ABSTRACT
Background
High-intensity Constraint-Induced Aphasia Therapy Plus (CIAT-Plus) and Multi-Modality Aphasia Therapy (M-MAT) are effective interventions for chronic post-stroke aphasia but challenging to provide in clinical practice. Providing these interventions may be more feasible at lower intensities, but comparative evidence is lacking. We therefore explored feasibility, acceptability, and preliminary efficacy of the treatments at a lower intensity.
Methods
A multisite, single-blinded, randomized Phase II trial was conducted within the Phase III COMPARE trial. Groups of participants with chronic aphasia from the usual care arm of the COMPARE trial were randomized to M-MAT or CIAT-Plus, delivered at the same dose as the COMPARE trial but at lower intensity (6 hours/week × 5 weeks rather than 15 hours/week × 2 weeks). Blinded assessors measured aphasia severity (Western Aphasia Battery-Revised Aphasia Quotient), word retrieval, connected speech, multimodal communication, functional communication, and quality of life immediately post interventions and after 12 weeks. Feasibility and acceptability were explored.
Results
Of 70 eligible participants, 77% consented to the trial; 78% of randomized participants completed intervention and 98% of assessment visits were conducted. Fatigue and distress ratings were low with no related withdrawals. Adverse events related to the trial (n = 4) were mild in severity. Statistically significant treatment effects were demonstrated on word retrieval and functional communication and both interventions were equally effective.
Conclusions
Low–moderateintensity CIAT-Plus and M-MAT were feasible and acceptable. Both interventions show preliminary efficacy at a low–moderate intensity. These results support a powered trial investigating these interventions at a low–moderate intensity.
Introduction
There is a considerable gap between effective high-intensity aphasia interventions published in research trials and the typically low-intensity treatment schedules provided in clinical practice.Citation1 Published high-intensity interventions for chronic aphasia frequently provide 15 hours of treatment per week,Citation2 or more.Citation3 By contrast, surveys of clinical practice from Australia, the United Kingdom, and the United States of America consistently show that, for community-dwelling individuals with aphasia, no more than 3 hours per week is offered, and frequently less.Citation1,Citation4–8 While intervention dose is a complex, multidimensional construct,Citation9 this demonstrates that intensity of treatment is one aspect of service delivery with a wide research-practice gap. Insufficient funding and resources are reported as the primary barrier to increasing therapy intensity in clinical practice,Citation1,Citation10 with clinicians managing caseloads with multiple demands and a limit to the resources they can dedicate to aphasia.Citation1,Citation11 Hence, comparing the effectiveness of lower and higher intensity interventions will ultimately be crucial to optimizing service design for best outcomes in people with aphasia and for determining the best allocation of funding.
There are plausible theoretical rationales for both scheduling approaches. The neuroplasticity principle that intensity matters suggests that higher intensity should be superior to lower.Citation12 However, most of the research underpinning this principle was conducted in motor tasks and with animals,Citation13 with questions remaining concerning its application to language recovery. Moreover, even in effective high-intensity aphasia interventions, there are high rates of non-respondersCitation14 and dropouts.Citation15 In contrast, cognitive psychology research provides evidence that more distributed practice results in greater recall for new learning, particularly over the long termCitation16; however, the applicability of research on learning new verbal information to rehabilitating impaired language is not yet established.Citation17 Consequently, given these conflicting hypotheses, direct experimental evidence is required within aphasia.
Two influential reviews of intensive therapies compared studies with different treatments and/or a different number of total treatment hours and consequently could not determine differential effects of amount, type, and distribution of therapy.Citation15,Citation18 Hence, Pierce et al. systematically reviewed studies that directly compared different intensities of treatment for chronic aphasia while controlling for treatment type and total hours provided.Citation19 While only eight studies matched these criteria, meta-analyses of expressive language outcomes suggested that neither higher or lower intensity scheduling was more effective. Similarly, three subsequently published studiesCitation20–22 reported mixed results for high- or low-intensity therapy. In addition, a network meta-analysis of aphasia interventionCitation23 provides further evidence of the complexity of treatment intensity: overall, the strongest treatment gains were associated with providing 2–4 or 9+ hours of treatment per week. Hence, the evidence remains unclear regarding whether higher or lower intensity therapy differ in their effectiveness for chronic aphasia. Data on outcomes at different treatment intensities are necessary to inform future research and clinical services.
We explored this issue by embedding a lower intensity sub-study in a recent high-intensity aphasia trial.Citation7 A lower intensity (i.e. more distributed) schedule may enable greater uptake of these treatments, if shown to be equally effective. Using this embedded sub-study, we aimed to explore the feasibility and acceptability of the treatments at a lower intensity and to explore preliminary efficacy.
Method
Study design
This Phase II parallel randomized trial was a nested sub-study comprised of participants from the usual care arm of the COMPARE trial.Citation7 COMPARE was a three-arm, single-blinded, randomized controlled trial of Constraint-Induced Aphasia Therapy Plus (CIAT-Plus), Multi-Modality Aphasia Therapy (M-MAT), and usual care in chronic aphasia (n = 216). Ethical approval was obtained at multiple sites (see online protocol).
Participants
Participants were recruited from the 70 participants in the Usual Care (control) arm of the COMPARE study, who were offered the opportunity to take part in this sub-study following completion of their final assessment in the COMPARE trial.
In COMPARE, participants, recruited directly from the community and from 19 hospital sites in Australia and New Zealand, were placed in groups of three according to aphasia severity (mild, moderate, severe based on Western Aphasia Battery – Revised Aphasia Quotient scores, WAB-R AQCitation24) and each group was randomized to M-MAT, CIAT-Plus or Usual Care (full details of the COMPARE trial are available in Rose et alCitation7). At the time of recruitment to the COMPARE trial, participants needed to be aged over 17 years, living in the community, and have chronic aphasia resulting from stroke (>6 months duration) confirmed by WAB-R-AQ < 93.8, fluent in English prior to their stroke, independent in toileting or have a caregiver who could assist, and have sufficient auditory comprehension to provide written informed consent with the use of supported communication strategies and simplified, aphasia accessible, consent documents. Participants were excluded if they had a neurological condition other than stroke, severe apraxia of speech or dysarthria, uncorrected sensory loss preventing participation, or an untreated mental health condition preventing adherence to the study protocol. Prior to inclusion in the sub-study, participants were re-screened on mental health, medical and medication criteria.
Randomization and blinding
When the participants in a usual care group all consented to the sub-study, the group was randomized into CIAT-Plus or M-MAT. If group members did not unanimously consent to the sub-study, additional groups were made up of consenting participants of the same aphasia severity and geographical area. Randomization was conducted via a computer algorithm from a central allocation system, using blocked randomization within each severity.Citation25 Simple randomization was used, meaning imbalances in the number of groups in each arm was possible. Trial managers were notified of the randomization result for each group via telephone using an external central allocation system. Blinding of participants and therapists in behavioral interventions is not possible, but assessors were blinded to participant allocation.
Interventions
The intervention protocols for CIAT-Plus and M-MAT were from the COMPARE trialCitation25 and are summarized in Appendix A. The present study protocol was registered within ANZCTR (ACTRN12615000618550) in 2015. It provided 2 hours × 3 days × 5 weeks (30 hours), in contrast to COMPARE participants in treatment arms who received 3 hours × 5 days × 2 weeks (30 hours). The low–moderate-intensity schedule of six hours per week was chosen as it contrasted strongly with the high intensity of 15 hours per week provided in COMPARE and has been used successfully in previous chronic aphasia intervention research,Citation26 Further, a typical clinical intensity (≤2 hours per week) at a matched total dose of 30 hours was not feasible as this would have extended total participation to 29 weeks, which was deemed potentially burdensome.
During the trial, participants were permitted to continue with their standard care (including speech therapy, physiotherapy, groups, etc.). However, any treatments that were not considered standard care (as judged by trial coordinators) were not permitted, for example, alternative therapy, herbal preparations, or other clinical trials. Standard care was recorded in a diary for 16 weeks during the interval between COMPARE assessments by the participant and/or their family. Diaries were not continued beyond the baseline period of this study so as not to increase the burden of participation.
Treatment fidelity
Treatment adherence was ensured using the COMPARE trial procedures.Citation7 Time logs by therapists measured intervention time, and the number of participant “turns” in each intervention session provided a more granular measure of dose. A turn involved an attempt at using the target noun or verb in an utterance within a card game, regardless of accuracy or whether cueing was required. Participants rated their fatigue and distress on visual analogue scales before and after each day of treatment, and if greater than zero, were asked if they were willing to continue with the study.
Testing and outcomes
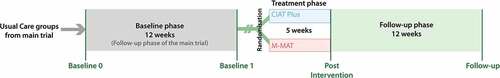
illustrates the study timeline. The post intervention and 12-week follow-up assessments from the COMPARE trial formed two pre-intervention baseline assessments for this sub-study, Baseline 0 and Baseline 1. Within 1 week of the conclusion of the sub-study intervention, a post-intervention assessment was completed and a follow-up assessment 12 weeks later. Feasibility was assessed by examining recruitment and retention rates, while acceptability was operationalized as the number of participants who completed the planned treatment and assessments as well as direct participant reports of fatigue and distress.
Outcome measures included aphasia severity (WAB-R AQCitation24), word retrieval (COMPARE naming battery, 80 treated, 100 untreated items)Citation7; connected speech (Content Information Unit (CIU) count and CIUs per minute)Citation27; multimodal communication (Scenario Test)Citation28; functional communication (Communicative Effectiveness Index – CETI)Citation29; and quality of life (Stroke and Aphasia Quality of Life Scale 39 g – SAQOL-39).Citation30
Participant eligibility and description assessment data were taken from baseline assessments in the COMPARE main trial to avoid unnecessary reassessment. A rigorous assessment fidelity protocol was implemented to ensure valid and reliable measurements.Citation25 For a small number of participants (n = 2), assessments were conducted via video call due to COVID-19 social distancing protocols; equivalence to face-to-face assessment has been established.Citation31,Citation32
Analysis
Statistical comparisons for baseline characteristics were not used as they are strongly discouraged by the CONSORT guidelines for reporting RCTs.Citation33 Instead, the standardized mean difference for continuous variables was calculated with any difference greater than 0.1 considered to show possible imbalance.Citation34
In this unpowered Phase II trial, mixed repeated measures ANOVAs (with Bonferroni correction) were conducted to explore within- and between-group change across all timepoints, using per protocol approach. ANOVA assumptions were checked (see Supplementary file) and post hoc testing applied where main effects or interactions were significant. Alpha was set at 0.05. ANOVA involves casewise deletion, where any data point that is missing for an outcome results in dropping of the participant’s data for that outcome; some analyses therefore contained fewer participants (see supplementary file).
Results
Feasibility
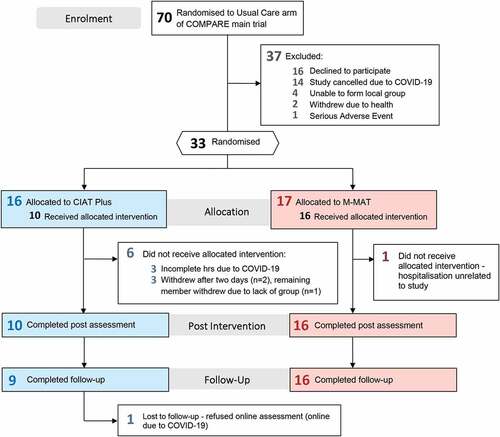
Of the 70 eligible participants, 54 consented to participate (77%). displays the CONSORT Flow Diagram and participant flow. Notably, 14 of 54 consenting participants were not randomized because of unplanned cessation of the trial due to COVID-19 social distancing protocols. Other participants were not randomized due to a lack of suitable nearby group members (n = 4) and unrelated health events (n = 3). Ultimately, 13 groups were randomized to CIAT-Plus (n = 16) or M-MAT (n = 17); 10 groups of three and 3 groups of two, comprised of 33 substudy participants plus three additional people with aphasia who complemented group sizes but did not contribute data.
Data from all participants who completed the allocated interventions were analyzed. Two assessment visits were not completed (2% of datapoints): Follow-Up assessments for one M-MAT group were conducted online due to COVID-19 social distancing protocols which one participant declined to participate in; one CIAT Plus participant missed the Baseline 0 assessment due to an unrelated illness.
Acceptability
A total of 16/17 M-MAT participants (94%) and 10/16 CIAT-Plus participants (63%) received the full allocated intervention. For four participants, failure to complete the interventions was unrelated to the trial: one M-MAT participant was admitted to hospital for an unrelated adverse event and one CIAT-Plus group (n = 3) was ceased due to COVID-19 precautions. In another group, two participants withdrew due to travel time and an interpersonal conflict, respectively, meaning the remaining participant could not continue.
Median fatigue ratings were 1/10 (IQR 2) at the start of day and 1 (IQR 3) at the end of day. Median distress ratings increased from 0/10 (IQR 1) to 1/10 (IQR 2). No participants discontinued the study due to fatigue or distress.
In total, 25 adverse events were recorded, with 21 unrelated to the study and four considered “probably” related: headaches in two participants. Three adverse events were considered severe, all unrelated to the study: food poisoning, elective surgery, and influenza.
Baseline Characteristics
shows sub-study participant characteristics at baseline of the COMPARE trial. Analysis showed that the M-MAT and CIAT-Plus arms were comparable in terms of aphasia severity, non-verbal reasoning, and short-term memory, while there were differences >0.1 SMD in age (mean difference 6.1 years), education (0.8 years) and several cognitive and linguistic measures. However, inspection of the raw mean differences did not suggest highly imbalanced groups. The Supplementary file contains visual representations of group characteristics in Beeswarm plots.
Table 1. Baseline participant characteristics.
Intervention
A mean of 28.7 hours of intervention was provided (SD 1.6) and each arm received a similar total dose (mean difference 0.6 hour difference) and mean session duration (mean difference 1 minute). Within treatment sessions, the mean number of language game turns was higher for CIAT-Plus participants (mean 26.3 per session) than M-MAT participants (21.2) due to the difference in cueing hierarchies.
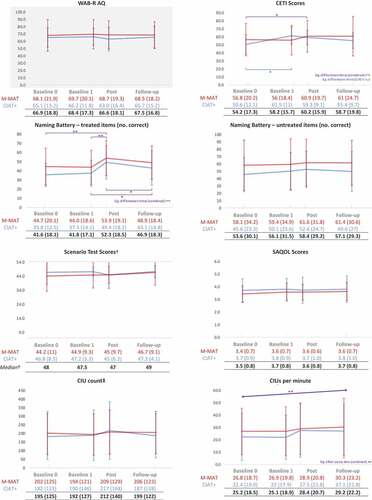
displays mean scores by group and time for all outcome measures. The main effect of time (i.e. change in scores across time for all participants) was significant for word retrieval of treated items (F = 14.392, p < 0.001, partial η2 = .407), CIUs per minute (F = 4.492, p = 0.007, partial η2 = 0.183) and functional communication (CETI) (F = 3.173, p = 0.031, partial η2 = 0.137). No other outcomes were significantly different across time. Post hoc analysis using pairwise comparisons of timepoints, found that, for word retrieval of treated items, Post Intervention was significantly greater than both Baselines (each p < .001) and follow-up (p = .040), and follow-up was significantly greater than Baseline 1 (p = .021). For CIUs per minute, there were no statistically significant differences between pairs of timepoints, while for the CETI, Post Intervention was significantly greater than Baseline 0 (p = .036).
Figure 3. Mean group changes (± SD) over time for CIAT-Plus and M-MAT.
Between-group differences
There were no significant main effects of group for any outcomes. There were no significant interactions between group and time except on the CETI (F = 3.006, p = .037, partial η2 = .131). For CIAT-Plus, there was a simple main effect of time (F = 8.692, p < .001, partial η2 = .521) but not for M-MAT. Further analysis of this interaction using pairwise comparisons of CIAT Plus CETI scores between timepoints revealed a statistically significant difference between Baseline 0 and Baseline 1 only (mean improvement 10.9, p = .004).
Comparison to high intensity (COMPARE)
shows the outcomes in this sub-study alongside those of the high intensity COMPARE trial that significantly improved over usual care. Word retrieval and functional communication were significant in both this sub-study and COMPARE, while there were different outcomes in quality of life and one connected speech measure.
Table 2. Significant outcomes across this trial and COMPARE.
Discussion
This Phase II randomized controlled trial established the feasibility, acceptability, and preliminary efficacy of low–moderate-intensity aphasia interventions.
The proportion from the COMPARE trial consenting to the sub-study was lower than anticipated (77%), yet feasible for a larger trial. This proportion was potentially due to the burden of assessment – by the time of consenting to this sub-study, participants had typically completed three assessment sessions for the main trial at several hours each without receiving any trial-provided intervention. In a standalone trial of these low–moderate-intensity interventions, there would be fewer assessments; therefore, we expect that recruitment would be feasible. In those participants who participated in the sub-study, 102/104 assessments were completed (99%), demonstrating a feasible assessment protocol. The completion rate for the intervention was lower than anticipated (26/33, 79%), though when cessations due to COVID-19 are excluded, the completion rate is 87%, suggesting an overall acceptable experience of the treatments at this schedule and a feasible trial protocol. Fatigue and distress remained very low for most participants and there were no withdrawals citing fatigue or distress. Further, adverse events recorded during the interventions were typically unrelated to the trial and mild in severity. Taken together, these outcomes demonstrate that participants were able to safely tolerate the treatment schedule. However, formal feedback from trial participants was not collected and could have strengthened the understanding of acceptability by providing insights on the decisions for withdrawal and participants’ overall experience. Therapy integrity was closely monitored and was 95% compliant overall, showing that the intervention protocols are feasible at this intensity. Qualitative data were not collected from therapists but would have been interesting to explore perspectives on feasibility for implementing these interventions in a clinical role.
Both treatments indicated preliminary efficacy at a low–moderate schedule for improving word retrieval of treated items and functional communication (CETI), with no difference in outcomes between CIAT-Plus and M-MAT. Results are encouraging for continuation in a larger trial. While this sub-study was not powered for comparison to the high-intensity treatments within the COMPARE trial, these pilot data indicate broadly similar outcomes. Specifically, for both intensities, there were significant changes in word retrieval and functional communication following interventions, and significantly improved word retrieval at follow-up. Most nonsignificant outcomes were also aligned – aphasia severity, multimodal communication, and CIU count were not significantly different for either intensity.
However, there were some discrepant results between intensities. COMPARE found a significant effect of both treatments on communication-related quality of life, whereas this study did not, while this sub-study found significant improvement in one discourse measure (CIUs/min) across time that was not present in the COMPARE trial. In addition, while CIAT Plus and M-MAT were equivalent at low–moderate intensity, the high-intensity COMPARE trial found some significant differences between the two treatments: there was greater improvement in word retrieval of treated items for CIAT Plus compared to M-MAT, and greater improvement in communication-related quality of life in M-MAT compared to CIAT Plus, though neither difference was present at 12-week follow-up assessments. A larger, powered trial of this low–moderate intensity would more definitively determine whether differences are a true effect of the varied intensities or measurement error. For example, if a powered trial confirmed the observation that CIUs/min improved more following low–moderate-intensity interventions, this could be evidence that distributed practice facilitates learning effects for this domain.
Overall, these preliminary results do not suggest an inferior or superior effect of lowering weekly intensity while controlling the total dose, in accordance with a previous systematic review of the limited available evidence on intensity.Citation19 One explanation for the equivocal findings of that review, and of contradictory findings published since then, is that the effects of altering intensity may differ across participants, treatment and outcome measures. Dose and intensity are complex constructs that likely produce different outcomes in different treatments,Citation19 and the RELEASE data recently showed a heterogeneous pattern of optimal intensity depending on the type of language outcome.Citation23 In addition, participant factors are partially predictive of recoveryCitation23,Citation35 and are also likely to interact with dose and intensity, meaning that the optimal intensity might differ across individuals. Confident predictions of optimal dose for individuals within different treatments and outcomes require a large amount of data. The sample size in the present study does not afford analysis of predictors such as aphasia severity or cognitive profile. A larger, powered trial of CIAT Plus and M-MAT at low–moderate intensity would provide important comparative data on the effect of intensity in these treatments and contribute to large-scale meta-analyses. Future research might also explore an intensity more closely resembling typical clinical practice (1–2 hours/week) to assess the relative effectiveness of modifying standard schedules. Exploration of cultural and linguistic adaptations of these treatments would also be valuable.
An established evidence base for lower intensity interventions would enable more rapid clinical implementation which would address the challenge of allocation of limited rehabilitation resources.
Supplemental Material
Download MS Word (936.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, JEP, upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10749357.2023.2196765
Additional information
Funding
References
- Cavanaugh R, Kravetz C, Jarold L, Quique Y, Turner R, Evans WS. Is there a research–practice dosage gap in aphasia rehabilitation? Am J Speech Lang Pathol. 2021;30(5):2115–2129. doi:10.1044/2021_ajslp-20-00257.
- Mozeiko J, Coelho CA, Myers EB. The role of intensity in constraint-induced language therapy for people with chronic aphasia. Aphasiology. 2015;30(4):339–363. doi:10.1080/02687038.2015.1070949.
- Szaflarski JP, Ball AL, Vannest J, et al. Constraint-induced aphasia therapy for treatment of chronic post-stroke aphasia: a randomized, blinded, controlled pilot trial. Med Sci Monit. 2015;21:2861–2869. doi:10.12659/msm.894291.
- Code C, Heron C. Services for aphasia, other acquired adult neurogenic communication and swallowing disorders in the United Kingdom, 2000. Disabil Rehabil. 2003;25(21):1231–1237. doi:10.1080/09638280310001599961.
- Mackenzie C, Le May M, Lendrem W, McGuirk E, Marshall J, Rossiter D. A survey of aphasia services in the United Kingdom. Eur J Disord Commun J Coll Speech Lang Ther Lond. 1993;28(1):43–61. doi:10.3109/13682829309033142.
- Palmer R, Witts H, Chater T, Starrfelt R, Starrfelt R. What speech and language therapy do community dwelling stroke survivors with aphasia receive in the UK? PLoS One. 2018;13(7):e0200096. doi:10.1371/journal.pone.0200096.
- Rose ML, Nickels L, Copland D, et al. Results of the COMPARE randomised controlled trial of constraint induced or multi-modality aphasia therapy compared with usual care in chronic post-stroke aphasia. J Neurol Neurosurg Psychiatry. 2022;93(6):573–581. doi:10.1136/jnnp-2021-328422.
- Verna A, Davidson B, Rose T. Speech-language pathology services for people with aphasia: a survey of current practice in Australia. Int J Speech Lang Pathol. 2009;11(3):191–206. doi:10.1080/17549500902726059.
- Harvey S, Rose ML, Brogan E, et al. Examining dose frameworks to improve aphasia rehabilitation research. Submitted.
- Page SJ, Wallace SE. Speech language pathologists’ opinions of constraint-induced language therapy. Top Stroke Rehabil. 2014;21(4):332–338. doi:10.1310/tsr2104-332.
- Code C, Petheram B. Delivering for aphasia. Int J Speech Lang Pathol. 2011;13(1):3–10. doi:10.3109/17549507.2010.520090.
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech, Lang Hear Res. 2008;51(1):S239. doi:10.1044/1092-4388(2008/018).
- Raymer AM, Beeson P, Holland AL, et al. Translational research in aphasia: from neuroscience to neurorehabilitation. J Speech, Lang Hear Res. 2008;51(1):S259–75. doi:10.1044/1092-4388(2008/020).
- Menahemi-Falkov M, Breitenstein C, Pierce JE, Hill AJ, O’Halloran R, Rose ML. A systematic review of maintenance following intensive therapy programs in chronic post-stroke aphasia: importance of individual response analysis. Disabil Rehabil. 2022;2021(20):1–16. doi:10.1080/09638288.2021.1955303.
- Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 2016;6(6):CD000425. doi:10.1002/14651858.cd000425.pub4.
- Dignam JK, Rodriguez AD, Copland DA. Copland D. evidence for intensive aphasia therapy: consideration of theories from neuroscience and cognitive psychology. PM&R. 2016;8(3):254–267. doi:10.1016/j.pmrj.2015.06.010.
- Middleton EL, Schuchard J, Rawson KA. A review of the application of distributed practice principles to naming treatment in aphasia. Top Lang Disord. 2020;40(1):36–53. doi:10.1097/tld.0000000000000202.
- Bhogal SK, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke J Cereb Circ. 2003;34(4):987–993. doi:10.1161/01.STR.0000062343.64383.D0.
- Pierce JE, O’halloran R, Menahemi-Falkov M, Togher L, Rose ML. Comparing higher and lower weekly treatment intensity for chronic aphasia: a systematic review and meta-analysis. Neuropsychol Rehabil. 2020;31(8):1289–1313. doi:10.1080/09602011.2020.1768127.
- Simic T, Leonard C, Laird L, Stewart S, Rochon E. The effects of intensity on a phonological treatment for anomia in post-stroke aphasia the effects of intensity on a phonological treatment for anomia in post-stroke apha-sia. J Commun Disord. 2021;93:106125. Published Online First: 2021. doi:10.1016/j.jcomdis.2021.106125.
- Monetta L, Lavoie M, Routhier S, Macoir J. Intensive and non-intensive treatment of lexical anomia are equally efficient in post-stroke aphasia. Neurocase. 2021;27(1):76–85. doi:10.1080/13554794.2020.1868534.
- Auclair-Ouellet N, Tittley L, Root K. Effect of an intensive comprehensive aphasia program on language and communication in chronic aphasia. Aphasiology. 2022;2021(11):1–21. doi:10.1080/02687038.2021.1959016.
- The REhabilitation and recovery of peopLE with Aphasia after StrokE (RELEASE) Collaborators, Brady MC, Ali M, et al. Dosage, intensity, and frequency of language therapy for aphasia: a systematic review–based, individual participant data network meta-analysis. Stroke. 2022;53(3):956–967. doi:10.1161/STROKEAHA.121.035216.
- Kertesz A. Western Aphasia Battery (Revised). New York: Grune & Stratton; 2007.
- Rose ML, Copland D, Nickels L, et al. Constraint-induced or multi-modal personalized aphasia rehabilitation (COMPARE): a randomized controlled trial for stroke-related chronic aphasia. Int J Stroke. 2019;14(9):972–976. doi:10.1177/1747493019870401.
- Dignam JK, Copland D, McKinnon E, et al. Intensive versus distributed aphasia therapy a nonrandomized, parallel-group, dosage-controlled study. Stroke. 2015;46(8):2206–2211. doi:10.1161/STROKEAHA.115.009522.
- Nicholas LE, Brookshire RH. A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. J Speech, Lang Hear Res. 1993;36(2):338–350. doi:10.1044/jshr.3602.338.
- van der Meulen I, van de Sandt-Koenderman WME, Duivenvoorden HJ, et al. Measuring verbal and non-verbal communication in aphasia: reliability, validity, and sensitivity to change of the Scenario Test. Int J Lang Commun Disord R Coll Speech Lang Ther. 2010;45(4):424–435. doi:10.3109/13682820903111952.
- Lomas J, Pickard L, Bester S, Elbard H, Finlayson A, Zoghaib C. The communicative effectiveness index: development and psychometric evaluation of a functional communication measure for adult aphasia. J Speech Hear Disord. 1989;54(1):113–124. doi:10.1044/jshd.5401.113.
- Hilari K, Lamping DL, Smith SC, Northcott S, Lamb A, Marshall J. Psychometric properties of the Stroke and Aphasia Quality of Life Scale (SAQOL-39) in a generic stroke population. Clin Rehabil. 2009;23(6):544–557. doi:10.1177/0269215508101729.
- Dekhtyar M, Braun EJ, Billot A, et al. Videoconference administration of the western aphasia battery–revised: feasibility and validity. Am J Speech Lang Pathol 2020;29:673–687. doi:10.1044/2019_ajslp-19-00023
- Caute A, Northcott S, Clarkson L, Pring T, Hilari K. Does mode of administration affect health-related quality-of-life outcomes after stroke? Int J Speech Lang Pathol. 2012;14(4):329–337. doi:10.3109/17549507.2012.663789.
- de Boer MR, Waterlander WE, Kuijper LDJ, Steenhuis IH, Twisk JW. Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. Int J Behav Nutr Phys Act. 2014;12(1):4. doi:10.1186/s12966-015-0162-z.
- Schober P, Vetter TR. Correct baseline comparisons in a randomized trial. Anesth Analg. 2019;129(3):639. doi:10.1213/ane.0000000000004211.
- Kiran S, Thompson CK. Neuroplasticity of language networks in aphasia: advances, updates, and future challenges. Front Neurol. 2019;10:S225. doi:10.3389/fneur.2019.00295.
Appendix A
CIAT-Plus overview
CIAT-Plus is a variant of the original constraint treatment for aphasia, CIAT (Pulvermüller et al., 2001). Language is stimulated, and spoken communication practised, via interactive group card games which use picture cards. There were six card games which all require participants to name or request cards in order to play – Fish, Bingo, Memory, Snap, Who Am I, and I Went Shopping. Three levels of cards (easy, moderate, hard) were available depending on the group’s severity allocation, each with two sets of 16 verbs and three sets of 16 nouns. Thus, there were 80 items (48 nouns; 32 verbs) treated in each group, taken from the 180-item naming battery. Therapists alternated between nouns and verbs each hour. When at least two-thirds of participants reached 80% accurate production on a particular noun or verb set or 9 hours of treatment for that set had been provided, the next set of words was used.
Participant responses were shaped progressively through six linguistic levels. The lowest level requires a single word, while the highest level requires a complex sentence with elements including subordinate clauses, adjectives, and prepositions. details the linguistic levels for nouns and verbs.
Table A1. Linguistics levels for nouns and verbs.
Barriers were placed between participants to discourage the use of gestures or other non-verbal modalities to communicate, apart from games that required shared space; namely Snap, I Went Shopping and Memory. While participants were not specifically discouraged from using other modalities (e.g. gesture, finger writing) to self-cue, they were reminded not to use these to communicate with other participants. Pens and writing paper were not available during treatment.
In CIAT-Plus, when the participant was unable to produce the required utterance within approximately 10 seconds, the therapist provided a phonemic cue for the target word. If the phonemic cue was effective, the participant was asked to repeat the target word three times (see for cueing hierarchy). The phonemic cue step, effective or not, was always followed by the therapist showing the printed target word to the participant and reading it aloud. The participant was then asked to repeat the entire utterance (carrier phrase/sentence including the target word) three times. No other cueing was provided for word retrieval. Errors in the carrier phrase were prompted verbally (e.g. “You forgot to say who was dancing”) and if necessary, a written sentence frame was used to demonstrate the desired sentence elements and structure (e.g. “I have: The [subject] is/was [target] [verb] the/a [object]”).
CIAT-Plus builds upon CIAT by assigning home tasks to improve carryover of language skills into real life. The therapist assigned each participant a 15-minute home task at the end of each intervention day. Tasks aimed to target the vocabulary and linguistic levels treated that day and were incorporated into the participants’ existing plans for that day. For example, the participant may have been going to a supermarket or post office on the way home and the task may have included asking for items, requesting information about postage, etc. If the participant was going straight home, the task might have involved telephoning or video conferencing with a relative or friend or discussing a news item/social plan with their family member. Task assignments were recorded on a written log and sent home with the participant, and the outcomes discussed at the following intervention session.
M-MAT overview
M-MAT uses the same structure as CIAT-Plus in terms of card games, stimulus cards, and linguistic levels. Participants were also assigned home practice tasks as described in CIAT-Plus. However, M-MAT does not use visual barriers during any games. The cueing hierarchy () also differed in M-MAT. If the participant was unable to produce a target utterance, the therapist prompted them to produce an iconic gesture to see whether they could successfully self-cue. If still unsuccessful, the participant (a) copied the gesture modeled by the therapist, (b) drew the target word, and (c) read aloud and then copied out the written word. The target word was repeated aloud once with each of these steps. Following these cueing steps, the participant was then asked to repeat the entire utterance (carrier phrase/sentence including the target word) three times.
Table A2. Cueing hierarchies for CIAT-Plus and M-MAT.