Abstract
The effect of R. oligosporus on structural and functional properties of fermented soybean was studied. After 24 hours of fermentation, the amount of total free amino acids formed greatly increased in soybean (1.20 g/100 g soybean). The pH of the fermented soybean was neutral during the growth of the mold. The amount of g-amino butyric acid (GABA) gradually increased during fermentation of the soybean (21.4 mg/100 g soybean per 24 hours). The proteins (albumin, globulin, alkaline soluble) were rapidly degraded to amino acids and low-molecular-weight peptides. Instrumental texture of fermented soybean (48 hours) had higher weakness (7.14 N), modulus of elasticity (1.02 × 109 Pa) and surrender value (8.17 × 106 Pa); these values were significantly different (P < 0.05) compared with 24 and 72 hours fermentation. Cell structure of fermented soybean was proportionally disorganized during the course of fermentation. The cell walls, cytoplasm, and vacuoles could not be distinctly identified. R. oligosporus produced enzymes which hydrolyzed protein, lipid, and starch, providing growth substrates and playing a role in its metabolism. Metabolism of mold was attributed to soybean cell disorganization, and the ratio of mature mold became higher, which was indicated by dark color.
Keywords:
INTRODUCTION
Tempeh is a traditional fermented soybean and a staple food in Indonesia, where it serves as a cheap basic foodstuff with high nutrition, and it is consumed by most Indonesian people. It is manufactured with dehulled, soaked, and cooked soybeans inoculated with a mold, normally, of the genus Rhizopus. The cultured soybeans are bound together by newly grown thick white mycelia to form a cake. During the growth of mold, the functional properties of foods are formed as follows: protein is hydrolyzed to amino acids and peptides by proteolytic enzymes,[Citation1, Citation2] oligosaccharides is hydrolyzed to monosaccharides,[Citation3] phytic acid degraded to inorganic phosphates.[Citation4, [Citation5]
Traditional tempeh has been investigated for about fifty years for its vitamin,Citation[1, Citation6] protein,[Citation7, Citation8] fatty acids,[Citation8, Citation9, Citation10] and enzyme activity.[Citation1] Almost all published studies have been done to understand the functional properties; but, structural and rheological properties of tempeh during fermentation process have not yet been investigated. This information is useful for the process of tempeh making and shows necessary knowledge of the cell structure and texture of tempeh. Actually, with regard to food products, flavor and textures are of most importance and top priority aside from nutrition, because they decide whether a food has preference and competitiveness at market.
Various studies have been done on temperatures, fermentation time, and humidity regarding the functional properties of fermented products. However, the effect of R. oligosporus on structural and rheological properties is not yet understood. The main objective of this study was to focus on the formation of amino acids (including GABA), protein degradation, structural and functional properties of fermented soybean. In addition, GABA, an important amino acid, has pharmaceutical effects on human body as anti-stress and anti-hypertension agent. Therefore the change of amount of GABA during fermentation of soybean was also investigated in this study.
MATERIALS
Soybean (Tsurunoko cultivar) cultivated in Hokkaido, Japan, was obtained from a local market in Osaka district, as the variety is the most popular in Japan. Dried culture of R. oligosporus was obtained from LIPI, Bogor, Indonesia, at a level of 1 g/kg of dry seeds.
METHODS
Preparation of Fermented Soybean
Five hundred gram dehulled samples were soaked with 1 L of tap water for 30 minutes, then gently rinsed with an approximate amount (0.5 L) of tap water, drained, and cooked (45-60 minutes) in 2 L of tap water in an uncovered pan. After the removal of drain, the soybean was allowed to cool at room temperature, then inoculated with dried culture of R. oligosporus suspension. The sample mixture was placed in Petri dishes (15 cm i.d. × 5 cm) and incubated at 30°C, 75% humidity. It was incubated on different lengths of fermentation including 0, 24, 48, and 72 hours.
Amino Acid Analysis
After the fermentation process, the samples were freeze-dried, and pulverized using a milling apparatus (Retsch, Germany). Amino acids were extracted according to the modified method of Saikusa et al.[Citation11] 1.6 g of the flours with 4 mL of 8% trichloroacetic acid solution in test tubes (2 cm i.d. × 16 cm) were homogenized using a homogenizer for 5 minutes, then shake (100 strokes/min; 5 cm amplitude) at 30°C for 1 hour. The suspension was centrifuged at 6000 g for 15 minutes at 4°C. The supernatant was filtered using 0.45 μm membrane filter (Advantec Co., Ltd., Tokyo, Japan), then 20 μL of filtrate was injected into the column of an LC-11 Amino Acid Analyzer (Yanaco Co., Ltd., Kyoto, Japan)
Isolation and Determination of Protein
Proteins (albumin, globulin, alkaline-soluble and alcoholic-soluble) were extracted according to the method of Ju et al.[Citation12]—one hundred gram of flour was defatted with hexane for 2 hours, and then dried at room temperature for 24 hours. The flour was extracted twice by shaking with 200 mL of deionized water for 4 hours at 20°C and centrifuged at 6000 g for 10 minutes. Supernatant was precipitated by the 2.5-fold addition of ethanol containing 10 mM β-mercaptoethanol. The precipitated albumin was collected by centrifugation at 6000 g for 10 minutes, and then dried under reduced pressure. After the water extraction, the resultant flour was extracted twice with 200 mL of 5% NaCl for 4 hours at 20°C and then centrifuged at 6000 g for 10 minutes. Globulin was collected by the solvent precipitation method, described as albumin extraction, and followed by twice extraction of the precipitate under vortex mixing with 250 mL of 0.02 M NaOH (to pH 11.0) at 20°C for 30 minutes, then centrifuged at 6000 g for 10 minutes (alkaline soluble protein). Then the alkaline soluble protein in the supernatant solution was precipitated by the 3-fold addition of ethanol, and then the precipitate was washed with acetone and ethyl ether. Alcoholic soluble protein was extracted twice with 100 mL of 70% ethanol for 2 hours at room temperature and then centrifuged at 6000 g for 10 minutes. It was dialyzed by a dialysis membrane (Wako Pure Chemicals Industries, Ltd.), and it was evaporated to concentrate it. Soluble protein was determined according to the method of Bradford.[Citation13] Bovine serum albumin was used as a standard protein. Ten to twenty μL of samples were put in a test tube and 1 mL of Bradford reagent (Biorad-Laboratories, USA) was added.
Determination of pH and Color
The pH of duplicate samples of fermented soybean was measured with a pH meter (Horiba F-12, Kyoto, Japan). One gram of sample was mixed with 5 times the amount of distilled water, then pH was measured after stirring for 10 minutes. Color (L*, a*, and b*) of fermented soybean was measured by using Minolta Chromameter (Model CR-13, Osaka, Japan) according to the method of Ìçöz et al.[Citation14] with modification. The L* value indicates lightness, and the a* and b* values indicate hue and chroma (a* from green to red; b* from blue to yellow). The sample flour was put into the plastic dish (diameter 40 mm). The average of color parameter for all areas were reported. Three samples were used in each analysis.
Instrumental Texture Measurement
Instrumental texture of the fermented soybean was investigated immediately after incubation (24, 48, and 72 hours) using a texture analysis with a V-shaped blade (Fudoh Rheometer, Rheotech Co., Ltd., Tokyo, Japan). A cubic sample (1×1×1 cm3) was perpendicularly oriented to the blade on smooth platform. The crosshead speed was 6 mm/sec and the plunger was stopped at 98% in depth of sample thickness. The data were processed using a computer program, Rheosoft TR-06 (Rheotech Co., Ltd.).[Citation15] Three replicates were taken within 5 minutes for each sample.
Scanning Electron Microscopy (SEM)
The sample preparation of SEM was carried out according to Morita et al.[Citation16] The samples (50×50×10 mm3) were oxidized using 2% of OsO4 in water for 12 hours. After removal the cubic sample from the solution, the OsO4 gas was completely removed under reduced pressure in a desiccator containing NaOH. Samples were put on a stage and coated with Pt-Pd for 4 minutes and observed with SEM model S-800 (Hitachi Co., Ltd., Tokyo, Japan).
Statistical Analysis
The results were processed by the SPSS software (V.11.0 for Windows, SPSS, Chicago IL). One way analysis of variance and a Duncan's test were used to analyze the results. Differences were considered significant at P < 0.05, unless otherwise specified. All samples were run in triplicate.
RESULTS AND DISCUSSION
Formation of Amino Acids During Fermentation of Soybean
The formation of free amino acids of soybean during fermentation is shown in . The total amount of amino acid of soybean increased proportionally to fermentation time. The formation of total free amino acids of soybean became 3–10 folds of the control. These observations agreed well with the previous report of amino acid in fermented products from remaining protein of soybean.[Citation2] Therefore, R. oligosporus hydrolyzed protein into amino acids and small peptides. Composition of free amino acids of fermented soybean is presented in . Mostly, the higher amount of free amino acids was formed within 24 to 72 hours fermentation. The essential amino acids of fermented soybean were higher at the 72 hours incubation, except for threonine, which was stable. Lysine fluctuated, which had lower amount at 48 hours. Semi-essential amino acids such as arginine, histidine, and tyrosine were unstable; they had a higher amount of 21.5, 50.6, and 38.1 mg/100 g soybean, respectively, after 72 hours of fermentation. The amount of glycine gradually increased during the course of fermentation. Non-essential amino acids, especially alanine, aspartic acid, cysteine, proline, and serine rapidly increased during fermentation. Other amino acids, such as asparagin, glutamine, ornithine, and GABA fluctuated: but, they showed higher amounts at the 72 hours fermentation mark, except that GABA displaced a higher amount at 24 hours. The synthesis of GABA depends on the amount of glutamic acid as basic and precursor amino acids in the metabolism of amino acid. GABA is generally synthesized from 2-oxoglutarate via glutamate, then decarboxylated to GABA by glutamate decarboxylase.[Citation11, Citation15]
Table 1 Amount of free amino acids (mg/100 g soybean)in the fermented soybean using R. oligosporus.
Degradation of Soybean Proteins
Albumin content of soybeanisshown in Soybean albumin degraded sharply up to 24 hr after inoculation of the mold, and then held the level until 72 hours. The holding level indicated that the most amount of albumin in soybean (substrate) was not completely degraded because the maximum mold production arrived at 72 hours, and the proteolytic activity was restricted. The level of albumin that was approximately 3-4 folds of the control degraded to the amino acids and small peptides. Soybean globulin also rapidly degraded approximately 9 folds more than those of the control during the course of fermentation; it disappeared at 72 hours fermentation. In contrast, the amount of alkaline soluble protein became lower when R. oligosporus was inoculated, but degradation activity of the protein was much lower than albumin and globulin, where the degradation ratio of the alkaline soluble protein became approximately 2 folds at 72 hours of fermentation. The amount of alcoholic soluble protein was quite variable; it was higher at 24 and 48 hours, then lower after 72 hours.
Table 2 Degradation of proteins during fermentation of soybean
The authors are currently studying on the degradation or hydrolysis products extracted from fermented soybean. In general, the proteins were degraded or hydrolyzed during the fermentation process. However, the decrease of various kinds of proteins (albumin, globulin and alkaline soluble) suggested that the mold rapidly degraded these proteins, and utilizes amino acids and low-molecular-weight peptides for its own growth. As a result, the intermediate-sized products of protein were formed and accumulated. Except for amino acids and peptides, the hydrolyzed proteins were assimilated into mold biomass production, and then oxidized.[Citation2] The mold grows rapidly up to 24 hours, then undergoes a maturing process. The ratio of immature and mature mold affected the activities of enzymes, because mature mold had a lower production of enzymes—indicated by the color change to brown. Immature mold was white in color and it has maximum production at 24 hours fermentation (). This phenomenon might be dependent on the characteristics of protein (as energy source for assimilation) and proteolytic enzymes. The amount and kinds of substrates in media could provide the rapid spreads of mycelium and protein products hydrolyzed by enzyme.
Table 3 Color properties of soybean during the growth of R. oligosporus.
Color Value and pH
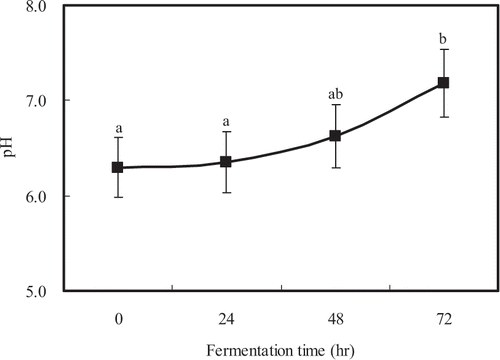
The unique phenomenon occurred in the change of pH during the growth of R. oligosporus. The pH of control soybean was 6.30, then it gradually raised during the regeneration of mold, where pH ranged around neutral (6.30 to 7.18), but the control was not significantly different (P < 0.05) at 24 and 48 hours (). During the growth of mold in soybean, the pH of medium changed greatly, because water-soluble organic acids were produced from lipids,[Citation17] proteins,[Citation2, Citation8] and oligosaccharides.[Citation3] The optimal condition for the accumulation of total amounts of free amino acids of soybean was pH 7.18 (). However, the large amount of GABA accumulated at pH 6.5 and 24 hours fermentation (21.4 mg/100 g soybean). GABA and some free amino acids showed large accumulation under slightly acidic conditions and small accumulation under basic conditions, coinciding with pH-activity responses, reported for glutamate decarboxylase (optimum pH 5.9) and GABA-pyruvate transaminase (optimum pH 8.9) from radish leaves.[Citation6]
Figure 1 Change of pH during fermentation. Values followed by the same letter are not significantly different (P < 0.05).
Soybean had originally different colors during the course of fermentation process, in which L*, a* and b* values of fermented soybean were significantly different (P < 0.05) from that of the control. The L* value of the control exhibited high lightness values of approximately 83.02, and then the lightness glimmered to 72.00. During the growth of the mold, the chromaticity coordinates in soybean, showed that the value of a* and b* increased from +2.1 to +3.8 and +15.4 to +19.3, respectively (). The color values (lightness and hue) of the fermented soybean developed from light to dark, indicating the growth of the mold had a distinct effect on L* values and chromaticity coordinates (a* and b*). When the ratio of mature mold became higher than immature one, the development of flour color gradually glimmered.
Instrumental Texture Properties
Instrumental texture properties of fermented soybean are shown in . In this case, unfermented soybean could not be used as a control because it was very hard and breakable, and the mycelium had disappeared. The complete fermentation of soybean was reached during the approximately 24 hours process, and mycelium spread among the interstice of soybean grain. This means that the tempeh is ready to cook or fry to consume it. The result of texture property tests showed that weakness (7.14 N), modulus of elasticity (1.02 × 109 Pa) and surrender value (8.17 × 106 Pa) of 48 hr fermented soybean were significantly higher (P < 0.05) than those of other fermentation lengths. As a result, the 24 hours fermented soybean was softer, and had a compact form, but mycelium was overgrown and has link network among soybean grains becoming strong after 48 hours. The increase of mature mold softened the texture of fermented soybean at 72 hours fermentation because the mycelium network was not so strong and much mycelia were not regenerated.
Table 4 Instrumental texture properties of soybean during fermentationby R. oligosporus.
Electron Microscopy
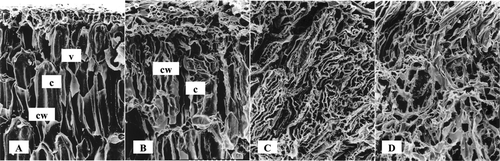
Structure of fermented soybean cells was observed using SEM in 60 (μm magnification (). The description of the control soybean cell could be distinguishable between cell wall (cw), cytoplasm (c) and vacuole (v), but the cell structure was irregular after 48 hours of inoculation of R. oligosporus. Cytoplasm of fermented soybean (24 hours) became longer and thinner than that of the control because the cell structure was degraded and somewhat difficult to determine. It became irregular after 48 and 72 hours of fermentation. In addition to the increase in the amount of essential components, such as amino acids and peptides, R. oligosporus also degraded the cell structures of soybean. Lipases, amylases and proteases involved in the disorganization of cell, these hydrolyzed substrates to provide and supply energy for growth.
CONCLUSIONS
R. oligosporus affected physical and functional properties of the fermented soybean. It has some effects on essential components (amino acids, peptides) and cell structure (texture and organization), which may be due to the enzyme activities during the fermentation process. As a result, R. oligosporus produced some enzymes in the fermented soybean, which hydrolyzed protein into substrates used for R. oligosporus's metabolism. This allowed the cell structures to degrade during the fermentation process. In addition, R. oligosporus was found to assimilate protein (albumin, globulin and alkaline soluble) as the energy source for the growth of mold. The color of the fermented soybean was changed after fermentation caused by mold growth. Furthermore, the pH of soybean became neutral (pH 7.0). Fermented soybeans (tempeh) are a big prospect as a food product to provide nutrition to humans. So, the modified processing, by using some kinds of fungi or bacteria or fermentation conditions such as temperature and humidity, is needed to produce good quality, end-use product.
Notes
L*, indicates lightness; a* and b* are the chromaticity coordinates (+a* is the red direction, −a* is the green direction, +b* is the yellow direction, −b* is the blue direction).
REFERENCES
- Wang , H.L. and Hasseltine , C.W. 1966 . Wheat tempeh . Cereal Chem , 43 : 563 – 570 . [CSA]
- Sparringa , R.A. and Owens , J.D. 1999 . Protein Utilization during soybean tempeh fermentation . J. Agric. Food Chem. , 47 : 4375 – 4378 . [INFOTRIEVE] [CSA] [CROSSREF]
- Rehms , H. and Barz , W. 1995 . Degradation of stachyose, raffinose, melibiose and sucrose by different tempe-producing Rhizopus fungi . Appl. Microbiol. Biotechnol. , 44 : 47 – 52 . [INFOTRIEVE] [CSA]
- Sutardi and Buckle , K.A. 1988 . Characterization of extra- and intracellular phytases from Rhizopus oligosporus used in tempeh production . Int. J. Food Microbiol. , 6 : 67 – 79 . [INFOTRIEVE] [CSA] [CROSSREF]
- Hachmeister , K.A. and Fung , D.Y. 1993 . Tempeh: a mold–modified indigenous fermented food made from soybeans and/or cereal grains . Crit. Rev. Microbiol. , 19 : 137 – 188 . [INFOTRIEVE] [CSA]
- Keuth , S. and Bisping , B. 1994 . Vitamin B12 production by Citrobacter freundii or Klebsiella pneumoniae during tempeh fermentation and proof of enterotoxin absence by PCR . Appl. Environ. Microbiol. , : 1495 – 1499 . [CSA]
- Fudiyansyah , N. , Petterson , D.S. , Bell , R.R. and Fairbrother , A.H. 1995 . A nutritional, chemical and sensory evaluation of lupin (L. angustifolius) tempeh . Int. Food Sci. Technol. , 30 : 297 – 305 . [CSA]
- Hering , L. , Bisping , B. and Rehm , H.J. 1991 . Pattern and formation of fatty acids at tempeh fermentation by several strains of Rhyzopus . sp. Fat Sci. Technol. , 93 : 303 – 308 . [CSA]
- De Reu , J.C. , Rombouts , F.M. and Nout , M.J.R. 1995 . Influence of acidity and initial substrate temperature on germination of Rhizopus oligosporus sporangiospore during tempe manufacture . J. Appl. Bacteriol. , 78 : 200 – 208 . [CSA]
- Hesseltine , C.W. and Wang , H.L. 1967 . Traditional fermented foods . Biotechnol. Bioeng. , 9 : 275 – 288 . [CSA] [CROSSREF]
- Saikusa , T. , Horino , T. and Mori , Y. 1994 . Distribution of free amino acids in rice kernel and kernel fraction and the effect of water soaking on the distribution . J. Agric. Food Chem. , 42 : 1122 – 1125 . [CSA] [CROSSREF]
- Ju , Z.Y. , Hettiarachchy , N.S. and Rath , N. 2001 . Extraction, Denaturation and Hydrophobic Properties of Rice Flour Proteins . J. Food Science. , 66 : 229 – 232 . [CSA]
- Ausubel , F.M. , Brent , R. , Kingston , R.E. , Moore , D.D. , Seidman , J.G. , Smith , J.A. and Struhl , K. 1999 . Short protocols in molecular biology , 10 – 19 . New York : John Wiley and Sons, Inc. .
- Ìçöz , D. , Sumnu , G. and Sahin , S. 2004 . Color and texture development during microwave and conventional baking of breads . Int. J. Food Prop. , 7 : 201 – 213 . [CSA] [CROSSREF]
- Watanabe , M. , Maeda , T. , Tsukahara , K. , Kayahara , H. and Morita , N. 2004 . Application of pregerminated brown rice for breadmaking . Cereal Chem , 81 : 450 – 455 . [CSA]
- Morita , N. , Nakata , K. , Hamauzu , Z. and Toyosawa , I. 1996 . Effect of alpha-glucosyl rutin as improvers for wheat dough and breadmaking . Cereal Chem , 73 : 99 – 104 . [CSA]
- Baum , G. , Chen , Y. , Arazi , T. , Takatsuji , H. and Fromm , H. 1993 . A plant glutamate decarboxylase containing a calmodulin binding domain . J. Biol. Chem. , 268 : 19610 – 19617 . [INFOTRIEVE] [CSA]