Abstract
The active components of largely consumed dietary onions (Allium species) in Turkey were extracted with water and ethanol, separately heated in a drying oven (at 50°C, for 1–4 h) and in a microwave oven (at 90 W, for 1–4 min), and their total antioxidant capacity was determined with different electron transfer-based assays. Five different onion species/aerial parts, namely yellow, red, white, fresh green leaf, and fresh green root, were measured for total antioxidant capacity with different methods, the hierarchic order in aqueous extracts being: CERAC: yellow-skinned > red-skinned > spring-root > spring-leaf > white-skinned onion; CUPRAC: spring-leaf > red-skinned > spring-root > yellow-skinned > white-skinned onion; Folin-Ciocalteau method: spring-leaf > spring-root > red-skinned > yellow-skinned > white-skinned onion. Using all three methods, white onion, showed the lowest total antioxidant capacity, while with respect to two methods (i.e., CUPRAC and Folin-Ciocalteau), spring onion-leaf showed the highest value. In ethanolic extracts, white onion exhibited the lowest total antioxidant capacity using two methods (CUPRAC and Folin-Ciocalteau). Of the heat-processed onions, the highest CERAC and Folin-Ciocalteau total antioxidant capacity values were obtained for red-skinned onions, while the highest CUPRAC value was for spring onion leaves. All three assays marked white-skinned onion as the lowest total antioxidant capacity content of heat-processed products. The change in total antioxidant capacity caused by both heating processes was not drastic; spring onion leaves essentially maintained its total antioxidant capacity level after 4 min microwave or 4 h drying oven heating. Onion processing by heat treatment did not cause a drastic loss in antioxidant values, favourable for traditional cooking practices.
INTRODUCTION
Food, drugs, cosmetics, smoke, radiation, and other environmental/chemical factors introduced by modern life result in the production of prooxidants and free radicals in the organism. When natural antioxidant defences of the organism (of enzymatic, non-enzymatic, or dietary origin) are overwhelmed by an excessive generation of reactive oxygen species, a situation of oxidative stress occurs, in which cellular and extracellular macromolecules (proteins, lipids, and nucleic acids) can suffer oxidative damage, causing tissue injury.Citation[1, Citation2] Consumption of food naturally bearing antioxidant activity (e.g., various food plants, fruits, and vegetables) is the most efficient way of combating such tissue injuries, undesired transformations, and health risks. An antioxidant is a molecule capable of slowing or preventing the undesired oxidation of biological macromolecules, such as lipids, proteins, and DNA. The protective effect of fruits and vegetables against chronic diseases is attributed to their phytochemical (essentially phenolic) content and subsequent antioxidant activity.
Plants of genus Allium are a common ingredient of human nutrition. They are used as food or spices, and are especially appreciated because of their reputed antibacterial and other biological activities.Citation[3] Allium species are often applied in traditional medicine in the treatment of cardiovascular diseases, as well as for the prevention of symptoms of infective diseases. Some of the observed effects may be explained by the antioxidant activity of these species.Citation[4] Onions (Allium cepa L.) are consumed in everyday cooking all over the world. The use of these vegetables dates back to ancient times. The dietary intake of onion has been reported to diminish the risk of cardiovascular disease, and cancers of various organs such as stomach, colon, oesophagus, prostate, bladder, liver, lungs, breast, skin, and brain.Citation[5] Onion extracts have remarkable radical scavenging power and have been shown to inhibit proliferation of leukemia cells.Citation[6] These biological activities have been reviewed, indicating the compounds responsible for each one of them.Citation[7, Citation8] Onions possess a high level of antioxidant activity, which is mainly attributed to the flavonoid and organo-sulfur content. Cysteine is the dietary origin for the physiological synthesis of glutathione, the primary antioxidant tripeptide in mammalian cells responsible for antioxidant defence, and cysteine is found in onion. This vegetable contains both flavonoids, alkenyl cysteine sulphoxides (ACSOs), and alkyl sulfides as biologically active compounds.Citation[9] Onion is one of the major sources of dietary flavonoids (such as quercetin, kaempferol, and their glycosides)Citation[10] in many countries, which are present either as sugar conjugates or as aglycones. The major flavonoid found in onion is quercetin, present in conjugated form as quercetin 4′-O-β-glycopyranoside, quercetin 3,4′-O-β-diglycopyranoside, and quercetin 3,7,4′-O-β-triglycopyranoside.Citation[5, Citation11] The dry outer layers of onion, usually wasted before cooking, contain large amounts of quercetin, quercetin glycosides, and their oxidation products.Citation[12] Flavonoid consumption has been associated with a reduced risk of cancer, heart disease, and diabetes. While anthocyanins as a subclass of flavonoids give certain onion species their pink-red colour, quercetin and the related flavonol derivatives give yellow and brown colours. Onions owe their characteristic odour and taste mainly to ACSOs and alkyl sulfides.
Several analytical methods have been developed to measure total antioxidant capacity (TAC) and total antioxidant activity.Citation[13, Citation14] A basic classification of TAC or activity assays is the type of reaction: Electron transfer (ET)- and hydrogen atom transfer (HAT)-based assays.Citation[15] ET-based assays include ABTS/TEAC (Trolox equivalent antioxidant capacity),Citation[16, Citation17] DPPH,Citation[18] Folin-Ciocalteau reagent,Citation[19, Citation20] FRAP (ferric ion reducing antioxidant power),Citation21−31 Citation Citation−23] CUPRAC (cupric reducing antioxidant capacity)Citation24−26 Citation Citation−26] and CERAC (Cerium (IV) ions reducing antioxidant capacity).Citation[27] Generally, HAT-based assays for chain-breaking antioxidants are more adequate in kinetic principle, and more sensitive, but at the same time are more time-consuming and require a significant experience in chemical kinetics for application. Consequently, ET-based assays are more practical, flexible, and suitable for routine testing of natural products; these commonly provide more information on the capability of natural products to reduce reactive species and scavenge stable free radicals. However, it is questionable whether the data obtained with ET-based assays give quantitative information on the capability of natural products to retard or inhibit oxidative processes. The other problem concerning the application of ET-based assays is their poor repeatability. Since such assay results are highly dependent on the protocol, the reagent concentrations, pH, and time of incubation need to be strictly controlled for standardization.
Since TAC variation with cooking is strongly dependent on the type of food plant, different heat treatment procedures were investigated for their effect on the antioxidant activity/capacity of selected vegetables and fruits.Citation[8, Citation28–36 Citation Citation Citation Citation Citation Citation Citation Citation−36] Phenolic compounds are generally assumed to undergo significant change during heat treatment of food plants. Onions having a significant variety of phenolics, flavonoids, and ACSOs are generally consumed after cooking rather than fresh. In their pioneering work on Polish garlic and white and red onions, Gorinstein et al.Citation[37] subjected the samples to blanching, boiling, frying, and microwaving for different periods of time, and then measured antioxidant activity as well as bioactive compounds (polyphenols, flavonoids, flavanols, anthocyanins, tannins, and ascorbic acid). They found that blanching and frying and then microwaving of garlic and onions did not significantly decrease the amounts of the bioactive compounds and the level of antioxidant activity. In another study, Gorinstein et al.Citation[38] subjected garlic and white and red onions to bleaching and boiling, and measured the changes in total antioxidant capacity (TAC) as well as in bioactive compounds, and concluded by comparative controls that bleaching for 90” of the studied vegetables most fully preserved contents of bioactive compounds and the level of antioxidant capacity. Therefore, the objective of this study was to measure the original TAC and to evaluate the effect of thermal processing on the antioxidant properties of dietary onions (Allium species) grown in Turkey by TAC measurement of the raw and thermally processed onions.
MATERIALS AND METHODS
Chemicals and Instrumentation
Quercetin was purchased from Sigma (Steinheim, Germany); Cerium(IV)sulfate tetrahydrate (Ce(SO4)2.4H2O), Folin-Ciocalteau phenol reagent, Na2CO3, NaKC4H4O6, CuCl2.2H2O, CuSO4, H2SO4, CH3COONH4, C2H5OH (96%, by wt.) were purchased from E. Merck (Darmstadt, Germany); NaOH, CH3COOH was purchased from Riedel-de Haën (Steinheim, Germany); and neocuproine (2,9-dimethyl-1,10-phenanthroline) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Commercial samples of onions were supplied from the the local food market in Istanbul, Turkey. All polyphenolic compounds and vitamin solutions were freshly prepared in 96% ethanol at 1 mM concentration.
The UV–Vis spectra recording and absorbance measurements were made with the use of a Varian Cary 100 model UV/Vis spectrophotometer (Mulgrave, Victoria, Australia) equipped with a pair of matched quartz cuvettes of 1-cm light path. Liquid sampling at 5–50 μL and 200–500 μL were made with a Genex Beta-type (Torquay, Devon, United Kingdom) variable and Brand Transferpette-type fixed-volume micropipettes (Essex, CT, USA), respectively. The samples were blended with a Magic Bullet blender (CiXi Delijia Electrical Appliance Co. Ltd., China); liquid sample equilibration was performed using a rotary shaker (Edmund Bühler GmbH, Tübingen, Germany), and heating was carried out in a Heraeus drying oven (Heraeus Instruments, UK) or in a Bosch microwave oven (Bosch GmbH, Germany), depending on the purpose.
Preparation of Onion Samples
The onion varieties listed as white-skinned (Albion), yellow-skinned (Rijnsburger), red-skinned (variety Red Baron), and spring onion were purchased from local markets. Each onion sample was skinned and chopped in a blender. After crushing in an onion chopper, an amount of 5 g was weighed in a flask, 50 mL of water or ethanol was added, and shaken for 30 min in a stirrer at 350 rpm. A clear extract was collected after filtration through a Whatman blue-band filter paper.
Drying and Microwave Heating of Onion Samples
Five grams of chopped onion were heat-treated either by heating in a microwave oven (at 90 W power) for 1, 2, and 4 min, or by drying in an ordinary oven kept at 50°C (at atmospheric pressure) for 1, 2, and 4 h. Fifty mL of water were added to each flask of the heated onions, and shaken in a stirrer at 350 rpm for 30 min. Finally, a clear onion extract was collected after filtration through a Whatman blue-band filter paper (i.e., the final extract contained the soluble ingredients from 5 g solid matter in 50 mL).
CERAC (Ce(IV) Reducing) Assay of Total Antioxidant Capacity
Preparation of solutions
A cerium(IV) sulfate solution containing 2.0 × 10−3 M Ce(IV) was prepared by dissolving 0.2 g Ce(SO4)2·4H2O in 50 mL of distilled water, adding 41 mL of concentrated H2SO4, and thoroughly mixing with the aid of a magnetic stirrer until total dissolution at room temperature. This solution was transfered to a 250-mL flask, and diluted to the mark with distilled water. The working solution at 1.0 × 10−3 M of quercetin was prepared in ethanol.
Procedure
One milliliter of 2.0 × 10−3 M Ce(IV) solution was added to 1 mL of 1.0 × 10−5 M quercetin solution, and the mixture was diluted to 10 mL with distilled water. After shaking for a few minutes, the solution was allowed to stand for 30 min at room temperature. The absorbance of the reaction mixture was measured at 320 nm against a blank composed of distilled water. The difference in absorption (ΔA) arising from Ce(IV) between the blank (containing the same amount of Ce(IV)) and phenolics-reacted solution was recorded, ΔA being proportional to phenolic antioxidant (e.g., quercetin) concentration. Since the difference in absorbance of reagent and sample solution (ΔA) is measured, a slight initial absorbance of the sample at 320 nm could be tolerated as long as the Ce(IV)-oxidized products did not strongly absorb at the same wavelength.Citation[27]
CUPRAC Assay of Total Antioxidant Capacity
Preparation of solutions
Copper(II) chloride stock solution (1.0 × 10−2 M) was prepared by dissolving 0.4262 g of cupric chloride dihydrate in distilled water, and diluting to a final volume of 250 mL. Ammonium acetate (NH4Ac) buffer at pH 7 was prepared by dissolving 19.27 g NH4Ac in water and diluting to 250 mL. Neocuproine solution (3.0 × 10−3 M) was prepared daily by dissolving 0.15 g neocuproine in 96% ethanol, and diluting to 250 mL with the same solvent.Citation[24, Citation25]
Procedure
To a test tube were added 1 mL CuCl2 solution (1.0 × 10−2 M), 1 mL neocuproine alcoholic solution (3.0 × 10−3 M), and 1 mL NH4Ac buffer solution, followed by mixing; (x) mL antioxidant followed by (1.1-x) mL water were then added (total volume, 4.1 mL) and mixed well. Absorbance against a reagent blank was measured at 450 nm after 30 min.Citation[24, Citation25]
Folin-Ciocalteau Method of Total Phenolics Assay
Preparation of solutions
Folin-Ciocalteau phenol reagent was diluted at a volume ratio of 1:3 with water prior to use. Lowry A solution was prepared from sodium carbonate such that the strength of Na2CO3 in 0.1 M NaOH solution was 2% (w/v). Lowry B solution was prepared from copper(II) sulfate such that the strength of CuSO4 in 1% sodium potassium tartrate (NaKC4H4O6) solution was 0.5% (w/v). Lowry C solution was prepared by freshly mixing 50 mL Lowry A with 1 mL Lowry B.Citation[20]
Procedure
To (x) mL phenolic sample solution was added (2.0-x) mL H2O. An aliquot of 2.5 mL Lowry C solution was added, and the mixture was let to stand for 10 min. At the end of this period, 0.25 mL Folin-Ciocalteau reagent was added, and 30 more min were allowed for stabilization of the blue colour formed. The absorbance against a reagent blank was read at 750 nm.Citation[20]
RESULTS
The Calibration Line for Quercetin in the CERAC Method
To five different quercetin (QR) solutions at concentrations in the range of 2.0 × 10−5 to 1.0 × 10−6 M, 1.0 mL of 2.0 × 10−3 M Ce(IV) solution was added, and the obtained absorbances—at 320 nm—after 30 min (A 320) were recorded against QR concentrations (CQR ). The regression equation was found as A 320 = −(1.10 × 105) CQR + 0.9998, with squared linear correlation coefficient r 2 = 0.9894. Other antioxidant assays were likewise applied using quercetin as the reference compound. The molar absorptivity values of quercetin (ϵ, calculated from the slope of the calibration line drawn as absorbance versus concentration, in the units of Lmol−1 cm−1) obtained in the CERAC, CUPRAC, and Folin-Ciocalteau methods were (1.10 ± 0.04) × 105, (7.40 ± 0.06) × 104, and (2.15 ± 0.05) × 104, respectively. Since the molar absorptivity of a spectrophotometric method is closely related to its sensitivity, CERAC was the most sensitive method toward quercetin.
Application of TAC Assays to Onion Sample Extracts
The TAC measurements were carried out in 50 mL of water (or ethanol) extracts of 5 g chopped onion samples (white-skinned [Albion], yellow-skinned [Rijnsburger], red-skinned [variety Red Baron], and spring onion. An example calculation of TAC according to the CERAC method is given by EquationEq. (1):
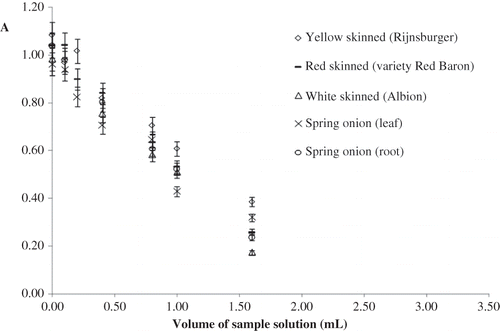
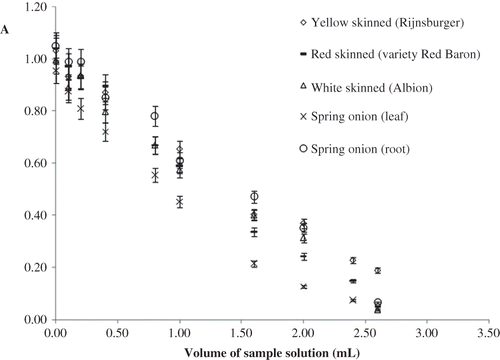
The change of absorbance at 320 nm—due to Ce(IV)—with the volume of aqueous and ethanolic onion extract as a result of the reaction of extracts (of onion varieties) with 2.0 × 10−4 M Ce(IV) solution in the CERAC method are shown in and , respectively. Slight changes may occur in the slopes of these linear curves with respect to onion variety and solvent, as electron transfer-based antioxidant assays are known to be solvent-dependent.Citation[39] The TAC values of white-skinned (Albion), yellow-skinned (Rijnsburger), red-skinned (variety Red Baron), and spring onion species, extracted with water or EtOH, and determined with CERAC, CUPRAC, and Folin-Ciocalteau methods are depicted in .
Table 1 The TAC of white-skinned (Albion), yellow-skinned (Rijnsburger), red-skinned (variety Red Baron), and spring onion species, extracted with water or EtOH, and determined with CERAC, CUPRAC, and Folin-Ciocalteau methods
Antioxidant Capacity as a Function of Different Heating Procedures
The traditional cuisine practices in Turkey generally involve consumption of cooked onions. Therefore, it is desirable to measure the possible change of TAC of onion varieties as a function of different heating procedures. The changes in TAC of onions were followed using CERAC, CUPRAC, and Folin-Ciocalteau assays. The residual weight (g) and the percentage of weight loss of microwave- and oven-heated onions are tabulated in . The corresponding TAC changes (in quercetin equivalents, based on fresh weight of onions) for microwave-heated () and oven-heated onions () measured with three different methods are also shown.
DISCUSSION
Most vegetables are commonly cooked before they are consumed, and cooking is known to induce significant changes in chemical composition influencing the concentration and bioavailability of bioactive compounds of vegetables. Both positive and negative effects have been reported depending on morphological and nutritional characteristics of vegetable species.Citation[30, Citation40−42 Citation Citation−42] In addition, different heating conditions (e.g., length of heating time and cooking temperatures) may have different effects on the antioxidant properties of vegetables.Citation40–42 Citation Citation−42] Cooked vegetables are traditionally associated with a reduced nutritional quality due to the loss of heat-sensitive compounds including antioxidants, and heating can result in oxidation, thermal degradation, leaching, and other events that lead to lower levels of antioxidants in processed foods compared with fresh foods.Citation[43] In this study, it is noteworthy that onion processing by heat treatment did not cause a drastic loss in antioxidant values.
In a highly cited review by Prior et al.,Citation[44] it has been proposed that “three methods, namely ORAC, Folin-Ciocalteau phenolics assay, and ABTS/TEAC, should be standardized for use in the routine quality control and measurement of antioxidant capacity of dietary supplements and other botanicals”; thus, the Folin-Ciocalteau assay, well responding to almost all water-soluble antioxidants in addition to phenolics, was used as a TAC assay in this work along with CERAC and CUPRAC. The order of TAC measured in aqueous extracts of onions using different methods were as follows: CERAC: yellow-skinned > red-skinned > spring-root > spring-leaf > white-skinned onion; CUPRAC: spring-leaf > red-skinned > spring-root > yellow-skinned > white-skinned onion; Folin-Ciocalteau method: spring-leaf > spring-root > red-skinned > yellow-skinned > white-skinned onion. Using all three methods, white onion showed the lowest TAC while with respect to two methods (i.e., CUPRAC and Folin-Ciocalteau), spring onion-leaf showed the highest value ().
The order of TAC measured in ethanolic extracts of onions were: CERAC: red-skinned > yellow-skinned > white-skinned > spring-root > spring-leaf; CUPRAC: spring-root > spring-leaf > red-skinned > yellow-skinned > white-skinned onion; Folin-Ciocalteau method: red-skinned ≥ spring-root > spring-leaf > yellow-skinned > white-skinned onion. Again, white onion exhibited the lowest TAC using two methods (CUPRAC and Folin-Ciocalteau). CUPRAC and Folin-Ciocalteau methods were in agreement in labeling the ethanolic extracts of red-skinned and spring onion-root highest whereas of white-skinned onion lowest (); these two methods were shown not to differ significantly in measurements of TAC order of many other plant extracts,Citation[45, Citation46] as both methods are highly responsive to phenolic antioxidants involving the oxidation of phenolic –OH to the corresponding quinone. Naturally, the degree of responsiveness of phenolic antioxidants to the applied assays were different in water and ethanol extracts, due to variations in the hydrophilicity of phenolics; it must be remembered that the TAC measurements with ET-based assays are highly dependent on the type of extracting solvent.Citation[39]
The percentages in weight loss of microwave-heated onions were (8.8 ± 3.2)%, (25.4 ± 4.4)%, and (50.3 ± 4.8)% as a result of 1, 2, and 4 min heating, respectively (), however the losses in antioxidant values as measured by the three ET-based methods (based on fresh-weight of onions) were not very intensive (). The percentage weight loss of oven-heated (at 50°C) onions were (26.9 ± 4.2)%, (45.8 ± 5.4)%, (72.1 ± 5.8)% as a result of 1, 2, and 4 h drying, respectively (); however, again the losses in antioxidant values as measured by the three assays (based on fresh-weight of onions) were not relatively high (). Of the heat-processed onions, the highest CERAC and Folin-Ciocalteau TAC values were obtained for red-skinned onions, while the highest CUPRAC value was for spring onion leaves. For statistical comparison, n = 15 pairs of TAC data ( and ) were subjected to linear regression analysis, and the calculated t values (using the formula: t = │r│{(n − 2)/(1 − r 2)}1/2) were compared to the tabulated t values at the desired confidence level using a two-tailed t-test. When the calculated value of t was greater than the tabulated value, the “null hypothesis” was rejected to conclude that a significant correlation did exist.Citation[47] Regarding the TAC changes of microwave-heated onion varieties with respect to heating time (), Folin-Ciocalteau and CERAC values correlated with each other at 95% confidence level, while Folin-Ciocalteau and CUPRAC values correlated at 90% confidence level. For the TAC changes of oven-heated onion varieties as a function of time (), CERAC and CUPRAC values correlated at 90% confidence level, while both CERAC/Folin-Ciocalteau and CUPRAC/Folin-Ciocalteau correlations were found to exist at 95% confidence level. Using the paired data tabulated in , the highest correlation coefficient was found between CERAC and Folin-Ciocalteau values (for n – 2 = 13 degrees of freedom, at 95% confidence level): TACFolin-Ciocalteau = 0.700 TACCERAC + 6.46 × 10−4 (correlation coefficient: r = 0.7546). Naturally, only correlation analysis could be conducted for the values found with different methods, since these values could not be expected to be identical considering the different reaction mechanisms and redox potentials of the concerned TAC assay reagents.Citation[39]
Table 2 The percentage of weight loss of heating process for microwave- and oven-heated onions
Table 3 The TAC changes of microwave-heated onions at 90 W power as a function of time (heating periods 1, 2, and 4 min)
Table 4 The TAC changes of oven-heated onions (at 50°C) as a function of time (heating periods 1, 2, and 4 h)
All three assays marked white-skinned onion as the lowest TAC content of heat-processed products. Both the magnitude of trolox-equivalent TAC values of onion extracts and of the correlation coefficients for CUPRAC/Folin-Ciocalteau data pairs were also in accordance with the findings of Kaur et al.Citation[48] Although both heating processes (i.e., microwave and oven heating) caused a decrease in TAC, this change was not drastic (considering the fact that the reproducibility of all the performed TAC assay procedures were about ≤5%). Moreover, spring onion leaves essentially maintained its TAC level after 4 min microwave—or 4 h drying oven—heating. It may be concluded that onion processing by heat treatment does not cause a drastic loss in antioxidant values, in accordance with the important findings of Gorinstein and coworkers.Citation[37, Citation38] Likewise, Yin and ChengCitation[49] had reported that heat treatments at 65 or 100°C and acid treatment at pH 2 reduced the antioxidant activity for most foods, though a part of the Allium family essentially retained antioxidant capability. It is possible that similar to the mechanism observed in the ageing of garlic extract, heat treatment may modify unstable molecules with antioxidant activity and simultaneously increase stable and bioavailable water-soluble organosulfur compoundsCitation[50] so as not to yield serious losses in TAC. These findings may support the traditional cooking practices of Turkish and other leading world cuisines in regard to mild heat treatment and drying of onions.
REFERENCES
- Halliwell , B. and Gutteridge , J.M.C. 1989 . Free Radicals in Biology and Medicine , Oxford , , United Kingdom : Oxford University .
- Halliwell , B. and Aruoma , O.I. 1991 . DNA damage by oxygen-derived species: Its mechanisms and measurement in mammalian systems . FEBS Letters , 281 : 9 – 19 .
- Rahman , M.S. 2007 . Allicin and other functional active components in garlic: Health benefits and bioavailability . International Journal of Food Properties , 10 : 245 – 268 .
- Končić , M.Z. and Juga , M. 2011 . Antioxidant and bioadhesive properties of onions (Allium L., Alliaceae) processed under acidic conditions . International Journal of Food Properties , 14 : 92 – 101 .
- Corzo-Martinez , M. , Corzo , N. and Villamiel , M. 2007 . Biological properties of onions and garlic . Trends in Food Science & Technology , 18 : 609 – 625 .
- Fu , H.Y. 2004 . Free radical scavenging and leukemia cell growth inhibitory properties of onion powders treated by different heating processes . Journal of Food Science , 69 : SNQ50 – 54 .
- Kumari , K. , Mathew , B.C. and Augusti , K.T. 1995 . Antidiabetic and hypolipidemic effects of S-methyl cysteine sulfoxide isolated from Allium cepa Linn . Indian Journal of Biochemistry & Biophysics , 32 : 49 – 51 .
- Sun-Waterhouse , D. , Smith , B.G. , O'Connor , C.J. and Melton , L.D. 2008 . Effect of raw and cooked onion dietary fibre on the antioxidant activity of ascorbic acid and quercetin . Food Chemistry , 111 : 580 – 585 .
- Stajner , D. , Milic , N. , Canadanovic-Brunet , J. , Kapor , A. , Stajner , M. and Popovic , B.M. 2006 . Exploring Allium species as a source of potential medicinal agents . Phytotherapy Research , 20 : 581 – 584 .
- Vinson , J.A. , Hap , Y. , Su , X. and Zubik , L. 1998 . Phenolic antioxidant quantity and quality in foods: vegetables . Journal of Agricultural and Food Chemistry , 46 : 3630 – 3634 .
- Sellappan , S. and Akoh , C.C. 2002 . Flavonoids and antioxidant capacity of Georgia-grown Vidalia onions . Journal of Agricultural and Food Chemistry , 50 : 5338 – 5342 .
- Gulsen , A. , Makris , D.P. and Kefalas , P. 2007 . Biomimetic oxidation of quercetin: Isolation of a naturally occurring quercetin heterodimer and evaluation of its in vitro antioxidant properties . Food Research International , 40 : 7 – 14 .
- Cao , G. and Prior , R.L. 1998 . Comparison of different analytical methods for assesing total antioxidant capacityof human serum . Clinical Chemistry , 44 : 1309 – 1315 .
- Rice-Evans , C.A. 2000 . Measurement of total antioxidant activity as a marker of antioxidant status in vivo: Procedures and limitations . Free Radical Research , 33 : 59 – 66 .
- Huang , D. , Ou , B. and Prior , R.L. 2005 . The chemistry behind dietary antioxidant capacity assays . Journal of Agricultural and Food Chemistry , 53 : 1841 – 1856 .
- Miller , N.J. , Rice-evans , C.A. , Davies , M.J. , Copinathan , V. and Milner , A. 1993 . A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates . Clinical Science , 84 : 407 – 412 .
- Re , R. , Pellegrini , N. , Oroteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolarization assay . Free Radical Biology &Medicine , 26 : 1231 – 1237 .
- Jiménez-Escrig , A. , Jiménez-Jiménez , I. , Sánchez-Moreno , C. and Saura-Calixto , F. 2000 . Evaluation of free-radical scavenging of dietary carotenoids by the stable radical 2,2-diphenyl-1-picrylhydrazyl . Journal of the Science of Food and Agricultural , 80 : 1686 – 1690 .
- Folin , O. and Ciolcalteu , V. 1927 . On tyrosine and tryptophone determinations in proteins . Journal of Biological Chemistry , 73 : 627 – 650 .
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent . Methods in Enzymology , 299 : 6287 – 6293 .
- Benzie , I.F.F. and Strain , J.J. 1996 . The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Benzie , I.F.F. and Szeto , Y.T. 1999 . Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay . Journal of Agricultural and Food Chemistry , 47 : 633 – 636 .
- Pulido , R. , Bravo , L. and Saura-Calixto , F. 2000 . Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay . Journal of Agricultural and Food Chemistry , 48 : 3396 – 3402 .
- Apak , R. , Güçlü , K. , Özyürek , M. and Karademir , S.E. 2004 . Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method . Journal of Agricultural and Food Chemistry , 52 : 7970 – 7981 .
- Apak , R. , Güçlü , K. , Özyürek , M. , Karademir , S.E. and Altun , M. 2005 . Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method . Free Radical Research , 39 : 949 – 961 .
- Özyürek , M. , Çelik , S.E. , Berker , K.I. , Güçlü , K. , Tor , I. and Apak , R. 2007 . Sensitivity enhancement of CUPRAC and iron(III)-phenanthroline antioxidant assays by preconcentration of colored reaction products on a weakly acidic cation exchanger . Reactive and Functional Polymers , 67 : 1478 – 1486 .
- Ozyurt , D. , Demirata , B. and Apak , R. 2007 . Determination of total antioxidant capacity by a new spectrophotometric method based on Ce(IV) reducing capacity measurement . Talanta , 71 : 1155 – 1165 .
- Tepe , B. , Sökmen , M. , Akpulat , H.A. and Sökmen , A. 2005 . In vitro antioxidant activities of the methanol extracts of five allium species from Turkey . Food Chemistry , 92 : 89 – 92 .
- Lombard , K. , Peffley , E. , Geoffriau , E. , Thompson , L. and Herring , A. 2005 . Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation . Journal of Food Composition and Analysis , 18 : 571 – 581 .
- Turkmen , N. , Sari , F. and Velioglu , Y.S. 2005 . The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables . Food Chemistry , 93 : 713 – 718 .
- Nencini , C. , Cavallo , F. , Capasso , A. , Franchi , G.G. , Giorgio , G. and Lucia Micheli , L. 2007 . Evaluation of antioxidative properties of Allium species growing wild in Italy . Phytotherapy Research , 21 : 874 – 878 .
- Prakash , D. , Singh , B.N. and Upadhyay , G. 2007 . Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa) . Food Chemistry , 102 : 1389 – 1393 .
- Çelik , S.E. , Özyürek , M. , Altun , M. , Bektaşoğlu , B. , Güçlü , K. , Berker , K.I. , Özgökçe , F. and Apak , R. 2008 . Antioxidant capacities of herbal plants used in the manufacture of Van Herby cheese: ‘Otlu Peynir’ . International Journal of Food Properties , 11 : 747 – 761 .
- Faller , A.L.K. and Fialho , E. 2008 . The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking . Food Research International , 42 : 210 – 215 .
- Kuljarachanan , T. , Devahastin , S. and Chiewchan , N. 2009 . Evolution of antioxidant compounds in lime residues during drying . Food Chemistry , 113 : 944 – 949 .
- Rumbaoa , R.G.O. , Cornago , D.F. and Geronimo , I.M. 2009 . Phenolic content and antioxidant capacity of Philippine sweet potato (Ipomoea batatas) varieties . Food Chemistry , 113 : 1133 – 1138 .
- Gorinstein , S. , Leontowicz , H. , Leontowicz , M. , Namiesnik , J. , Najman , K. , Drzewiecki , J. , Cvikrova , M. , Martincova , O. , Katrich , E. and Trakhtenberg , S. 2008 . Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols . Journal of Agricultural and Food Chemistry , 56 : 4418 – 4426 .
- Gorinstein , S. , Jastrzebski , Z. , Leontowicz , H. , Leontowicz , M. , Namiesnik , J. , Najman , K. , Park , Y.S. , Heo , B.G. , Cho , J.Y. and Baeh , J.H. 2009 . Comparative control of the bioactivity of some frequently consumed vegetables subjected to different processing conditions . Food Control , 20 : 407 – 413 .
- Apak , R. , Güçlü , K. , Demirata , B. , Özyürek , M. , Çelik , S.E. , Bektasoglu , B. , Berker , K.I. and Ozyurt , D. 2007 . Comparative evaluation of total antioxidant capacity assays applied to phenolic compounds, and the CUPRAC assay . Molecules , 12 : 1496 – 1547 .
- Makris , D.P. and Rossiter , J.T. 2001 . Domestic processing of onion bulbs (Allium cepa) and asparagus spears (Asparagus officinalis): Effect on flavonol content and antioxidant status . Journal of Agricultural and Food Chemistry , 49 : 3216 – 3222 .
- Dewanto , V. , Wu , X. , Adom , K.K. and Liu , R.H. 2002 . Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity . Journal of Agricultural and Food Chemistry , 50 : 3010 – 3014 .
- Zhang , D. and Hamauzu , Y. 2004 . Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking . Food Chemistry , 88 : 503 – 509 .
- Kalt , W. 2005 . Effects of production and processing factors on major fruit and vegetable antioxidants . Journal of Food Science , 70 : R11 – R19 .
- Prior , R.L. , Wu , X and Schaich , K. 2007 . Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements . Journal of Agricultural and Food Chemistry , 53 : 4290 – 4302 .
- Apak , R. , Güçlü , K. , Özyürek , M. , Karademir , S.E. and Erçağ , E. 2006 . The cupric ion reducing antioxidant capacity (CUPRAC) and polyphenolic content of some herbal teas . International Journal of Food Sciences and Nutrition , 57 : 292 – 304 .
- Park , Y.S. , Jung , S.T. , Kang , S.G. , Heo , B.G. , Arancibia-Avila , P. , Toledo , F. , Drzewiecki , J. , Namiesnik , J. and Gorinstein , S. 2008 . Antioxidants and proteins in ethylene-treated kiwifruits . Food Chemistry , 107 : 640 – 648 .
- Miller , J.C. and Miller , J.N. 1993 . Statistics for Analytical Chemistry , 3rd , New York and London : Ellis Horwood PTR Prentice Hall .
- Kaur , C. , Singh , M. , Walia , S. , Joshi , S. and Munshi , A.D. 2010 . Variations in phenolics and antioxidants in Indian onions (Allium cepa L.) . Nutrition and Food Science , 40 : 6 – 19 .
- Yin , M.-C. and Cheng , W.-S. 1998 . Antioxidant activity of several Allium members . Journal of Agricultural and Food Chemistry , 46 : 4097 – 4101 .
- Borek , C. 2001 . Antioxidant health effects of aged garlic extract . Journal of Nutrition , 131 : 1010S – 1015S .