Abstract
The seasonal variations of fatty acids compositions in the muscle, liver, and ovarian tissues of mirror carp (Cyprinus carpio) living in Örenler Dam Lake were determined by using gas chromatography. The results obtained were subjected to statistical analyses by employing SPSS software and p < 0.05 was accepted as significant value. In the statistical analyses, multiple comparisons tests were performed. The results showed that monounsaturated fatty acids levels were found to be higher than that of polyunsaturated fatty acids and saturated fatty acids in all seasons. Palmitic and stearic acid levels were the highest in ovaries (19.95% in winter and 7.79% in summer, respectively). Oleic and palmitoleic levels were at the highest levels in liver tissues (34.53% in summer and 18.37% in winter, respectively). Docosahexaenoic (C22:6ω3, DHA), eicosapentaenoic (C20:5ω3, EPA), eicosatrienoic acid, and arachidonic (C20:4ω6, AA) levels were at the highest level as 17.27, 2.54, and 8.41% in ovaries in the winter and 6.37% in muscle tissues in the spring, respectively. ω3/ω6 ratios were 0.62–0.98 in muscle, 1.54–2.82 in the liver, and 2.82–3.89 in the ovaries. From the point of total fatty acid variations, the highest variations were observed as follows: muscle in spring (26.73%), liver in winter (33.85%), and ovaries in winter 36.38%.
INTRODUCTION
Many studies showed that food and nutritional habits cause a number of illnesses. That is why people who lived in developed countries pay attention to healthy foods intake in their daily life. It is now well known that hypercholesterolemia is a result of having excessive amounts of red meat. Nowadays, dieticians suggest consuming foods that contain rich polyunsaturated fatty acids in them. In this manner, fish and/or other water originated foods are of importance.[Citation1]
Fish meat is rich in polyunsaturated fatty acids containing ω3 fatty acids, linolenic, γ-linolenic, eicosatrienoic, eicosapentaenoic, and docosahexaenoic acids,[Citation2] which play very important roles in the prevention and curing of a number of disorders, such as heart and vein defects, rheumatoid arthritis, cancer, asthma, Alzheimer's disease, etc., and they also help the development of the brain and retina of newborns.[Citation3–7] On the other hand, Δ-12 and Δ-15 desaturase enzymes are not found in the human body, and linoleic and linolenic ω-fatty acids must be obtained from our food. Other ω fatty acids, eicosatrienoic, eicosapentaenoic, docosapentaenoic, and docosahexaenoic, can be synthesized from these two fatty acids by Δ-4, Δ-5, and Δ-6 desaturases in the appropriate tissues.[Citation8]
Age, species, sexual maturation, and environmental conditions are very effective on the storage and composition of fish oils. The chemical contents of the fish vary due to some environmental conditions, such as nutrition, temperature, salinity, etc.[Citation9–13] Additionally, seasonal variations of the factors mentioned could be effective in changing the fatty acid composition of fish.[Citation14–16]
Carp is an omnivorous fish and is nourished from the benthic part of its ambient. Their main nutrition is composed of planktons, plant residues, and fragments. Their ovulation period is between May and July, when the water temperatures reaches 18–20°C. They leave their eggs in the shallowly areas, which have an abundance of plants. The temperature of the water is a critical point for their ovulation. Therefore, they do not live in the waters situated in the highlands.[Citation17,Citation18]
Storage lipids of the carp vary in both reproduction and active periods, spring in particular, and these molecules are mobilized from one to another, such as liver, gonads, and muscles. Polyunsaturated fatty acids (PUFAs) are thought to help in forming healthy gametes and embryos.[Citation19]
The aim of the present study was to investigate the variations in fatty acid composition in its muscle, liver, and ovaries of mirror carp, Cyprinus carpio, living in Örenler Dam Lake. This species was chosen because it has an economical value and is consumed abundantly around the study area.
MATERIALS AND METHODS
Materials
Cyprinus carpio (L. 1758) specimens, 40 in total, were collected regularly between August 2005 and July 2006 from Örenler Dam Lake, Sandıklı borough of Afyonkarahisar province of Turkey. The lake has 4.4 km2 areas and is constructed for irrigation purposes in that region.
After collecting the specimens, they were transferred to the laboratory, then their metric measurements were recorded and their scales were taken for age determination. After opening their abdomen, sexual determinations were carried out, and ovaries and livers were removed carefully. Skeletons were cleaned and muscle tissue specimens were taken out between the lateral line and dorsal fin. All samples were wrapped with aluminum foil, labeled, and kept at −20°C until examination. Before the extraction procedure, samples were transferred to 4°C for the melting process for a while.
Extraction and Analyses of the Fatty Acids
In the extraction of fatty acids from the tissues studied, the basic method of Folch et al. (1957) was followed.[Citation20] For this, samples were homogenized in a chloroform/methanol (2/1, v/v) mixture. The method of CitationAOCS (1972) was employed in order to obtain the methyl esters of fatty acids by using BF3 (14%).[Citation21]
Fatty acid methyl esters (FAMEs) were analyzed on a HP (Hewlett Packard) Agilent 7890A model gas chromatography (GC), equipped with a flame ionization detector and fitted with a HP-88 capillary column (100 m, 0.25 mm i.d., and 0.20 μm). The oven temperature was programmed at an initial temperature of 170°C, was increased at a rate of 1°C per min to 208°C, was further increased at a rate of 2°C per min to 230°C, and was then held at that temperature for 5 min. The injector and flame ionization detector were set at 250°C. Nitrogen was used as a carrier gas. The injection volume was 1 μl with a split ratio of 1:50. Identification of normal fatty acids was carried out by comparing sample FAME peak relative retention times with those obtained for Supelco (37 mix) standards.
Statistical Analysis
Each reported result is the average value of three GC analyses. The results were given as means and standard deviations (±SD). Statistical analyses were performed by using SPSS 13.0 software, and multiple comparison tests were carried out. In the comparisons of seasons and groups, a one-way ANOVA test was performed and p < 0.05 value was accepted as statistical significant value.
RESULTS AND DISCUSSION
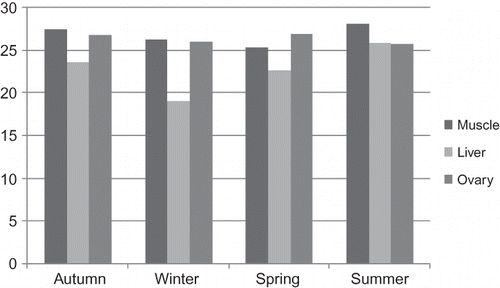
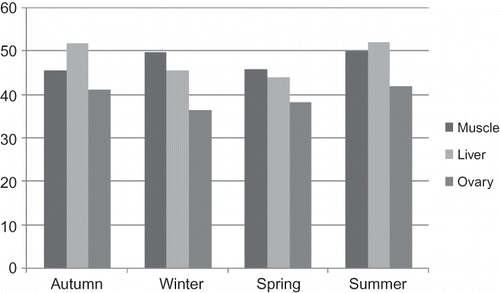
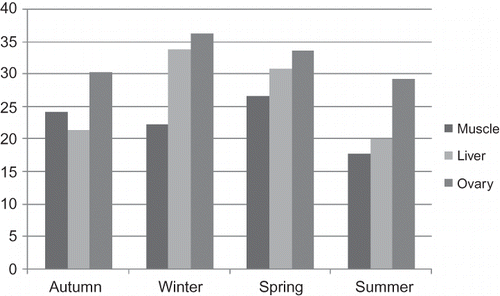
Seasonal variations of fork length, total weight, ovarian weight, condition factors, ages of the carps, and water temperatures of the lake's water were shown in . Average percent values of the fatty acids in the muscle tissues were shown in Tables showing the liver and ovaries' fatty acid percent variations, respectively. Total saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and PUFA ratio histograms were shown in .
Table 1 Average age, weight, height, and condition factor values of C. carpio
Table 2 Seasonal variations of fatty acids in the muscle of mirror carp (g/100 g fatty acids).Footnote*
Table 3 Seasonal variations of fatty acids in the liver of mirror carp (g/100 g fatty acids).Footnote*
Table 4 Seasonal variations of fatty acids in the ovariel tissues of mirror carp (g/100 g fatty acids).Footnote*
Their total weight, fork lengths, ages, condition factors, and water temperatures were shown in .
Muscle Tissue
A total of 27 different fatty acids, both saturated and unsaturated, were determined from the muscle tissue of C. carpio ( and ). Myristic acid (C14:0), palmitic (C16:0), stearic (C18:0), and arachidic (C20:0) acid were the most common SFAs. Palmitic acid was at the highest point in all seasons and its values in the autumn, winter, spring, and summer were 16.19, 15.39, 14.78, and 16.54%, respectively. Özogul et al. (2007), Mahmoud et al. (2007), and Hisar and Hisar (2003) reported that myristic, palmitic, and stearic acid were the main SFAs, and the palmitic acid was dominant in mirror carp's tissues.[Citation22–24] Their findings were similar to ours. Akpınar (1986) reported that the palmitic acid amount was at the highest amount (25.38%) in spring in C. Carpio's muscle tissue. Our findings showed that this fatty acid was at the highest amount in summer.[Citation25] On the other hand, total SFAs were at the highest in summer (28.13%) and at the lowest in spring (25.29%).
Myristoleic (C14:1), pentadecanoic (C15:1), palmitoleic (C16:1), heptadecanoic (C17:1), oleic (C18:1), and eicosenoic acid (C20:1) were the main MUFAs in the muscle tissue and oleic and palmitoleic acids amounts were the highest when compared to others. Total MUFA levels were at the top level in the summer (50.17%) (). Buchtova et al. (2004) reported the total MUFA levels were 54.8% in the muscle tissue of Tinca tinca.[Citation26]
The main PUFAs in the muscle tissue of C. carpio were as follows: ω3—linolenic (C18:3ω3), eicosatrienoic (C20:3ω3), eicosapentaenoic (C20:5ω3), and docosahexaenoic (C22:6ω3) acid, and ω6—linoleic (C18:2ω6), γ-linolenic (C18:3ω6), eicosadienoic (C20:2ω6), and arachidonic (C20:4ω6) acid (). These acids varied in the seasons. While their highest levels were in the spring (26.73%, ), they decreased to 17.87% in the summer.
Çelik et al. (2005) reported that Sander lucioperca's PUFA values were linoleic acid (4.12–9.08%), linolenic acid (1.45–0.49%), EPA (3.52–3.73%), and DHA (7.37–12.41%) living in Seyhan and Eğirdir lakes.[Citation27] Their total values were between 20.8 and 30.5%.
PUFAs do not only increase the quality of fish meat but also help them to both adapt to their ambient, such as temperature variations etc., and complete their reproduction activities successfully. As the temperature decreases, PUFAs' values increase remarkably or the other way around.[Citation28–30] These fatty acids make the ovulation easier to those ovulation periods beginning after 18–20°C of the water temperature. These organisms could be exemplified by carps. The abundance of the nutritional sources after slightly increasing the water temperature in spring might cause the increase of PUFAs. Both the water temperatures reach to the highest points and starting the reproduction activities let the fish consume enormous amounts of energy and this makes the PUFA level decrease. On the other hand, since these dam lakes are constructed for irrigational purposes, their water levels decrease rapidly. This event is also the cause of warming of the lake's water temperature. As a result of this condition, the oxygen amount also drops down and causes a big stress on fish. Therefore, these circumstances could be very effective in the decrease of PUFAs in the summer.
As it was observed, there is a contrary relation between SFAs and PUFAs. The more SFAs increase, the more PUFAs decrease.[Citation29] These could be explained as mentioned above. Additionally, as the carps enter the mating period SFAs could decrease due to formation of the ovaries.[Citation31–33] On the other hand, the variations in MUFAs were observed to be much higher than that of SFAs. In muscle tissues, the highest level of fatty acids belonged to the MUFAs, and there were statistically significant variations in the seasons studied (). The increase in the MUFAs could be because of the ability of the fish to synthesize these compounds.[Citation10,Citation27]
Liver Tissue
The data of liver's fatty acid composition of the mirror carp in the seasons were shown in . The most common SFAs in liver were C16:0 (17.50% summer), C18:0 (4.91% summer), C14:0 (1.48% spring), and C20:0 (1.11% summer). Akpınar (1986) reported that C16:0 was at the highest point in autumn (35.84%), and the lowest point was in summer (22.41%) in the common carp's liver.[Citation25] His findings are obviously higher than our findings. On the other hand, similarly, our findings seem to be lower than that of the literature reports on silver carp and Japanese catfish.[Citation34–36]
The main MUFAs of mirror carp's liver were C14:1, C15:1, C16:1, C17:1, C18:1, and C20:1. Among them, C16:1 and C18:1 were the dominant and their the highest values in the seasons were 18.37% for the first one in the winter and 34.53% for the latter in the summer, respectively.[Citation34–36]
The highest amount of the main ω3 PUFAs in the winter were C18:3ω3 (0.86%), C20:3ω3 (7.32%), C20:5ω3 (1.84%), and C22:6ω3(14.96%) (). ω6 fatty acids were C18:2ω6 (3.49% autumn), C18:3ω6 (3.13% summer), C20:2ω6 (0.76% winter), and C20:4ω6 (4.03% spring). The values given in the parentheses are their highest values. Cejas et al. (2004) reported from Diplodus sargus that while the total ω3 ratio was between 32.34 and 40.42%, the ω6 ratio was between 10.06 and 5.10%.[Citation37] The data of the present study were as follows: ω3 was at the highest level in the winter (24.98%) and ω6 was at the highest level in the spring (9.31%).
Excessive intake of the foods are stored as fat in the skeleton muscle, liver, ovaries, etc., and this fact affects the composition of the fatty acid levels in fish meat.[Citation38] Environmental conditions affect the variation of fatty acids in the liver as well as other parts of the fish. The reproduction period is one of the most effective phases in a fish life. As can be seen in Tables , PUFAs levels decreased while other fractions (SFAs and MUFAs) increased. These findings suggest that there is a regulation in the mobility and exchanging of the fatty acids from one organ to another.
Ovarian Tissue
The dominant SFAs in ovaries of mirror carp were C14:0 (0.93% autumn), C16:0 (19.95% winter), and C18:0 (7.79% summer) (). In total, the highest levels of SFAs were in autumn (26.9%) and the lowest in summer (25.73%). In this data, there was no statistically significant difference among the seasons' data obtained. Mukhopadhyay and Ghosh (2003) reported the lipid and fatty acid profiles in the ovaries of C. carpio.[Citation39] They found that the principal SFAs were C14:0, C16:0, C18:0, and C20:0 and their values were between 2.70–0.40%, 33.20–27.40%, 1.90–6.50%, and 0.30–2.20%, respectively. The ratios of SFAs were 38.2% in triacylglycerols and 36.5% in phospholipids. Shirai et al. (2001) studied the SFAs in both wild and cultured Silurus asotus ovaries and reported that C16 and C18 levels varied in cultured form but not in wilds.[Citation35]
Total MUFA levels were at the highest in summer (41.9%) and at the lowest in winter (36.41%) in ovarian tissue of mirror carp. Both C16:1 and C18:1 were the main constituents of MUFAs. The former one was dominant in spring (13.48%), and the latterone was dominant in summer (30.47%). Huynh et al. (2006) reported the total MUFAs ratio from Clupea harengus' ovarium tissue during the ovulation period as 26.82% containing C16:1 (4.00%), C17:1, and C18:1 (12.28%).[Citation40] Sushchik et al. (2007) studied Thymallus arcticus and found that the main MUFAs in autumn, spring, and summer months were as follows: C16:1 (1.42%–1.63%–0.85%), C18:1 (2.52%–3.98%–1.87%), and C20:1 (0.15%–0.21%–0.11%).[Citation41] Our findings and literature reports showed that MUFAs are the highest fatty acid groups in ovaries of the fish studied.[Citation42,Citation43]
MUFAs and tryacylglycerol ratios in common carp were reported to be 31.4 and 52.1%, respectively. Oleic acid was the dominant in MUFAs.[Citation39]
DHA were the main dominant fatty acids among the PUFAs in mirror carp's ovaries, presenting 17.27% in winter and 10.39% in summer. C20:3ω3, C20:4ω6, C18:2ω6, and C20:5ω3 followed these fatty acids. PUFAs ratio was at the highest level in winter as 36.38%, and their levels were lower than that of S. asotus's PUFAs.[Citation35]
Ovaries were supported by muscle and liver's fatty acids during the development of the eggs. The highest amount of PUFAs in ovaries in the winter and spring seasons should come from these two tissues mentioned. These facts were reported by several investigators,[Citation25,Citation42,Citation43] and our findings were similar to these reports.
In brief, it was found that the fatty acid compositions of three tissues of C. carpio varied significantly in some cases in different seasons. These variations could be because of ecologic and physiologic factors. On the other hand, age, species, sexual variations, and environmental factors are other effective factors in changing the fatty acid compositions.[Citation12–16,Citation34]
ACKNOWLEDGMENTS
The authors wish to thank to Dr. H. Shazly (Swansea, UK) for editing English of this paper.
REFERENCES
- Kaya , Y. , Duyar , A.H. and Erdem , E.M. 2004 . Balık yağ asitlerinin insan sağlığı için önemi . E.Ü., Su Ürünleri Dergisi , 21 ( 3–4 ) : 365 – 370 .
- Magali , C. , Francoise , C. , Henri , P. , Anne , P. and Marine , P. 1990 . Effects of salmon oil and corn oil on plasma lipid level and hepato-biliary cholesterol metabolism in rats . Biochimica et Biophysica Acta , 1046 : 40 – 45 .
- Simopoulos , A.P. 1991 . Omega-3 fatty acids in health and disease and in growth and development, a review . American Journal of Clinical Nutrition , 54 : 438 – 463 .
- Stone , J. 1996 . Fish consumption, fish oil, lipids and coronery hearty disease . American Heart Association , 94 : 2337 – 2340 .
- Kolanowski , W. , Swiderski , F. and Berger , S. 1999 . Possibilities of fish oil application for food products enrichment with omega-3 PUFA . International Journal of Food Science and Nutrition , 50 : 39 – 49 .
- Cunquer , J.A. 2000 . Fatty acid analysis of blood plasma of patient with Alzheimer's disease, other type of dementia, and cognitive impairment . Lipids , 35 : 1305 – 1311 .
- Arnold , L.G. 2001 . Alternative treatments for adult with ADHD analyses . The New York Academy of Science , 931 : 310 – 341 .
- Tocher , D.R. and Sargent , J.R. 1984 . Analyses of lipids and fatty acids in ripe roes of some Northwest European marine fish . Lipids , 19 ( 7 ) : 492 – 499 .
- Henderson , R.J. and Tocher , D.R. 1987 . The lipid composition and biochemistry of freshwater fish . Progress in Lipid Research , 26 ( 4 ) : 281 – 347 .
- Akpınar , M.A. and Aksoylar , M.Y. 1988 . Garro rufa Heckel, 1949'nın yağ asidi bileşimine sıcaklığın, besinsel yağ asitlerinin ve açlığın etkileri . Doga Türk Biyoloji , 12 ( 1 ) : 1 – 8 .
- Yılmaz , Ö , Konar , V. and Çelik , S. 1996 . Elazıg Hazar Gölü'nde yasayan Capoeta capoetaumbla (Heckel, 1843)'nın (Siraz) total lipit ve yag asidi miktarının aylara ve mevsimlere göre degisimi . Tr. J. of Biology , 20 : 245 – 257 .
- Bandarra , N.M. , Batista , I. , Nunes , M.L. and Empis , J.M. 2001 . Seasonal variation in the chemical composition of horse-mackerel (Tachurus tachurus) . European Food Research and Technology , 212 : 535 – 539 .
- Gökçe , A.M. , Tasbozan , O. , Çelik , M. and Tabakoglu , S.S. 2004 . Seasonal variations in proximate and fatty acid compositions of female common sole (Solea solea) . Food Chemistry , 88 : 419 – 423 .
- Kalyoncu , L. , Kissal , S. and Aktumsek , A. 2009 . Seasonal changes in the total fatty acid composition of Vimba, Vimba vimba tenella (Nordmann, 1840) in Egirdir Lake, Turkey . Food Chemistry , 116 ( 3 ) : 728 – 730 .
- Guler , G.O. , Kiztanir , B. , Aktumsek , A. , Citil , O.B. and Özparlak , H. 2008 . Determination of the seasonal changes on total fatty acid composition and omega 3/omega 6 ratios of carp (Cyprinus carpio L.) muscle lipids in Beysehir Lake (Turkey) . Food Chemistry , 108 ( 2 ) : 689 – 694 .
- Guler , G.O. , Aktumsek , A. , Citil , O.B. , Arslan , A. and Torlak , E. 2007 . Seasonal variations on total fatty acid composition of fillets of zander (Sander lucioperca) in Beysehir Lake (Turkey) . Food Chemistry , 103 ( 4 ) : 1241 – 1246 .
- Atay , D. and Çelikkale , M.S. 1983 . Sazan Üretim Teknigi . San Matbaası , : 185s
- Geldiay , R. and Balık , S. 1996 . “ Türkiye Tatlı Su Balıkları. Ege Üniversitesi Su ÜrünleriFak., Yayınları ” . In Ders Kitabı , Vol. 16 , Dizi no .
- Kara , C. and Çelik , M. 2000 . Fatty acid composition of gonad tissue in female and male Chondrostoma regium (Heckel, 1843) living in Ceyhan River, Kahramanmaraş-Turkey, C.Ü . Fen ve Müh. Derg. , 3 ( 1 ) : 160 – 166 .
- Folch , J. , Lees , M. and Stanley , A. 1957 . Simple method for the isolation and purification of total lipids from animal tissues . Journal of Biological Chemistry , 226 : 497 – 509 .
- AOCS . 1972 . Official Methods and Recommended Practices of the American Oil Chemists Society , 2nd , Champaign, Illinois : American Oil Chemists Society .
- Özogul , Y. and Özogul , F. 2007 . Fatty acid profiles of commercially important fish species from the Mediterranean, Aegean and Black Seas . Food Chemistry , 100 : 1634 – 1638 .
- Mahmoud , M.S.B. , Kawai , Y. , Yamazaki , K. , Miyashita , K. and Suzuki , T. 2007 . Effect of treatment with electrolyzed NaCl solutions and essential oil compounds on the proximate composition, amino acid and fatty acid composition of carp fillets . Food Chemistry , 101 : 1492 – 1498 .
- Hisar , O. and Hisar , A.S. 2003 . Farklı Su Sıcaklıklarında Tutulan Aynalı Sazanlarda(Cyprinus carpio) Eritrosit Hücrelerinin Ozmotik Kırılganlıkları ile Yag Asidi Kompozisyonları . Turkish Journal of Veterinary and Animal Sciences , 27 : 1277 – 1281 .
- Akpınar , M.A. 1996 . Cyprinus carpio L. (Osteichthyes: Cyprinidae)'nın Karaciğer ve kasındaki total lipit ve total yağ asidinin mevsimsel değişimi . C.Ü. Fen. Ed. Fak., Fen Bil. Derg , 4 : 33 – 42 .
- Buchtova , H. , Smutna , M. , Vorlova , L. , Svobodova , Z. and Flajshans , M. 2004 . Fatty acid composition of diploid and triploid populations of tench (Tinca tinca L.) . Acta Vet. Brno , 73 : 235 – 245 .
- Çelik , M. , Diler , A. and üçükgülmez , K . 2005 . A. A comparison of the proximate compositions and fatty acid profiles of zander (Sander lucioperca) from two different regions and climatic conditions . Food Chemistry , 92 : 637 – 641 .
- Hazel , J.R. 1979 . Influence of thermal acclimation on membrane lipid composition of rainbow trout liver . American Journal of Physiology , 236 : R91 – 101 .
- Farkas , T. 1984 . Adaptation of fatty acid composition to temperature—A study on carp (Cyprinus carpio L.) liver slices . Comparative Biochemistry and Physiology , 79B ( 4 ) : 531 – 535 .
- Cossins , A.R. 1994 . “ Homeoviscous adaptation of biological membranes and its functional significance ” . In Temperature Adaptation of Biological Membranes , Edited by: Cossins , A.R. 63 – 76 . London : Portland Press .
- Sengör , F.G. , Özden , Ö. , Nuray , E. , Tüter , M. and Aksoy , A.H. 2003 . Fatty acid compositions of flathead grey mullet (Mugil cephalus L., 1758) fillet, raw and beeswaxed caviar oils . Turkish Journal of Fisheries and Aquatic Science , 3 : 93 – 96 .
- Shirai , N. , Tereyama , M. and Takeda , H. 2002 . Effect of season on the fatty acid composition and free amino acid content of the sardine Sardinops melanostictus . Comparative Biochemistry and Physiology Part B , 131 : 387 – 393 .
- Özyurt , G. , Duysak , Ö. , Akamca , E. and Tureli , C. 2006 . Seasonal changes of fatty acids of cuttlefish Sepia officinalis L. (Mollusca: Cephalopoda) in the northeastern Mediterranean Sea . Food Chemistry , 95 : 382 – 385 .
- Agren , J. , Muje , P. , Hanninen , O. , Herranen , J. and Penttila , I. 1987 . Seasonal variation of lipid fatty acids of boreal freshwater fish species . Biochemical Physiology , 88 : 905 – 909 .
- Shirai , N. , Suzuki , H. , Toukairin , S. and Wadaa , S. 2001 . Spawning and season affect lipid content and fatty acid composition of ovary and liver in Japanese catfish (Silurus asotus) . Comparative Biochemistry and Physiology Part B , 129 : 185 – 195 .
- Aras , M.N. , Haliloglu , H. , Bayır , A. , Atamanalp , M. and Sirkecioglu , N.A. 2003 . Karasu Havzası, Yesildere Çayı Olgun Dere Alabalıkları (Salmo trutta macrostigma, Dumeril 1858)'nda farklı dokuların yağ asidi kompozisyonlarının karşılaştırılması . Turkish Journal of Veterinary And Animal Sciences , 27 : 887 – 892 .
- Cejas , R.J. , Almansa , E. , Jérez , S. , Bolaños , A. , Samper , M. and Lorenzo , A. 2004 . Lipid and fatty acid composition of muscle and liver from wild and captive mature female broodstocks of white seabream, Diplodus sargus . Comparative Biochemistry and Physiology Part B , 138 : 91 – 102 .
- Kiessling , A. , Pickova , J. , Johansson , L. , Asgárd , T. , Storebakken , T. and Kiessling , H.K. 2001 . Changes in fatty acid composition in muscle and adipose tissue of farmed rainbow trout (Oncorhynchus mykiss) in relation to ration and age . Food Chemistry , 73 : 271 – 284 .
- Mukhopadhyay , T. and Ghosh , S. 2003 . Lipid profile and fatty acid composition in eggs of common carp (Cyprinus carpio) . Journal of Oleo Science , 52 ( 8 ) : 439 – 442 .
- Huynh , M.D. , Kitts , D.D. , Hu , C. and Trites , A.W. 2007 . Comparison of fatty acid profiles of spawning and non-spawning pacific herring, Clupea harengus pallasi . Comparative Biochemistry and Physiology Part B , 146 ( 4 ) : 504 – 511 .
- Sushchik , N.N. , Gladyshev , I.M. and Kalachova , S.G. 2007 . Seasonal dynamics of fatty acid content of a common food fish from the Yenisei river, Siberian grayling, Thymallus arcticus . Food Chemistry , 104 : 1353 – 1358 .
- Ackman , R.G. 1967 . Characteristic of the fatty composition and biochemistry of some fresh-water fish oils and lipids in comparision with marine oils and lipids . Comparative Biochemistry and Physiology , 22 : 907 – 922 .
- Farkas , T. 1976 . Csengeri. Biosynthesis of fatty acids by the carp, Cyprinus carpio L., in relation to environmental temperature . Lipids , 11 ( 5 ) : 401 – 407 .