Abstract
Pulmonary inhalation administration is an ideal approach to locally treat lung disease and to achieve systemic administration for other diseases. However, the complex nature of the structural characteristics of the lungs often results in the difficulty in the development of lung inhalation preparations. Nanocrystals technology provides a potential formulation strategy for the pulmonary delivery of poorly soluble drugs, owing to the decreased particle size of drug, which is a potential approach to overcome the physiological barrier existing in the lungs and significantly increased bioavailability of drugs. The pulmonary inhalation administration has attracted considerable attentions in recent years. This review discusses the barriers for pulmonary drug delivery and the recent advance of the nanocrystals in pulmonary inhalation delivery. The presence of nanocrystals opens up new prospects for the development of novel pulmonary delivery system. The particle size control, physical instability, potential cytotoxicity, and clearance mechanism of inhaled nanocrystals based formulations are the major considerations in formulation development.
1. Introduction
Pulmonary inhalation administration not only is an ideal way to be used to locally treat lung disease, such as asthma, cystic fibrosis, chronic obstructive pulmonary disease, bronchitis, and lung cancer (Patton & Byron Citation2007; Mansour et al., Citation2009), but also to achieve systemic administration for other diseases, owing to the tremendous surface area (70–140 m2 in the adult human lung) of the lung and fast transport of actives across the respiratory epithelium (relatively high blood flow up to 5000 mL/min) (Yang et al., Citation2014; Elsayed & Shalash Citation2018). Furthermore, it can bypass the liver's first pass effect and possess low enzymatic activity, thereby reducing the dosage and side effects of medication, with respect to the other administration, such as oral administration or injection administration (Gonda Citation2006; de Kruijf & Ehrhardt Citation2017). Unfortunately, pulmonary formulations were strictly restricted by the physiological characteristics of the lungs, such as branching structure, mucociliary, and macrophages (Ling et al., Citation2014), as well as certain properties of the drugs like particle size and solubility (Loira-Pastoriza et al., Citation2014).
Nanotechnology has a huge advantage in pulmonary delivery for poorly soluble drug, which could overcome the numerous biological and physical barriers of the pulmonary, improve drug solubility, and prolong retention time in lung of drug to enhance bioavailability in the lungs (Sung et al., Citation2007; Roa et al., Citation2011; Muralidharan et al., Citation2015; Elsayed & AbouGhaly Citation2016; Mangal et al., Citation2017). The Arikayce® inhalation suspension as a liposome nanomedicine was approved by the FDA for the treatment of Mycobacterium avium complex lung disease (Shirley, Citation2019), which encouraged the development of more nanoparticles (NPs) based formulations for pulmonary drug delivery. It was well known that nanoformulations for pulmonary administration are classified into nanocarriers (liposomes, solid lipid NPs, nanostructured lipid carrier, micelles, and polymeric NPs) and polymer–drug conjugates (dendrimers and PEGylated NPs) (Xing et al., Citation2020). Nanocrystals as a free carrier-nanotechnology have obtained increasing attention for pulmonary administration of poorly soluble drugs owing to their improved dissolution rate and saturation solubility of poorly soluble drug, biological properties, and low toxicity (Gao et al., Citation2012; Pawar et al., Citation2014; Thakkar et al., Citation2017).
However, the complex physiological structures or barriers and clearance mechanisms in the respiratory tract could eliminate the foreign particles and hinder the effective delivery of drug (Yang et al., Citation2008). Moreover, the surface properties of NPs could also significantly influence their fate (adsorption or clearance) in the lung (Hu et al., Citation2013; Liu et al., Citation2020). Therefore, in this review, the physiological structure of lung and various clearance pathways of NPs in the lung are presented systemically. The advantages, advanced technologies for preparation of nanocrystal based pulmonary delivery system, as well as the applications of nanocrystals in pulmonary delivery system were highlighted. It is expected to provide reference for promoting the pulmonary administration of poorly soluble drugs.
The lungs are the organs that contact with the exchange of air between the organism and the outside world. They are divided into two main regions: the conducting airway region and the respiratory region. The airway is a continuous branch from the bronchi to the lungs and consists mainly of bronchi, bronchioles, and terminal bronchioles. As the bronchi continue to branch, the diameter of the tubes becomes smaller, the tube wall becomes thinner, and the structure of the tube wall changes gradually. The annular smooth muscles of the bronchi contract or relax under splanchnic nerves innervation and it is responsible for the regulation of airflow passage into the alveoli. This is the site where the lung tissue completes gas exchange consisting of respiratory bronchioles, alveolar ducts, lung sacs, and alveoli. The respiratory bronchiole is the transitional pipes between the pulmonary airway and the respiratory site. Each respiratory bronchiole branch is divided into 2–3 alveolar ducts. The alveolar sacs are the common opening of several alveoli and are connected to the alveolar ducts. The gut is the main site for the digestion and absorption of nutrients.
Alveoli, the terminal part of the bronchial tree, are the main site for the gas exchange. There are approximately 300–500 million alveoli in each lung (Yue et al., Citation2018).
The lung is composed of a single layer of epithelial cells with a large surface area (greater than 100 μm) and thin epithelium (0.2–1 μm). Adequate blood supply allows the drug to be quickly absorbed in the lungs (Patton & Byron Citation2007), which is more attractive than systemic drug delivery. In addition, the relatively low enzyme activity in the lungs can avoid the damage caused by the acid–base of the gastrointestinal tract during oral administration, and help maintain a high bioavailability (Yang et al., Citation2010), noninvasive lung delivery pathway can also improve patient compliance (Gonda Citation2006; Mansour et al., Citation2009).
2. The barriers to lung inhalation administration
2.1. Mucociliary clearance
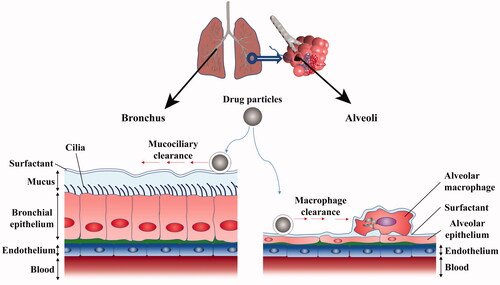
The absorption of drugs in the lungs is influenced by multiple barriers. Due to the different anatomical structures of lungs, the clearance mechanisms of drugs in different regions may be different as well (). Mucus clearance, which can help to capture the foreign dust and microorganisms, then removes them from the lungs. Therefore, the mucus clearance is the primary drug clearance mechanism for conducting airway region. The conducting airway region consists mainly of ciliated epithelial cells and goblet cell that together form the bilayer mucin periciliary layer (O' Donnell & Smyth Citation2011). Notably, the upper is a gel-forming mucins that form a viscous mucus layer and the submucous layer is ciliated sol layer with low viscosity, and the cilia are covered with a viscoelastic mucus (Button et al., Citation2012). The elastic mucus is usually comprised of hydrogel (95%), mucin (2%), glycoprotein, lipid, and salt, which are secreted by the goblet cell and the submucosal glands (Sanders et al., Citation2009). The thickness of the mucus in the trachea is 10–30 μm, and 2–5 μm in the bronchus. The mucin is the main functional component of elastic mucus with the concentration range of mucin between 1 and 5%. Mucin producing the mucus has astringency and adhesion characteristics, thereby adhering foreign objects such as drugs (Bansil & Turner Citation2006; O' Donnell & Smyth Citation2011). Mucin is a hydrophilic linear peptide chain consisting of 8–169 amino acids of repeated proline, threonine, and serine, which is called as the PTS (proline, threonine, and serine) domain. The hydrophobic region composed of cysteine is called as the non-PTS region. Mucin monomers associate with the several other monomers via cysteine bridges in aqueous media, forming a mucin fiber mesh-like structure with variable porosity of 50–1800 nm with the different spacings diameters (Araújo et al., Citation2018). However, when the drugs cannot cross the mesh-like structure, the drugs are wrapped by viscous mucus, the swinging cilia may push the mucus which wrapped with drugs to the pharynx (Button et al., Citation2012). It enables the drugs to be swallowed or coughed out. With the help of the mucociliary clearance mechanism, the defense system of the lungs can remove foreign bodies and captured particles in the mucus within 15 minutes to two hours (Mansour et al., Citation2009). It has been found that the tracheal mucociliary clearance rate of healthy young people was about 4–20 mm/min (García-Díaz et al., Citation2018).
2.2. Phagocytosis by pulmonary macrophages
Pulmonary macrophages are one of the most common cells in the lung which are derived mainly from mononuclear cells produced by bone marrow and exist in four subgroups, and are widely distributed in pulmonary blood vessels, alveoli, interstitial, etc.
Pulmonary macrophages can play an important role in normal physiological process of the lungs and the occurrence and development of diseases process. It is generally in a microenvironment with relatively high oxygen partial pressure and has frequent contact with foreign bodies, and has physiological characteristics such as adhesion, deformation, migration, and phagocytosis (Praphakar et al., Citation2018). After the recognition procedure of the foreign bodies by the macrophages, the foreign bodies could be engulfed by macrophages and migrate to the respiratory tract and the foreign bodies were washed out by the effect of mucus. The phagocytosis of pulmonary macrophages is primarily associated with the size of phagocytic particles, the optimal particles size ranges for endocytosis was 1–3 μm. The particles in this range will be eliminated by macrophages. Due to active endocytosis of lung macrophages, the drug absorption after pulmonary administration is hindered, especially for macromolecular protein. Moreover, the phagocytosis of pulmonary macrophages results in the decrease of efficacy and concentration of drug. The lysosomes and other enzyme systems possessed by lung macrophages can lead to inactivation of macromolecular protein by degradation (Lu et al., Citation2018). The alveolar macrophages are the main drug clearance mechanism in the respiratory region of the lung (Liu et al., Citation2020). The alveolar macrophages account for more than 90% of the airway immune cells (Lee et al., Citation2015), and there are 8–12 alveolar macrophages in each alveolar. The inhaled microorganisms or foreign bodies can be readily engulfed by alveolar macrophages and then transported to phagosomes, thereby triggering microorganisms or foreign bodies degradation (Alblas et al., Citation1981). It was reported that in order to achieve the same hypoglycemic effect as subcutaneous insulin, the drug concentration by pulmonary administration must be increased by 7–8 times, because more than 1/3 of insulin is cleared by pulmonary macrophages (Praphakar et al., Citation2018).
2.3. Effects of pulmonary surfactant
The pulmonary surfactant (PS) is synthesized and secreted by alveolar type II epithelial cells, and forms a surfactant membrane at the respiratory gas–liquid interface (Johnson et al., Citation2006; Button et al., Citation2012). Its main function is to reduce the surface tension in gas–liquid interface, and maintain the alveolar morphological stability and protect the alveolar epithelial cells, and PS is also involved in defensive function of lung host (Perez-Gil & Weaver Citation2010). The composition of PS is particularly complex, including 90% lipid and 10% protein (Arick et al., Citation2015). Among the various lipids found in PS, the dipalmitoyl lecithin (DPPC) is the most abundant component, together with several other phospholipids, accounting for 80% of lung surfactant lipids (Yu & Possmayer Citation2003). In contrast, cholesterol, phosphatidylinositol (PI), phosphatidylglycerol (PG), and acidic phospholipids were composed of 20% of lipids in PS (Wüstneck et al., Citation2005). PS is also involved in the process of drug clearance, and the proteins in PS can promote the adhesion and aggregation of drugs, making it easier to be cleared by mucosal cilia or recognized by macrophages and monocytes for phagocytosis (Ordonez et al., Citation2017). The proteins found in PS have four different forms according to previously literature reports (Goerke, Citation1998), the surface active proteins SP-B and SP-C are small hydrophobic proteins, which played an important role in maintaining and regulating the physiological function of PS (Ding et al., Citation2001; Whitsett & Weaver Citation2002). The SP-A and SP-D are large hydrophilic glycoproteins that can enhance the tendency and phagocytosis of macrophages. When the fungal infection occurs in the lung, SP-A and SP-D bind to fungal ligands in a Ca2+ dependent manner and regulate or promote the secretion of cytokines in order to enhance macrophage phagocytosis (Ordonez et al., Citation2017).
3. Aerodynamic requirements for lung inhalation
The aerodynamic characteristics of inhalable microparticles are the main external factors affecting the pulmonary inhalation of drugs. The size distribution of the inhalable particles is usually expressed by aerodynamic diameter, which varies according to the shape, size and density of the objects. The aerodynamic diameter of inhalable particles determines whether they can be deposited in the lungs. There are three main mechanisms for its deposition in the lung: inertial collision, gravity sedimentation, and Brownian diffusion. Inertial collision is a collision of particles with a mass median aerodynamic diameter (MMAD) greater than 5 μm on the surface of the oropharynx and upper respiratory tract due to inertia effect. Gravity sedimentation is main mechanism of deposition on the surface of trachea, bronchus and alveoli. When the MMAD of particles is in the range of 1–5 μm, gravity sedimentation can occur. But when the particle MMAD is less than 0.5 μm, the sedimentation effect is not as effective as Brownian’s diffusion. The particles with MMAD less than or equal to about 0.5 μm are mainly deposited in small airways and alveoli owing to Brownian’s diffusion. Meanwhile, the deposition of particles is affected by the action of breath holding which can facilitate deposition (Rogueda & Traini Citation2007; Wei et al., Citation2018). In order to successfully deposit in the deep lung, the particles must be small enough to avoid deposition in the upper respiratory tract due to inertial collision, and the particles must be large enough in order to avoid being exhaled during exhalation. The particles with MMAD less than 0.5 μm would be discharged with the airflow through Brownian’s motion, usually up to 80%. Therefore, in order to obtain effective lung deposition, the MMAD of particles should be in the range of 1–5 μm.
4. Advantages of nanocrystals technology for lung inhalation of poorly soluble drug
In recent years, nanocrystals have become an intense focus of research of nanotechnology. Drug nanocrystals were generally pure drug particles with a particle size of 1–1000 nm, which can be stabilized by surfactants or stabilizers without the need of carrier materials. Drug nanocrystals can enhance the adhesion to biological membranes, increase the saturated solubility and dissolution of the drug and improve the bioavailability by virtue of its large specific surface area. The nanocrystals technology can be used as intermediate preparation technology, which is extensively employed in various routes of drug delivery (such as oral, intravenous, pulmonary, percutaneous, and ocular administration, etc.). Compared with other nanotechnology, nanocrystals technology has unique advantages in pulmonary drug delivery, which brings hope for the treatment of various lung diseases. Its main features are as follows:
4.1. High drug loading and good safety
Nanocrystals have high drug loading compared with other carrier based NPs. With the improvement of high drug loading and bioavailability, drugs nanocrystals can improve drug safety by reducing the dosage. The preparation of poorly water-soluble drug solutions requires a large number of surfactants or organic solvents to dissolve them. However, these surfactants and organic solvents have certain adverse effects on cells.
Nanocrystal formulations could solve these problems because they are usually prepared without using large amounts of organic solvents, thereby reducing co-solvent interference and potential toxicological effects in the body. Moreover, the properties of nanocrystals help to increase the saturated solubility of the drug, have a higher drug loading capacity, without the need for higher concentrations of surfactants, and show better bioavailability compared with other nanoformulations (Müller et al., Citation2011; Kannan et al., Citation2013). Nanocrystals minimize the excessive use of harmful non-aqueous solvents, while the increase of drug loading and bioavailability reduces the dose and enhances the safety of lung administration. Rundfeldt et al. have found that itraconazole nanocrystals suspension is used in inhaled treatment of cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Its long-lasting lung tissue concentration is much higher than the lowest inhibitory concentration of Aspergillus strain, so that patients only need to be administered once a day to reduce systemic exposure (Rundfeldt et al., Citation2013).
4.2. Enhanced mucus-penetrating ability
After the drug enters the lung, it is easy to be removed by mucociliary. At the same time, high viscoelasticity and adhesive mucus effectively prevent the delivery of nano drugs to the patient's lungs. It is found that rod-shaped nanocrystals can enhance the diffusion of drugs in mucus, and rod-shaped NPs have higher diffusion rate than spherical NPs in biological mucus medium. Wang et al. have shown that the aspect ratio of nanorods and their adhesion to host media play a key role in the hopping diffusion behavior of nanorods. In viscous polymer solution and gels, hopping diffusion makes the diffusion rate of nanorods higher than that of spherical NPs (Wang et al., Citation2018). Costabile et al. studied C109 nanocrystals with rod-shaped structure. C109 is an effective but poorly soluble filamentous temperature-sensitive protein Z (FtsZ) inhibitor, which has a good inhibitory effect on the central softening of the high-risk pathogen in patients with cystic fibrosis. The results show that C109 nanocrystals can smoothly penetrate the mucus of artificial cystic fibrosis for diffusion and exert therapeutic effect (Costabile et al., Citation2020).
4.3. Double drug release in the lungs
Nanocrystal drugs show immediate and prolonged release behaviors after topical administration. The high surface area of nanocrystals increases the saturation solubility and dissolution of the drug, which helps to obtain an initial high drug concentration to achieve absorption and trigger action (Gao et al., Citation2008). The high surface area of drug nanocrystals also helps to increase their interaction with biofilms and extend their residence time on the biofilm. In addition, the longer the residence time of these drug particles in the lungs, the longer the drug release will be (Ali et al., Citation2011).
4.4. Reduced macrophage clearance
The optimal particle size for macrophage endocytosis is 1–3 μm, while the particle size of nanocrystals greatly reduces the possibility of macrophage clearance. Simultaneous studies have shown that NPs could minimize the clearance of macrophages (Oberdörster et al., Citation1994, Citation2005), and inhalation of drug nanocrystals can lead to deep lung deposition and smaller airway penetration, resulting in more uniform drug distribution, higher drug deposition rate, and more precise drug efficacy (Zheng & Bosch Citation1997).
5. Production of nanocrystals based pulmonary inhalation system
Nanocrystals based pulmonary inhalation delivery systems are a research hotspot for new drug delivery technologies. Due to the characteristics and advantages of nanocrystal technology, it has been widely used in pulmonary inhalation drug delivery systems for poorly soluble drugs, such as nanosuspension-based aerosol, dry powder inhaler, the inhalable nanocomposite particle, and so on.
5.1. Production method of drug nanocrystals
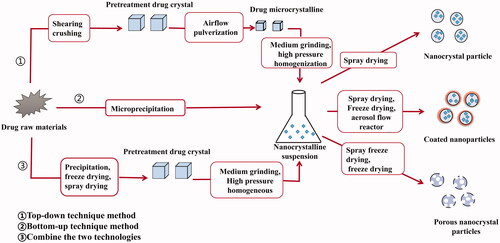
The production methods of drug nanocrystals are mainly divided into two categories: bottom-up technology and top-down technology, including micro-precipitation method, media milling method, high pressure homogenization method, precipitation–homogenization combinative technology, etc. ().
5.1.1. Bottom-up technology
Bottom-up technique, also known as ‘precipitation’, is a method in which the drug is completely dissolved in one solvent and then added to another non-solvent. The main approaches for the preparation of nanocrystals are micro-precipitation and supercritical fluid (Sinha et al., Citation2013; Yue et al., Citation2018). The key of this technique is to control the crystal structure of the drug to avoid agglomeration and to control the particle size in the nanometer range. Controlling the crystal structure of the drug to avoid agglomeration and the particle size in the nanometer range are the key factors of this technique. Advantages of this technique include: easy preparation, lower costs, and preparation done in steps. However, its poor reproducibility makes it difficult to scale up, and it is easy to residual the organic solvent because of the need for organic solvents in the preparation process. Thus, this method was not suitable for the drug that is insoluble in water and non-aqueous solvents (Du et al., Citation2015). Xiong et al. prepared resveratrol nanocrystal suspensions with an average particle size of 222.54 ± 1.66 nm and a polydispersity coefficient of 0.125 ± 0.035 using this technique (Xiong et al., Citation2020).
5.1.2. Top-down technology
Top-down techniques, also known as ‘dispersion’, in which the raw material comprises of larger solid particles than the resulting NPs and mechanical processes are the fundamental mechanism leading to particle size reduction. This method mainly includes media milling, high-pressure homogenization, and microfluidization method (Malamatari et al., Citation2018; Yue et al., Citation2018). Currently, the top-down technology is the major route of the research and development of listed products (Liu et al., Citation2018). The advantage of the top-down approaches is easy preparation.
Top-down approaches are simple, easy to industrialize and reproducible processes, easy preparation, easily adaptable to industrialized production and re-use. However, the large number of cycles required to reach the desired drug particle size and the potential for aggregation of drug particles over time result in poor physical stability. Huang et al. prepared harmine nanocrystal suspension with an average particle size of 179.21 ± 0.01 nm and the zeta potentials were all between −41.4 ± 1.08 mV, which indicated that the HAR-NS had good stability (Huang et al., Citation2021) ().
Table 1. Examples of nanocrystal preparation.
5.1.3. Combination method
In the process of nanocrystals preparation, it is difficult to achieve the requirements of uniform particle size and good stability by a single preparation technique. Therefore, combination technologies have also been developed that integrate a pretreatment step with a subsequent high energy step, like high pressure homogenization (Jermain et al., Citation2018). The combination of bottom-up and top-down technologies can make full use of their respective advantages to improve drug nanosizing efficiency and narrow particle size distribution to prepare safe and effective formulations. However, the high production cost and the complex production processes of the combined techniques limit their industrialization. Tao et al. (Citation2016) prepared the resveratrol nanocrystal suspensions by combination technology and obtained the resveratrol nanocrystals with an average particle size of 192 nm, which was much smaller than the average particle size of 569 nm prepared by high-pressure homogenization.
5.2. Production of nanocrystals based inhalable nanocomposite particles
The preparation process of inhalable nanocrystals based delivery system mainly includes two main steps: drug nanonization and solidification. Solidification methods for converting nanosuspensions into inhalable nanocomposite particles include spray-drying technology, freeze-drying technology, spray-freeze drying technology, and so on.
5.2.1. Spray-drying
Spray-drying is a single-step process that converts a liquid (solution, suspension, or emulsion) directly into dry particles (Alhajj et al., Citation2021). The main principle of spray-drying is the atomization of the liquid feedstock into fine droplets through the nozzle, and evaporate the solvent through the hot drying gas (Raula et al., Citation2013). During the spray-drying process, the particle properties (e.g. particle size distribution, shape, residual humidity, and density) can be controlled by adjusting the formulation and process parameters (e.g. feed rate and inlet temperature). The spray-drying technology has become the basic particle engineering technology for the development of inhalable preparations. Also, from the industrial perspective, due to its faster and more cost-effective than freeze-drying, spray-drying is more popular for inhalable preparations. Therefore, spray-drying of nanosuspensions can be used as a platform for pulmonary administration of poorly water-soluble drugs. Pomázi et al. (Citation2013) developed meloxicam inhalable nanocrystal aggregates embedded in mannitol and other excipients (polymer and l-leucine) by applying high-pressure homogenization and spray-drying technology. It was found that due to the presence of the amino acid l-leucine, the adhesion between particles was reduced and the aerosolization of nanocrystals-aggregates was enhanced (Sou et al., Citation2011). The nanocrystal-aggregates of meloxicam are crystalline and exhibit enhanced in vitro nebulization (FPF >53%, MMAD <3.52 µm) and solubility properties. The itraconazole nanocrystals-aggregates could be an effective strategy for the treatment of pulmonary aspergillosis (Rundfeldt et al., Citation2013).
5.2.2. Freeze-drying
The freeze-drying (lyophilization) is a classical process for removing water from high value products (such as antibiotics, enzymes, vaccines, etc.). In order not to cause excessive damage to the active ingredients, the addition of water or other suitable aqueous diluents before freeze-drying increased the storage stability and restorability of drugs (Jakubowska & Lulek Citation2021).
The process of freeze-drying is to first freeze the liquid solution or suspension under atmospheric pressure and then heat it under vacuum in order to remove ice crystals by sublimation. Finally, the high-porosity powder with low moisture content was obtained. The freeze-drying process consists of three steps: freezing, primary drying, and secondary drying (Siow et al., Citation2016). Freeze-drying and spray-drying are most commonly used techniques for solidification of nanosuspension as they are easily applied in practice, their scale up production and high industrial acceptability. In order to form inhalable microparticles (NP-agglomerates), El-Gendy et al. (Citation2009) used freeze-drying to solidify the nanosuspension, which was obtained through a controlled flocculation process (protocol under the name NanoClustersΤΜ, owned by Savara Pharmaceuticals, Austin, TX). Budesonide nanocrystals-agglomerates prepared by this method showed enhanced dissolution rate when compared with the raw drug, with an MMAD of 2.1 ± 1.8 µm (mean standard deviation) in terms of aerosolization, which is aided by the penetration of the particles deeper into the lungs.
5.2.3. Spray-freeze-drying
The spray-freeze-drying is an important alternative technology for spray-drying, which has been already used for the preparation of thermolabile inhalable particles, especially proteins (Rouse et al., Citation2007). During the spray-freeze drying process, the liquid is fed and atomized by a nozzle, and the atomized droplets are rapidly frozen in liquid nitrogen to remove the freezing solvent (water) by sublimation and obtain a powdered product. Compared with spray-drying, the spray-freeze-drying is a low-temperature drying process that produces porous particles (Yue et al., Citation2018). Voriconazole powder prepared by spray-freeze-drying exhibited porous and spherical structure, and displayed crystalline characteristics (Liao et al., Citation2019). However, the spray-freeze-drying particles are partially composed of amorphous solids with a large specific surface area, and this state is hygroscopic which can lead to changes in physicochemical properties by humidity when the particles are stored over the long-term or under high humidity conditions such as in the lungs (Tanaka et al., Citation2020). Cocrystal technique has been used to solve such problems (Duggirala et al., Citation2016; Al-Obaidi et al., Citation2021).
5.2.4. Aerosol flow reactor
The aerosol flow reactor patented by Teicos Pharma (Espoo, Finland) has been used for the solidification of nanosuspensions. It is a simple and efficient one-step continuous process that produces particles directly in the desirable particle size range. In detail, the drug solution was atomized by ultrasonic to create droplets, then the droplets suspended in the carrier gas were passed (usually N2) through a heated tubular laminar flow reactor where the evaporation occurs and finally the particles are collected (Torvela et al., Citation2011). Studies have found that changing the temperature of the reactor tube had an effect on the particle morphology. Specifically, the particle size increases significantly with increasing reactor temperatures of 160 °C due to formation of hollow NPs. This situation has reversed at the temperature above 160 °C, further increase in temperature results in decreasing particle size and shows smooth surfaces attributed to the collapse of the hollow structure of the NPs (Eerikäinen et al., Citation2003). Laaksonen et al. (Citation2011) prepared indomethacin nanosuspensions by wet-bead milling method using Poloxamer 188 as stabilizer. The nanocrystals diluted with aqueous solution of mannitol and leucine were granulated by the aerosol flow reactor. In this way, the fast-dissolving composite microparticles of indomethacin nanocrystals embedded in a mannitol matrix with a leucine-rich coating layer were produced.
6. Application of nanocrystals based pulmonary inhalation system
6.1. Inhalable nanocrystals based aerosol
The inhalable nanocrystals based aerosol (INA) produces aerosol through an inhaler or aerosol inhaler that delivers the drug nanocrystals to the lungs after inhalation administration, as they are highly fine particles, whose distribution is more evenly on the surface of the lungs. The main advantages of INA are that they do not require propellants, are easy to produce, and compounds are not limited by solubility (Chiang et al., Citation2009), making them more acceptable to patients. Kraft et al. prepared budesonide INA for the treatment of bronchial asthma and tested its pharmacokinetic parameters in vivo. These results showed that there was no significant difference in AUC between INA and commercially inhaled budesonide suspension (Pulmicort Respules), but the Cmax was 1212 pg/mL and 662 pg/mL, and the Tmax was 8.4 min and 14.4 min, respectively, indicating that the INA was more effective (Kraft et al., Citation2004). Irene et al. prepared coenzyme Q10 INA with an average particle size lower than 100 nm, which overcomes the disadvantage of poor water solubility and has good atomization performance (Rossi et al., Citation2018).
6.2. Inhalable nanocrystals based composite microparticle (INCM)
Nanocrystals based nanocomposite particle is composed of ‘primary particles’ and ‘secondary particles’. The ‘primary particles’ are inhalable composite microparticles loaded with nanocrystals particles, which are easily deposited in the lungs and reduce the phagocytosis of function pulmonary macrophages. The nanocrystals as secondary particles are dispersed by rehydration after inhalation. The INCM is composed of biodegradable matrix that is rapidly soluble in lung fluid and releases nanocrystals immediately (Luo et al., Citation2020). INCM is a promising drug pulmonary delivery system for poorly soluble drug, which can not only overcome the disadvantage of low deposition rate of nanocrystals in the deep lung, but also improve the lung deposition of drug nanocrystals owing to excellent aerodynamic requirements of inhalable microparticles (Abd Elwakil et al. Citation2018). Pomazi et al. developed INCM using mannitol and other excipients (polymers and l-leucine) as matrix formers, and found that the presence of amino acids l-leucine reduced the adhesion between INCM and thus enhanced the aerosol of INCM (Sou et al., Citation2011). The results showed that meloxicam INCM was crystalline with a fine particle content greater than 53% and the aerodynamics particle size was less than 3.52 μm. Ninety percent of meloxicam could be dissolved and released within 5 min, indicating its enhanced aerosolization and dissolution performance in vitro (Pomázi et al., Citation2013). Chen et al. (Citation2021) reported a design of novel inhalable nanocrystals embedded microparticles for pulmonary delivery of breviscapine (BVC). The TPGS modified BVC nanocrystals (BVC-NC@TPGS) were fabricated by high pressure homogenization, and further converted into nanocrystals-embedded microparticles (BVC-NEP) via spray-drying. BVC-NEP with 10% TPGS as stabilizer and 200% Mannitol (MN) as matrix formers presented the highest FPF value and most excellent flowability as well as redispersibility. The in vivo pharmacokinetic results illustrated that the AUC(0–∞) of the inhalable BVC-NEP/MN was 6.29 times (p<.05) as high as that of the coarse BVC, and not significantly from that of BVC injection ().
Table 2. Examples of nanocrystals pulmonary inhalation system.
6.3. Inhalable nanocrystals based adhesive microparticles
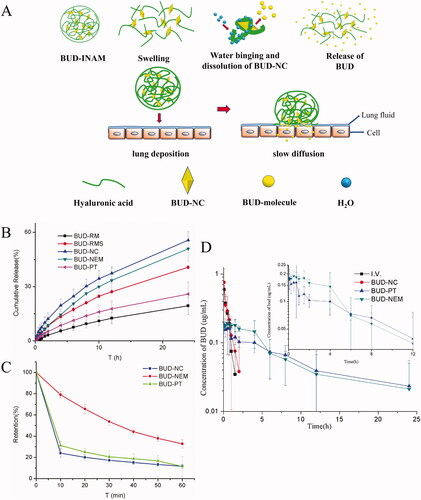
Microparticles are susceptible to the clearance by mucus cilia, which affects the efficiency of inhalable drug particles. Inhalable nanocrystals based adhesive microparticles (INAM) can enhance adhesion to the mucus layer by loading the nanocrystals into inhalable polymer materials with the function of mucus adhesion, and avoid being cleared by mucus, thereby prolonging the retention time in the lungs. Liu et al. prepared ultrafine budesonide nanocrystals by wet-milling method and loaded them into hyaluronic acid particles by spray-drying method to prepare an inhalable budesonide INAM. Budesonide INAM hydrates the swelling after lung deposition and then release the drug (). After pulmonary administration in rats, the pharmacokinetic results showed that the AUC0–24 h, Cmax, Tmax, and T1/2 of budesonide nanocrystals and INAM were 2.85 ± 1.57 and 0.54 ± 0.37 μg·h·mL−1, 0.62 ± 0.56 and 1.15 ± 0.60 h, 0.20 ± 0.04 and 0.63 ± 0.35, 2.56 ± 8.39 and 0.98 ± 1.11 h, respectively. These results indicated that a significant increase in the bioavailability of budesonide INAM. Meanwhile, the drug clearance rate of INAM was 167.62 ± 68.34 mL·h−1·g−1, which was much lower than the 1184.34 ± 725.09 mL·h−1·g−1 of budesonide nanocrystals suspension () (Liu et al., Citation2018). This can be attributed to the adhesion of hyaluronic acid to mucus, which resists mucociliary clearance by anchoring polymer chains to mucociliary components, thus prolonging the retention time of active substances in the lungs (Rouse et al., Citation2007). Meanwhile, the highly viscous inhalable nanocrystals based adhesion particles decreased drug release rate and reduced systemic exposure.
Figure 3. (A) Schematic diagram of BUD-INAM in vitro and in vivo release of BUD, (B) in vitro release profile, (C) retention on the porcine tracheal mucosa surface as a function of time, and (D) plasma concentration–time profiles. Adapted with permission from Liu et al. (Citation2018).
6.4. Inhalable mucus-penetrating nanocrystals (IMN)
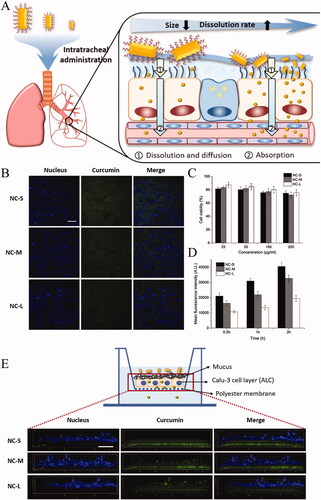
The treatment of many lung diseases requires that inhalable particles must be able to penetrate the lung mucus and reach the bronchial epithelial cells. Nanocrystals based mucus-penetrating particles are to functionalize the surface of nanocrystals, so that they can quickly penetrate the mucus barrier and be absorbed into the blood. Once the drug is administered to the lungs, undissolved particles may be rapidly cleared by the mucociliary or engulfed by alveolar macrophages. INMP will not be easily captured by the mucosal network structures, thus reducing the possibility of being cleared. Gabriella et al. reported that C109 mucus-penetrating nanocrystals (C109-IMN) modified by tocopherol polyethylene glycol 1000 succinate (TPGS) were prepared to improve the inhalation effect of C109 (an effective but insoluble FtsZ inhibitor) (). The results showed that C109-IMN could rapidly penetrate the artificial cystic fibrosis mucus due to the presence of PEG shells modified by TPGS and the optimized aspect ratio () (Costabile et al., Citation2020). He et al. (Citation2020) fabricated three different sized curcumin nanocrystals (CUR-NC) modified by poloxamer 188 for pulmonary delivery and studied the size effect on the mucus-penetrating process in vitro. The results demonstrated that a reduction in the crystal size of hydrophobic drugs could improve plasma concentrations and systemic absorption. The multiple particle tracking experiments revealed that CUR-NC with particle size of 246.16 ± 21.98 nm had larger mean squared displacement during diffusion in simulated mucus. Moreover, CUR-NC also possessed the enhanced cellular uptake and transport efficiency in Calu-3 cells (). The improved penetration ability of small nanocrystals could be attributed to the increase in the mucus diffusion ability, cellular uptake and transport in lung epithelium cells.
Figure 4. (A) Schematic diagram of PEGylated mucus-penetrating nanocrystals and lung treatment in vivo, (B) in vitro release profile, (C) inhibition ability B. cenocepacia J2315 biofilm, cytotoxicity of C109 formulations to wild type 16HBE (D) and CF (CFBE41o−) bronchial epithelial cells (E). Adapted with permission from Costabile et al. (Citation2020).
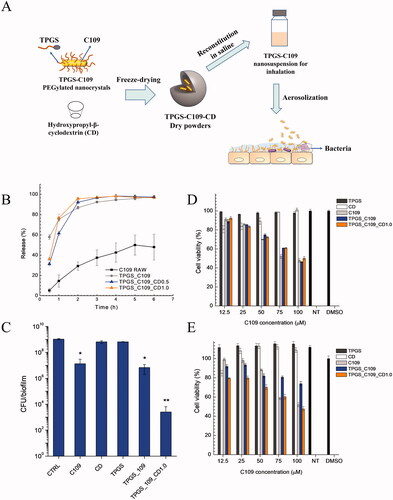
Figure 5. (A) Schematic illustration of curcumin nanocrystals on dissolution, airway mucosa penetration, lung tissue distribution, and absorption by pulmonary delivery. (B) Confocal microscopy images of calu-3 cells after incubation with CUR-NCs for 2 h (the scale bar is 50 μm); (C) calu-3 cell viability upon exposure to NC formulations at different drug concentrations; (D) mean fluorescence intensity of cells determined by flow cytometry (n = 3); (E) z-stack confocal images of the calu-3 cell layer with the polyester membrane after transport of NCs formulations for 1 h (the scale bar in xy plane was 50 μm). Adapted with permission from He et al. (Citation2020).
7. Outlook
Nanocrystals have been widely used in the oral, topical and parenteral route of drug delivery. To date, more than 20 nanocrystal-based formulations are in the market and in clinical trials. Nanocrystals also provide an effective strategy for pulmonary delivery of poorly soluble drugs. Nanocrystal-based inhalation formulations can be applied to the administration of topical as well as systemic disease by immediate and controlled release of the drugs, which can improve the dissolution rate and saturation solubility of poorly soluble drug and reduce the pulmonary clearance as well as enhance the adsorption of drug. These breakthroughs have inspired the researchers and industry to develop nanocrystal-based inhalation formulation. With the development of nanocrystals technologies for manufacturing drug inhalable formulations, we can predict many nanocrystal-based drug products on market in the near future.
8. Expert opinion
The presence of nanocrystals opens up new prospects for the development of novel pulmonary delivery system. The particle size control, physical instability, potential cytotoxicity, and clearance mechanism of inhaled nanocrystals based formulations are the major considerations in formulation development. To our knowledge, there are no marketed therapeutics related to nanocrystals-based drug inhalable formulations. To take full use of the advantages of nanocrystals for pulmonary drug delivery and develop commercially relevant products, extensive research efforts should be dedicated to the following research aspects in the future:
Novel formulation strategy that enhances the physical stability of nanocrystals aerosols and dry powder formulation, such as critical processing parameters and suitable matrix formers/carriers.
The release rate of nanocrystals from carriers/matrix formers or the rate of de-agglomeration in nanocrystals embedded composite particles, and their correlation to the pulmonary pharmacokinetics after inhalation administration.
The effects of particle size/morphology and surface chemistry on potential cytotoxicity and clearance rate of inhalable nanocrystals.
Disclosure statement
No potential conflict of interest was declared by the author(s).
Additional information
Funding
References
- Abd Elwakil MM, Mabrouk MT, Helmy MW, et al. (2018). Inhalable lactoferrin–chondroitin nanocomposites for combined delivery of doxorubicin and ellagic acid to lung carcinoma. Nanomedicine 13:2015–35.
- Abdelbary AA, Al-Mahallawi AM, Abdelrahim ME, et al. (2015). Preparation, optimization, and in vitro simulated inhalation delivery of carvedilol nanoparticles loaded on a coarse carrier intended for pulmonary administration. Int J Nanomedicine 10:6339–53.
- Alblas ABvO, Linden-Schrever B, Furth R. (1981). Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intravenous administration of heat-killed bacillus Calmette–Guérin. J Exp Med 154:235–52.
- Alhajj N, O'Reilly NJ, Cathcart H. (2021). Designing enhanced spray dried particles for inhalation: a review of the impact of excipients and processing parameters on particle properties. Powder Technol 384:313–31.
- Ali H, York P, Ali A, et al. (2011). Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J Control Release 149:175–81.
- Al-Obaidi H, Granger A, Hibbard T, et al. (2021). Pulmonary drug delivery of antimicrobials and anticancer drugs using solid dispersions. Pharmaceutics 13:1056.
- Araújo F, Martins C, Azevedo C, et al. (2018). Chemical modification of drug molecules as strategy to reduce interactions with mucus. Adv Drug Deliv Rev 124:98–106.
- Arick DQ, Choi YH, Kim HC, et al. (2015). Effects of nanoparticles on the mechanical functioning of the lung. Adv Colloid Interface Sci 225:218–28.
- Bansil R, Turner BS. (2006). Mucin structure, aggregation, physiological functions and biomedical applications. Curr Opin Colloid Interface Sci 11:164–70.
- Basu A, Guti S, Kundu S, et al. (2020). Oral andrographolide nanocrystals protect liver from paracetamol induced injury in mice. J Drug Deliv Sci Technol 55:101406.
- Bhavna , Ahmad FJ, Khar RK, et al. (2009). Techniques to develop and characterize nanosized formulation for salbutamol sulfate. J Mater Sci Mater Med 20:S71–S6.
- Button B, Cai LH, Ehre C, et al. (2012). A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337:937–41.
- Chang D, Ma Y, Cao G, et al. (2018). Improved oral bioavailability for lutein by nanocrystal technology: formulation development, in vitro and in vivo evaluation. Artif Cells Nanomed Biotechnol 46:1018–24.
- Chen Y, Gui Y, Luo Y, et al. (2021). Design and evaluation of inhalable nanocrystals embedded microparticles with enhanced redispersibility and bioavailability for breviscapine. Powder Technol 377:128–38.
- Chiang PC, Alsup JW, Lai Y, et al. (2009). Evaluation of aerosol delivery of nanosuspension for pre-clinical pulmonary drug delivery. Nanoscale Res Lett 4:254–61.
- Cipolla D, Wu H, Eastman S, et al. (2016). Tuning ciprofloxacin release profiles from liposomally encapsulated nanocrystalline drug. Pharm Res 33:2748–62.
- Costabile G, D’Angelo I, Rampioni G, et al. (2015). Toward repositioning niclosamide for antivirulence therapy of Pseudomonas aeruginosa lung infections: development of inhalable formulations through nanosuspension technology. Mol Pharm 12:237–58.
- Costabile G, Provenzano R, Azzalin A, et al. (2020). PEGylated mucus-penetrating nanocrystals for lung delivery of a new FtsZ inhibitor against Burkholderia cenocepacia infection. Nanomedicine 23:102113.
- de Kruijf W, Ehrhardt C. (2017). Inhalation delivery of complex drugs—the next steps. Curr Opin Pharmacol 36:52–7.
- Ding J, Takamoto DY, von Nahmen A, et al. (2001). Effects of lung surfactant proteins, SP-B and SP-C, and palmitic acid on monolayer stability. Biophys J 80:2262–72.
- Du J, Li X, Zhao H, et al. (2015). Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int J Pharm 495:738–49.
- Duggirala NK, Perry ML, Almarsson O, et al. (2016). Pharmaceutical cocrystals: along the path to improved medicines. Chem Commun 52:640–55.
- Eerikäinen H, Watanabe W, Kauppinen EI, et al. (2003). Aerosol flow reactor method for synthesis of drug nanoparticles. Eur J Pharm Biopharm 55:357–60.
- El-Gendy N, Gorman EM, Munson EJ, et al. (2009). Budesonide nanoparticle agglomerates as dry powder aerosols with rapid dissolution. J Pharm Sci 98:2731–46.
- Elsayed I, AbouGhaly MH. (2016). Inhalable nanocomposite microparticles: preparation, characterization and factors affecting formulation. Expert Opin Drug Deliv 13:207–22.
- Elsayed MMA, Shalash AO. (2018). Modeling the performance of carrier-based dry powder inhalation formulations: where are we, and how to get there? J Control Release 279:251–61.
- Gao L, Zhang D, Chen M. (2008). Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J Nanopart Res 10:845–62.
- Gao L, Liu G, Ma J, et al. (2012). Drug nanocrystals: in vivo performances. J Control Release 160:418–30.
- García-Díaz M, Birch D, Wan F, et al. (2018). The role of mucus as an invisible cloak to transepithelial drug delivery by nanoparticles. Adv Drug Deliv Rev 124:107–24.
- Goerke J. (1998). Pulmonary surfactant: functions and molecular composition. Biochim Biophys Acta 1408:79–89.
- Gonda I. (2006). Systemic delivery of drugs to humans via inhalation. J Aerosol Med 19:47–53.
- He Y, Liang Y, Mak JCW, et al. (2020). Size effect of curcumin nanocrystals on dissolution, airway mucosa penetration, lung tissue distribution and absorption by pulmonary delivery. Colloids Surf B Biointerfaces 186:110703.
- Hu G, Jiao B, Shi X, et al. (2013). Physicochemical properties of nanoparticles regulate translocation across pulmonary surfactant monolayer and formation of lipoprotein corona. ACS Nano 7:10525–33.
- Huang G, Xie J, Shuai S, et al. (2021). Nose-to-brain delivery of drug nanocrystals by using Ca2+ responsive deacetylated gellan gum based in situ-nanogel. Int J Pharm 594:120182.
- Jakubowska E, Lulek J. (2021). The application of freeze-drying as a production method of drug nanocrystals and solid dispersions—a review. J Drug Deliv Sci Technol 62:102357.
- Jermain SV, Brough C, Williams RO. (2018). Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery – an update. Int J Pharm 535:379–92.
- Johnson M, Bao H, Helms M, et al. (2006). Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci U S A 103:4964–9.
- Kannan R, Xu Q, Kambhampati S. (2013). Nanotechnology approaches for ocular drug delivery. Middle East Afr J Ophthalmol 20:26–37.
- Kraft WK, Steiger B, Beussink D, et al. (2004). The pharmacokinetics of nebulized nanocrystal budesonide suspension in healthy volunteers. J Clin Pharmacol 44:67–72.
- Laaksonen T, Liu P, Rahikkala A, et al. (2011). Intact nanoparticulate indomethacin in fast-dissolving carrier particles by combined wet milling and aerosol flow reactor methods. Pharm Res 28:2403–11.
- Lee WH, Loo CY, Traini D, et al. (2015). Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin Drug Deliv 12:1009–26.
- Lei Y, Kong Y, Sui H, et al. (2016). Enhanced oral bioavailability of glycyrrhetinic acid via nanocrystal formulation. Drug Deliv Transl Res 6:519–25.
- Li Y, Wang D, Lu S, et al. (2018). Pramipexole nanocrystals for transdermal permeation: characterization and its enhancement micro-mechanism. Eur J Pharm Sci 124:80–8.
- Liao Q, Yip L, Chow MYT, et al. (2019). Porous and highly dispersible voriconazole dry powders produced by spray freeze drying for pulmonary delivery with efficient lung deposition. Int J Pharm 560:144–54.
- Ling X, Shen Y, Sun CM, et al. (2014). Current progress on pulmonary drug delivery. J Pharm Res 33:711–3.
- Liu Q, Guan J, Qin L, et al. (2020). Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov Today 25:150–9.
- Liu Q, Guan J, Sun Z, et al. (2019). Influence of stabilizer type and concentration on the lung deposition and retention of resveratrol nanosuspension-in-microparticles. Int J Pharm 569:118562.
- Liu T, Han M, Tian F, et al. (2018). Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: in vitro and in vivo evaluation. Carbohydr Polym 181:1143–52.
- Liu T, Yao G, Zhang X, et al. (2018). Systematical investigation of different drug nanocrystal technologies to produce fast dissolving meloxicam tablets. AAPS PharmSciTech 19:783–91.
- Liu Y, Liu W, Xiong S, et al. (2020). Highly stabilized nanocrystals delivering ginkgolide B in protecting against the Parkinson's disease. Int J Pharm 577:119053.
- Loira-Pastoriza C, Todoroff J, Vanbever R. (2014). Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev 75:81–91.
- Lu Y, Zhao D, Li N, et al. (2018). Research advances of controlled release formulations for pulmonary delivery. J Pharm Res 37:469–72.
- Luo Y, Zhang Z, Huang G, et al. (2020). Roles of maltodextrin and inulin as matrix formers on particle performance of inhalable drug nanocrystal-embedded microparticles. Carbohydr Polym 235:115937.
- Malamatari M, Taylor KMG, Malamataris S, et al. (2018). Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov Today 23:534–47.
- Manca ML, Lai F, Pireddu R, et al. (2020). Impact of nanosizing on dermal delivery and antioxidant activity of quercetin nanocrystals. J Drug Deliv Sci Technol 55:101482.
- Mangal S, Gao W, Li T, et al. (2017). Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: challenges and opportunities. Acta Pharmacol Sin 38:782–97.
- Mansour HM, Rhee YS, Wu X. (2009). Nanomedicine in pulmonary delivery. Int J Nanomedicine 4:299–319.
- Mehanna MM, Mohyeldin SM, Elgindy NA. (2019). Rifampicin-carbohydrate spray-dried nanocomposite: a futuristic multiparticulate platform for pulmonary delivery. Int J Nanomedicine 14:9089–112.
- Müller R, Shegokar R, Gohla S, et al. (2011). Nanocrystals: production, cellular drug delivery, current and future products. Fundam Biomed Technol 5:411–32.
- Muralidharan P, Malapit M, Mallory E, et al. (2015). Inhalable nanoparticulate powders for respiratory delivery. Nanomedicine 11:1189–99.
- Ni R, Zhao J, Liu Q, et al. (2017). Nanocrystals embedded in chitosan-based respirable swellable microparticles as dry powder for sustained pulmonary drug delivery. Eur J Pharm Sci 99:137–46.
- Oberdörster G, Ferin J, Lehnert BE. (1994). Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect 102:173–9.
- Oberdörster G, Oberdörster E, Oberdörster J. (2005). Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–39.
- Onoue S, Aoki Y, Kawabata Y, et al. (2011). Development of inhalable nanocrystalline solid dispersion of tranilast for airway inflammatory diseases. J Pharm Sci 100:622–33.
- Ordonez SR, Veldhuizen EJA, van Eijk M, et al. (2017). Role of soluble innate effector molecules in pulmonary defense against fungal pathogens. Front Microbiol 8:2098.
- Ostrander KD, Bosch HW, Bondanza DM. (1999). An in-vitro assessment of a NANOCRYSTAL beclomethasone dipropionate colloidal dispersion via ultrasonic nebulization. Eur J Pharm Biopharm 48:207–15.
- Patton JS, Byron PR. (2007). Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov 6:67–74.
- Pawar VK, Singh Y, Meher JG, et al. (2014). Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release 183:51–66.
- Pelikh O, Eckert RW, Pinnapireddy SR, et al. (2021). Hair follicle targeting with curcumin nanocrystals: influence of the formulation properties on the penetration efficacy. J Control Release 329:598–613.
- Perez-Gil J, Weaver TE. (2010). Pulmonary surfactant pathophysiology: current models and open questions. Physiology 25:132–41.
- Pomázi A, Buttini F, Ambrus R, et al. (2013). Effect of polymers for aerosolization properties of mannitol-based microcomposites containing meloxicam. Eur Polym J 49:2518–27.
- Praphakar RA, Shakila H, Azger Dusthackeer VN, et al. (2018). A mannose-conjugated multi-layered polymeric nanocarrier system for controlled and targeted release on alveolar macrophages. Polym Chem 9:668.
- Raula J, Rahikkala A, Halkola T, et al. (2013). Coated particle assemblies for the concomitant pulmonary administration of budesonide and salbutamol sulphate. Int J Pharm 441:248–54.
- Roa WH, Azarmi S, Al-Hallak MH, et al. (2011). Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. J Control Release 150:49–55.
- Rogueda PG, Traini D. (2007). The nanoscale in pulmonary delivery. Part 1: deposition, fate, toxicology and effects. Expert Opin Drug Deliv 4:595–606.
- Rossi I, Sonvico F, McConville JT, et al. (2018). Nebulized coenzyme Q10 nanosuspensions: a versatile approach for pulmonary antioxidant therapy. Eur J Pharm Sci 113:159–70.
- Rouse JJ, Whateley TL, Thomas M, et al. (2007). Controlled drug delivery to the lung: influence of hyaluronic acid solution conformation on its adsorption to hydrophobic drug particles. Int J Pharm 330:175–82.
- Rundfeldt C, Steckel H, Scherliess H, et al. (2013). Inhalable highly concentrated itraconazole nanosuspension for the treatment of bronchopulmonary aspergillosis. Eur J Pharm Biopharm 83:44–53.
- Sanders N, Rudolph C, Braeckmans K, et al. (2009). Extracellular barriers in respiratory gene therapy. Adv Drug Deliv Rev 61:115–27.
- Shirley M. (2019). Amikacin liposome inhalation suspension: a review in Mycobacterium avium complex lung disease. Drugs 79:555–62.
- Sinha B, Müller RH, Möschwitzer JP. (2013). Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm 453:126–41.
- Siow CR, Wan Sia Heng P, Chan LW. (2016). Application of freeze-drying in the development of oral drug delivery systems. Expert Opin Drug Deliv 13:1595–608.
- O' Donnell KP, Smyth HDC. (2011). Macro- and microstructure of the airways for drug delivery. In: Smyth H, Hickey A, eds. Controlled pulmonary drug delivery. Advances in Delivery Science and Technology. Springer, New York, NY, 1–19.
- Song S, Guo J, Li H. (2013). Preparation of a high-efficiency nebulizer of betamethasone dipropionate by high pressure microfluidization. Journal of Controlled Release 172:e66.
- Sou T, Orlando L, McIntosh MP, et al. (2011). Investigating the interactions of amino acid components on a mannitol-based spray-dried powder formulation for pulmonary delivery: a design of experiment approach. Int J Pharm 421:220–9.
- Sung JC, Pulliam BL, Edwards DA. (2007). Nanoparticles for drug delivery to the lungs. Trends Biotechnol 25:563–70.
- Tanaka R, Hattori Y, Otsuka M, et al. (2020). Application of spray freeze drying to theophylline–oxalic acid cocrystal engineering for inhaled dry powder technology. Drug Dev Ind Pharm 46:179–87.
- Tao L, Müller R, Mschwitzer JP. (2016). Systematical investigation of a combinative particle size reduction technology for production of resveratrol nanosuspensions. AAPS PharmSciTech 18:1–9.
- Thakkar S, Shah V, Misra M, et al. (2017). Nanocrystal based drug delivery system: conventional and current scenario. Recent Pat Nanotechnol 11:130–45.
- Torvela T, Lähde A, Mönkäre J, et al. (2011). Low-temperature aerosol flow reactor method for preparation of surface stabilized pharmaceutical nanocarriers. J Aerosol Sci 42:645–56.
- Wang J, Yang Y, Yu M, et al. (2018). Diffusion of rod-like nanoparticles in non-adhesive and adhesive porous polymeric gels. J Mech Phys Solids 112:431–57.
- Wei S, Xie J, Luo Y, et al. (2018). Hyaluronic acid based nanocrystals hydrogels for enhanced topical delivery of drug: a case study. Carbohydr Polym 202:64–71.
- Whitsett JA, Weaver TE. (2002). Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 347:2141–8.
- Wüstneck R, Perez-Gil J, Wüstneck N, et al. (2005). Interfacial properties of pulmonary surfactant layers. Adv Colloid Interface Sci 117:33–58.
- Xing Y, Lu P, Xue Z, et al. (2020). Nano-strategies for improving the bioavailability of inhaled pharmaceutical formulations. Mini Rev Med Chem 20:1258–71.
- Xiong S, Liu W, Zhou Y, et al. (2020). Enhancement of oral bioavailability and anti-Parkinsonian efficacy of resveratrol through a nanocrystal formulation. Asian J Pharm Sci 15:518–28.
- Yamasaki K, Kwok PC, Fukushige K, et al. (2011). Enhanced dissolution of inhalable cyclosporine nano-matrix particles with mannitol as matrix former. Int J Pharm 420:34–42.
- Yang MY, Chan JG, Chan HK. (2014). Pulmonary drug delivery by powder aerosols. J Control Release 193:228–40.
- Yang W, Johnston KP, Williams RO 3rd. (2010). Comparison of bioavailability of amorphous versus crystalline itraconazole nanoparticles via pulmonary administration in rats. Eur J Pharm Biopharm 75:33–41.
- Yang W, Peters JI, Williams RO. (2008). Inhaled nanoparticles—a current review. Int J Pharm 356:239–47.
- Yu Q, Wu X, Zhu Q, et al. (2018). Enhanced transdermal delivery of meloxicam by nanocrystals: preparation, in vitro and in vivo evaluation. Asian J Pharm Sci 13:518–26.
- Yu SH, Possmayer F. (2003). Lipid compositional analysis of pulmonary surfactant monolayers and monolayer-associated reservoirs. J Lipid Res 44:621–9.
- Yue PF, Liu Y, Xie J, et al. (2018). Review and prospect on preparation technology of drug nanocrystals in the past thirty years. Acta Pharm Sin B 53:529–37.
- Zheng JY, Bosch HW. (1997). Sterile filtration of nanocrystal drug formulations. Drug Dev Ind Pharm 23:1087–93.