molecular-weight thiols during the imbibition of pea seeds. Physiol Plant 1993;88:557–562.
- Barba-Espin G, Diaz-Vivancos P, Clemente-Moreno MJ, Albacete A, Faize L, Faize M, et al. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ 2010;33:981–994.
- El-Maarouf-Bouteau H, Sajjad Y, Bazin J, Langlade N, Cristescu SM, Balzergue S, et al. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ 2015;38:364–374.
- Ishibashi Y, Tawaratsumida T, Kondo K, Kasa S, Sakamoto M, Aoki N, et al. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol 2012;158:1705–1714.
- Kranner I, Roach T, Beckett RP, Whitaker C, Minibayeva FV. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J Plant Physiol 2010;167:805–811.
- Roach T, Beckett RP, Minibayeva FV, Colville L, Whitaker C, Chen HY, et al. Extracellular superoxide production, viability and redox poise in response to desiccation in recalcitrant Castanea sativa seeds. Plant Cell Environ 2010;33:59–75.
- Roach T, Kranner I. Extracellular superoxide production associated with secondary root growth following desiccation of Pisum sativum seedlings. J Plant Physiol 2011;168:1870–1873.
- Bailly C, El-Maarouf-Bouteau H, Corbineau F. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 2008;331:806–814.
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Bio Med 2001;30:1191–1212.
- Zagorchev L, Seal CE, Kranner I, Odjakova M. Redox state of low-molecular-weight thiols and disulphides during somatic embryogenesis of salt-treated suspension cultures of Dactylis glomerata L. Free Radic Res 2012;46:656–664.
- Xie YJ, Zhang C, Lai DW, Sun Y, Samma MK, Zhang J, Shen WB. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J Plant Physiol 2014;171:53–62.
- Birtić S, Colville L, Pritchard HW, Pearce SR, Kranner I. Mathematically combined half-cell reduction potentials of low-molecular-weight thiols as markers of seed ageing. Free Radic Res 2011;45:1093–1102.
- Kranner I. Determination of glutathione, glutathione disulfide and two related enzymes, grutathione reductase and glucose-6-phosphate dehydrogenase, in fungal and plant cells. In: Varma A, ed. Mychorriza manual. Berlin: Springer; 1998:227–241.
- Bailly C, Kranner I. Analyses of reactive oxygen species and antioxidants in relation to seed longevity and germination. Berlin: Springer; 2011:343–367.
- Laker MF, Hofmann AF, Meeuse BJD. Spectrophotometric determination of urinary oxalate with oxalate oxidase prepared from moss. Clin Chem 1980;26:827–830.
- Clarke JM. Measurement of relative water-uptake rates of wheat seeds using agar media. Can J Plant Sci 1980;60:1035–1038.
- Rathjen JR, Strounina EV, Mares DJ. Water movement into dormant and non-dormant wheat (Triticum aestivum L.) grains. J Exp Bot 2009;60:1619–1631.
- Klapheck S, Chrost B, Starke J, Zimmermann H. γ-Glutamylcysteinylserine: a new homolog of glutathione in plants of the family Poaceae. Bot Acta 1992;105:174–179.
- Domínguez F, Moreno J, Cejudo FJ. A gibberellin-induced nuclease is localized in the nucleus of wheat aleurone cells undergoing programmed cell death. J Biol Chem 2004;279:11530–11536.
- Domínguez F, Moreno J, Cejudo FJ. The scutellum of germinated wheat grains undergoes programmed cell death: identification of an acidic nuclease involved in nucleus dismantling. J Exp Bot 2012;63:5475–5485.
- Rauser WE, Schupp R, Rennenberg H. Cysteine, γ-glutamylcysteine, and glutathione levels in maize seedlings - distribution and translocation in normal and cadmium-exposed plants. Plant Physiol 1991;97:128–138.
- Kobrehel K, Wong JH, Balogh A, Kiss F, Yee BC, Buchanan BB. Specific reduction of wheat storage proteins by thioredoxin h. Plant Physiol 1992;99:919–924.
- Dhindsa RS. Glutathione status and protein synthesis during drought and subsequent rehydration in Tortula ruralis. Plant Physiol 1987;83:816–819.
- Dhindsa RS. Drought stress, enzymes of glutathione metabolism, oxidation injury, and protein-synthesis in Tortula ruralis. Plant Physiol 1991;95:648–651.
- Tommasi F, Paciolla C, de Pinto MC, De Gara L. A comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. J Exp Bot 2001;52:1647–1654.
- Osman LPMS, Waring RH. Cysteine, its metabolism and toxicity. Sulphur Reports 1997;22:155–172.
- Stipanuk MH, Dominy JE, Lee JI, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr 2006;136:1652–1659.
- Ohtsu I, Wiriyathanawudhiwong N, Morigasaki S, Nakatani T, Kadokura H, Takagi H. The L-Cysteine/L-cystine shuttle system provides reducing equivalents to the periplasm in Escherichia coli. J Biol Chem 2010;285:17479–17487.
- Diaz-Vivancos P, de Simone A, Kiddie G, Foyer CH. Glutathione-linking cell proliferation to oxidative stress. Free Radic Biol Med 2015;89:1154–1164.
- Meyer AJ. The integration of glutathione homeostasis and redox signaling. J Plant Physiol 2008;165:1390–1403.
- Caliskan M, Cuming AC. Spatial specificity of H2O2-generating oxalate oxidase gene expression during wheat embryo germination. Plant J 1998;15:165–171.
- Bielawski W, Joy KW. Properties of glutathione reductase from chloroplasts and roots of pea. Phytochemistry 1986;25:2261–2265.
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell. 2000;12:97–109.
- Marquez-Garcia B, Njo M, Beeckman T, Goormachtig S, Foyer CH. A new role for glutathione in the regulation of root architecture linked to strigolactones. Plant Cell Environ 2014;37:488–498.
- Sánchez-Fernández R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, et al. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc Natl Acad Sci USA 1997;94:2745-2750.
- Stasolla C. Glutathione redox regulation of in vitro embryogenesis. Plant Physiol Biochem 2010;48:319–327.
- Belmonte MF, Donald G, Reid DM, Yeung EC, Stasolla C. Alterations of the glutathione redox state improve apical meristem structure and somatic embryo quality in white spruce (Picea glauca). J Exp Bot 2005;56:2355–2364.
- Drzymala A, Prabucka B, Bielawski W. Carboxypeptidase I from triticale grains and the hydrolysis of salt-soluble fractions of storage proteins. Plant Physiol Biochem 2012;58:195–204.
- Shi C, Xu LL. Characters of cysteine endopeptidases in wheat endosperm during seed germination and subsequent seedling growth. J Integr Plant Biol 2009;51:52–57.
- Circu ML, Aw TY. Intestinal redox biology and oxidative stress. Semin Cell Dev Biol 2012;23:729–737.
- Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta 2008;1780:1271–1290.
- Lozano RM, Wong JH, Yee BC, Peters A, Kobrehel K, Buchanan BB. New evidence for a role for thioredoxin h in germination and seedling development. Planta 1996;200:100–106.
- Gelhaye E, Rouhier N, Jacquot JP. Evidence for a subgroup of thioredoxin h that requires GSH/Grx for its reduction. FEBS Lett 2003;555:443–448.
- Reichheld JP, Khafif M, Riondet C, Droux M, Bonnard G, Meyer Y. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell 2007;19:1851–1865.
- Stone JR, Yang SP. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 2006;8:243–270.
- Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 2008;4:549–561.
- Lane BG, Dunwell JM, Ray JA, Schmitt MR, Cuming AC. Germin, a protein marker of early plant development, is an oxalate oxidase. J Biol Chem 1993;268:12239–12242.
- Lane BG, Cuming AC, Fregeau J, Carpita NC, Hurkman WJ, Bernier F, et al. Germin isoforms are discrete temporal markers of wheat development. Pseudogermin is a uniquely thermostable water-soluble oligomeric protein in ungerminated embryos and like germin in germinated embryos, it is incorporated into cell walls. Eur J Biochem 1992;209:961–969.
- Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 2001;125:1591–1602.

