Abstract
This study was undertaken to determine the total phenols, total flavonoids, and major phenolic compounds in the polar (methanol, 80% methanol, and aqueous) extracts of propolis collected from the Greek mainland (West Macedonia) and the Greek island, Rhodes. The antioxidant properties of the propolis extracts were also evaluated by using free 2,2′-diphenyl-1-picrylhydrazyl radical scavenging assay and ferric reducing antioxidant power assay. The results showed that propolis from West Macedonia was found to be the strongest radical scavenger and ferric reducing agent (mean IC50 0.179 and 0.009 mg/ml, respectively). Methanol (mean IC50 0.181 mg/ml) and 80% methanol extracts (mean IC50 0.138 mg/ml) of propolis from West Macedonia showed higher radical scavenging activity than the synthetic antioxidant butylated hydroxytoluene (mean IC50 0.207 mg/ml), while exhibiting similar levels of reducing activity (IC50 0.0099 mg/ml and 0.0085 mg/ml, respectively) with flavonol quercetin (mean IC50 0.0101 mg/ml). In addition, analysis by high performance liquid chromatography showed that West Macedonia propolis, contained the highest amount of phenolic compounds: phenolic acids (caffeic acid, caffeic acid phenethylester, ferulic acid, p-coumaric acid) and flavonoids (quercetin, galangin, luteolin, apigenin). Caffeic acid (0.639–4.172 mg/g propolis) and galangin (1.317–8.551 mg/g propolis) were found to be the predominant phenolic compounds in these propolis extracts.
INTRODUCTION
Propolis (bee glue) is the generic name for an adhesive, resinous natural product collected by honeybees (Apis mellifera L.) from various plant sources. Bees transform and use it to seal the holes, to smooth out the internal walls of the hive, and to protect the entrance against intruders.[Citation1] It has been used in folk medicine since ancient times in many countries and recently it was reported to possess various biological activities, such as antibacterial, antifungal, antiviral, anti-inflammatory, anticancer, antiangiogenesis, and antioxidant activities.[Citation2 − Citation5]
Propolis contains a variety of chemical compounds, such as flavonoid aglycones, phenolic acids and their esters, phenolic aldehydes, alcohols and ketones, steroids, coumarins, amino acids, and inorganic compounds.[Citation6] The chemical composition of propolis is affected by botanical and geographical factors; therefore, the variations in propolis bioactivity are expected to be associated with the chemical diversity of its constituents.[Citation5,Citation7]
Polyphenols, such as phenolic acid derivatives and flavonoids, appear to be the main propolis components responsible for its biological and pharmacological properties.[Citation5,Citation8 − Citation10] They are beneficial to human health due to various biological effects, such as free radical scavenging, metal chelation, and modulation of enzymatic activity.[Citation11 − Citation13] Hydroxycinnamic acids, such as caffeic acid and its derivatives, ferulic acid, and caffeic acid phenethylester, exhibit antioxidant, antimutagenic, anti-inflammatory, and antimicrobial activities.[Citation14 − Citation17] Hydroxybenzoic acids, such as protocatechuic acid and gallic acid, were strong antioxidants in emulsion and lipid systems.[Citation18]
Many flavonoids have been found to inhibit the lipid peroxidation and the oxidation of low-density lipoproteins.[Citation13,Citation19] Within the flavonoid family, quercetin is the most potent radical scavenger.[Citation20,Citation21] It has been reported that it also exhibits anti-inflammatory, anticarcinogenic, and antiaggregatory effects.[Citation22 − Citation24] Galangin, in medicinal plants, honey, and propolis exist in high concentrations and exhibit various pharmacological activities.[Citation25 − Citation27] The antioxidant activity of luteolin has been associated with its capacity to scavenge reactive oxygen and nitrogen species, to chelate transition metals, to inhibit pro-oxidant enzymes, and to induce antioxidant enzymes.[Citation28 − Citation30] Apigenin is a natural flavonoid that has been shown to possess anti-inflammatory and anticarcinogenic effects for skin and free radical scavenging properties in vitro systems.[Citation31]
The antioxidant and antimicrobial as well as other biological properties of propolis have been studied so far by using ethanol and, less frequently, methanol to obtain propolis extracts and their active constituents.[Citation10,Citation32 − Citation36] Studies concerning biological properties of water extracts of propolis are recently increasing.[Citation37 − Citation39] However, there are only a few studies of reactive oxygen species and phenolic profiles in relation to water extracts of propolis.[Citation40 − Citation42] The Greek flora showed high biodiversity with many endemic plants at various Greek islands as well as in mainland. To date, limited information is available on the bioactive properties and chemical composition of Greek propolis extracts.[Citation8,Citation43,Citation44]
The aims of this study were: (a) to determine the total phenols (TP), total flavonoids (TF), and the major phenolic components (phenolic acids and flavonoids) in polar (100% methanol, 80% methanol and aqueous) extracts of propolis samples collected from two different geographical areas, the Greek mainland (West Macedonia) and the Greek island (Rhodes), by using spectrophotometric and HPLC-ultraviolet/visible (UV) methods; and (b) to evaluate the antioxidant properties of the propolis extracts and standard phenolic compounds by using free radical scavenging (DPPH) and ferric reducing antioxidant power (FRAP) assays.
MATERIALS AND METHODS
Materials
Raw native propolis samples were obtained from the West North of Macedonia (Kastoria) and Dodecanese islands (Rhodes). They were stored in closed vessels in the dark at 4°C. The reagents Folin-Ciocalteau, ferric chloride, DPPH · , butylated hydroxytoluene (BHT), (purum >95% HPLC), ferulic acid, protocatechuic acid, quercetin, caffeic acid, gallic acid (purum ACS 98%), aluminum chloride, and sodium acetate were obtained from Sigma-Aldrich (Steinheim, Germany). Ascorbic acid (99.7%) was obtained from Riedel-de Häen (Seelze, Germany). TPTZ (2,4,6-Tris-(2-pyridyl)-S-triazine) was from Alfa Aesar (Karlsruhe, Germany). Anhydrous sodium carbonate was from Applichem (Darmstadt, Germany). Methanol, acetic acid, phosphoric acid, n-hexane (analytical grade) and acetonitrile (HPLC grade) were purchased from Merck (Darmstadt, Germany).
Preparation of Propolis Extracts
Crude propolis samples were frozen (-20°C), ground in a chilled grinder, and small amounts (10 g) were extracted under stirring with a 10-fold volume of the solvent (100 mL) for 24 h at room temperature. Hexane was first used to remove the waxes and other nonpolar substances from the crude material. The extraction solvents were methanol, methanol 80% (v/v), and water. Each extraction procedure was repeated two times. Then the mixtures were filtered, the solvents were removed using a rotary evaporator (40°C), and then freeze-dried. The propolis extracts obtained were kept in the dark at 4°C until use.
Determination of Antioxidant Activity
2,2’-Diphenyl-1-picrylhydrazyl radical scavenging assay (DPPH. assay)
The antioxidant activity of the propolis extracts, BHT, gallic, caffeic, ferulic, protocatechuic acids, and quercetin was measured in terms of hydrogen donating or radical scavenging activity using DPPH · .[Citation45] DPPH · is a stable free radical, violet in solution, and upon reduction by hydrogen or electron donation becomes in diphenylpicrylhydrazine, a stable diamagnetic molecule, yellow colored. Aliquots of 0.1 mL of various concentrations of the extracts (0.05–5 g/L), BHT (0.06–2 g/L), gallic acid (0.008–0.08 g/L), ferulic acid (0.04–0.9 g/L), protocatechuic acid (0.008–0.5 g/L), caffeic acid (0.008–0.2 g/L), and quercetin (0.015–0.1 g/L) were added to 3 mL of methanolic solution of DPPH · (6 × 10−5 M). After a 45-min incubation period at room temperature, the absorbance (abs) was read against a control at 517 nm. The percentage of the DPPH · radical scavenging activity caused by the samples was calculated as follows:
The extract/compound effective concentration providing 50% RSA (IC50) was calculated from the plot of % RSA against extract/compound concentration (mg/ml). All determinations were performed in triplicate.
Ferric reducing antioxidant power assay (FRAP)
This assay measures the change in absorbance at 593 nm owing to the formation of a blue-colored Fe2+-tripyridyltriazine complex compound from a colorless Fe3+-tripyridyltriazine by the action of electron donating antioxidants.[Citation46] First, 0.2 ml of various concentrations of the propolis extracts (0.002–0.9 g/L), ascorbic acid (0.001–0.1 g/L), gallic acid (0.0008–0.08 g/L), ferulic acid (0.01–0.5 g/L), protocatechuic acid (0.009–0.5 g/L), caffeic acid (0.006–0.08 g/L), and quercetin (0.0006–0.1 g/L) was added to 3 mL of FRAP reagent (10 parts of 300 mM sodium acetate buffer at pH 3.6, 1 part of 10 mM TPTZ (2,4,6-tripyridyl-S-triazine) solution and 1 part of 20 mM ferric chloride) and the reaction mixture was incubated in a water bath at 37°C. The increase in absorbance at 593 nm was measured after 30 min. The percentage of the ferric reducing/antioxidant power caused by the samples was calculated in the following way:
WhereA 0 is the absorbance of the control reaction (containing all reagents except the extract/compound) at 0 min and A 1 is the absorbance of the extract/compound at 30 min. The extract/compound effective concentration providing 50% FRAP activity (IC50) was calculated using the plot of % FRAP against extract/compound concentration (mg/ml). All determinations were performed in triplicate.
Determination of Total Phenols (TP) by Folin-Ciocalteau Method
The concentration of total phenols in extracts was measured at 760 nm, based on a colorimetric oxidation/reduction reaction. The oxidizing agent was Folin-Ciocalteau reagent.[Citation47] The sample (5 mL) was transferred to a 50-mL volumetric flask containing 6 mL of H2O to which was subsequently added 2.5 mL of undiluted Folin-Ciocalteau reagent. After 3 min, 5 mL of 20% aqueous Na2CO3 were added and the volume was made up to 50 mL with H2O. The controls contained all the reagents except the extract. After 1 h, the absorbance was measured at 760 nm. A standard graph was obtained by repeating the same procedure for all gallic acid (GA) solutions (20–150 ppm). The results were expressed in mg GA per g of propolis and were presented as means of triplicates.
Determination of Total Flavonoids (TF) by Aluminum Chloride Method
The concentration of total flavonoids in the propolis extracts was measured spectrophotometrically at 440 nm.[Citation48] Two ml of the sample (10 g/L) was transferred to a 10-mL volumetric flask containing 2 mL of AlCl3 (20 g/L in ethanol) and 6 mL of CH3COONa (50 g/L in ethanol). The controls contained all the reagents except for the extract. After 2.5 h at 20°C the absorbance was measured at 440 nm. The same procedure was repeated for the standard quercetin (QE) solutions (25–250 ppm) and the results were expressed in mg QE per g of propolis and were presented as means of triplicates.
Phenolic Compounds Using HPLC
A high-performance liquid chromatograph consisting of a Thermo Finnigan SCM 1000 solvent degasser (Thermo Fisher Scientific Inc., MA, USA), a Thermo Finnigann Spectra System P2000 pump model (Thermo Fisher Scientific Inc.), a column oven, and a Fasma 525 UV-Vis detector (Rigas Labs, Thessaloniki, Greece) was used. The column, a Kromasil 100 C18 (250 × 4.6 mm) (Mainz, Germany) was maintained at 28°C. For the determination of phenolic acids in methanol and methanol 80% (v/v) propolis extracts, an isocratic elution was used: acetonitrile as eluent A and acetic acid 3.5% in water as eluent B (23/77, v/v). The flow rate was 1.0 mL/ min. Detection wavelength was 280 nm. The sample injection volume was 10 μL. Working standard solutions of caffeic acid (10–500 ppm), ferulic acid (10–100 ppm), and p-coumaric acid (20–100 ppm) were injected into the HPLC and peak area responses obtained. The data were stored and processed with ChromQuest chromatographic software (Scientific Software Inc., San Jose, CA, USA). Quantification was carried out from integrated peak areas of the West Macedonia and Rhodes propolis extracts (at 2.5 and 5 mg/mL, respectively). The results were expressed as mg of phenolic acid per g of propolis and were presented as means of triplicates.
For the determination of flavonoids and caffeic acid phenethylester in methanol and methanol 80% (v/v) propolis extracts isocratic elution was also used: methanol as eluent A and phosphoric acid 0.4% in water as eluent B (40/60, v/v). The flow rate was 0.8 mL/ min. Detection wavelength was 365 nm. The sample injection volume was 10 μL. Quercetin (2.5–100 ppm) was used as external standard. Quantification was carried out from integrated peak areas of the West Macedonia and Rhodes propolis extracts (at 2.5 and 5 mg/mL, respectively). The results were expressed as mg of quercetin per g of propolis and were presented as means of triplicates.
Statistical Analysis
Significant effects were evaluated by using the analysis of variance (one- and two-way ANOVA) (Minitab Inc., State College, PA, USA). The probability value of P < 0.05 was used as the criterion for significant differences.
RESULTS AND DISCUSSION
The DPPH scavenging activities (expressed as IC50 values) of methanol, 80% methanol, aqueous propolis extracts, and BHT are shown in Fig. 1a. The results from two-way analysis of variance showed that the methanol and 80% methanol extracts from both geographic regions showed higher radical scavenging activity than the aqueous extracts. Propolis from West Macedonia was found to be the strongest radical scavenger (IC50: 0.179 mg/ml). All of its extracts had superior or equal activity to BHT (IC50: 0.207 mg/mL). In particular the 80% methanol extract from West Macedonia showed the highest activity (IC50: 0.138 mg/mL), while the aqueous extract from Rhodes the lowest (IC50: 1.557 mg/mL). In similar studies, the DPPH · activity of methanol, ethanol, and aqueous propolis extracts from different areas of Argentina, Turkey, Portugal, and India presented IC50 values between 0.006 and 0.062 mg/mL.[Citation7,Citation9,Citation41,Citation42,Citation49]
Figure 1 (a) DPPH assay. (b) FRAP assay: MEOH MAC, MEOH RHOD: methanol extracts of West Macedonia and Rhodes; 80MEOH MAC, 80MEOH RHOD: 80% methanol extracts of West Macedonia and Rhodes; H2O MAC, H2O RHOD: aqueous extracts of West Macedonia and Rhodes propolis, respectively; BHT: butyl-hydroxy-toluene. (c) DPPH assay. (d) FRAP assay: GA: gallic acid; CA: caffeic acid; PRA: protocatechuic acid; FRA: ferulic acid; QE: quercetin; BHT: butyl-hudroxy-toluene; AO: ascorbic acid. (e) % DPPH. (f) % FRAP: Galangin, apigenin, luteolin, and CAPE: caffeic acid phenethyl ester.
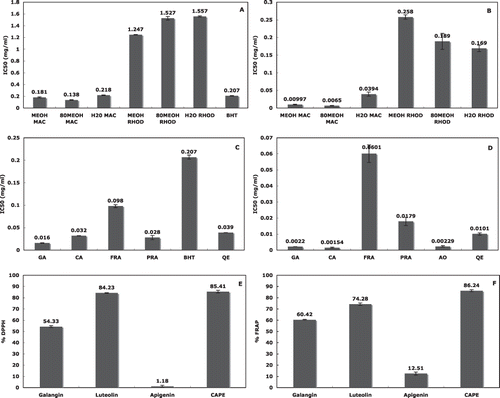
Fe(III)-reduction is often used as an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant action. The FRAP activities expressed as IC50 values of methanol, 80% methanol, and aqueous propolis extracts from West Macedonia and Rhodes, are shown in Fig. 1b. The results showed that the 80% methanol extracts presented higher reducing activity than methanol and aqueous extracts. In particular, the 80% methanol extract from West Macedonia had the highest activity (IC50: 0.0065 mg/ml) while the aqueous extract from Rhodes the lowest (IC50: 0.1690 mg/ml). The ferric reducing activities of the methanol extracts from two different regions of Portugal were similar to our results.[Citation49]
The results from the two-way analysis of variance (solvent, geographic origin) (P < 0.05), showed that the radical scavenging and ferric reducing properties of propolis were affected more by the geographical origin than the solvents used for the extraction. Similar studies have also indicated the significant contribution of the geographical origin to the antioxidant activity of propolis.[Citation3,Citation8] The DPPH · and FRAP activities of ethanol propolis extracts from the Greek mainland were found to be higher than that of Greek islands.[Citation8] In another study, Brazilian and Chinese aqueous propolis extracts exhibited stronger DPPH · activity than the corresponding methanol extracts, whereas in the case of Dutch and Peruvian propolis, the methanol extracts exhibited stronger DPPH · activity than the aqueous extracts.[Citation3]
The DPPH · and FRAP activities expressed as IC50 values of gallic acid, caffeic acid, protocatechuic acid, and ferulic acid, as well as of quercetin, are shown in Figs. 1c and 1d. BHT and ascorbic acid were also tested for comparison. According to the results of our previous study,[Citation50] all of the examined phenolic compounds were remarkable DPPH scavengers (IC50: 0.016–0.098 mg/ml). They were stronger than the synthetic antioxidant BHT (IC50: 0.207 mg/ml). Gallic acid exhibited the highest radical scavenging activity, followed by protocatechuic acid, caffeic acid, quercetin, and ferulic acid. Gallic acid and caffeic acid were found to be equal or even better reducing agents than the well known, as strong reducing agent, ascorbic acid.
In accordance to these results, phenolic compounds with o-diphenol structure, such as protocatechuic and caffeic acid derivatives, were found to exhibit high antiradical activity.[Citation51] Ferulic acid, the methoxy derivative of caffeic acid, was found to protect DNA and lipids against oxidation caused by reactive oxygen species.[Citation16,Citation17,Citation52] Gallic acid, a trihydroxy-benzoic acid, was also a strong antioxidant in emulsion or lipid systems.[Citation18] It is almost as effective as the tocopherol analogue Trolox and even more effective than several water soluble antioxidants, such as ascorbic acid.[Citation27,Citation53]
Quercetin is the most potent radical scavenger in the flavonoid family.[Citation20,Citation21] The antioxidative capacities of quercetin are attributed to the presence of two antioxidant pharmacophores within the molecule that have the optimal configuration for free radical scavenging activity, i.e., the catechol group in the B ring and the hydroxyl group at position 3 of the AC ring.[Citation54] Moreover, quercetin is suggested to substantially empower the endogenous antioxidant shield due to its contribution to the total plasma antioxidant capacity, which is 6.24 times higher than the reference antioxidant, Trolox.[Citation55]
The results of Figs. 1b and 1d also showed that the methanol (IC50: 0.0099 mg/ml) and 80% methanol propolis extracts (IC50: 0.0065 mg/ml) from West Macedonia exhibited higher reducing activity than protocatechuic acid (IC50: 0.0179 mg/ml) or quercetin (IC50: 0.0101 mg/ml), well known for their strong biological properties. The aqueous extract (IC50: 0.0394 mg/ml) had a higher reducing activity than ferulic acid (IC50: 0.0601 mg/ml). In a similar study, the ethanol and aqueous propolis extracts from India were found to contain antioxidant agents as strong as gallic acid and ascorbic acid.[Citation42]
The results of % DPPH · scavenging and % FRAP activity of apigenin, luteolin, galangin, and caffeic acid phenethyl ester at 500 ppm are shown in Figs. 1e and 1f. Caffeic acid phenethyl ester (85.41, 86.24%) and flavone luteolin (84.23, 74.28%) with o-diphenol groups in their structures showed higher DPPH · and FRAP activities than flavonol galangin (54.33, 60.42%), while flavone apigenin exhibited the lowest activity (1.18, 12.51%).
The antioxidant activity of luteolin has been associated with its capacity to scavenge reactive oxygen and nitrogen species, to chelate transition metals that may induce oxidative damage through the Fenton reaction, to inhibit pro-oxidant enzymes, and to induce antioxidant enzymes.[Citation28 − Citation30] Caffeic acid phenethyl ester (CAPE) contains a well described antioxidant structural moiety, the catechol ring, that largely accounts for its free radical scavenging properties.[Citation15,Citation56,Citation57]
Folin-Ciocalteau and aluminum chloride colorimetric methods have been applied to determine total phenol and flavonoid contents in methanol, 80% methanol, and aqueous extracts of propolis samples and the results are shown in . Propolis from West Macedonia had the highest concentration of total phenols and total flavonoids (105.36 and 49.27 mg/g). In particular, the 80% methanol extract from West Macedonia showed the highest amount (179.99 and 88.26 mg/g), while the aqueous extract from Rhodes showed the lowest (2.33 and 0.25 mg/g). By using colorimetric analysis, the methanol, ethanol, and aqueous propolis extracts from various geographical origins (Argentina, Australia, China, Hungary, New Zealand, and India) were found to possess similar levels of total phenols (31.2–299 mg/g) and total flavonoids (2.5–176 mg/g).[Citation5,Citation9,Citation42] Furthermore, the methanol and 80% methanol propolis extracts from West Macedonia and Rhodes, which showed high antioxidant activities, were examined for the presence of free phenolic acids and flavonoids by using simple and rapid isocratic reversed phase (RP)-HPLC methods. The chromatographic conditions were first determined by using mixtures of phenolic acids: gallic acid, protocatechuic acid, caffeic acid, caffeic acid phenethyl ester, ferulic acid, and p-coumaric acid and flavonoid aglycones: quercetin, apigenin, luteolin, and galangin as standards. The identification of the chromatographic peaks in the extracts was confirmed by comparing the retention times and spiking with the corresponding phenolic standards.
Table 1 Total phenol (mg GA/g propolis) and total flavonoid (mg QE/g propolis) contenta
Linear regression analysis was also used to evaluate the calibration curve of each phenolic standard as a function of its concentration. All of the standards showed good linearity (0.9902–0.9998) in a relatively wide concentration range. Repeatability expressed as coefficient of variation (CV%) of multiple independent determinations ranged from 0.42 to 5.57%. The accuracy of the method was evaluated with a recovery test. Samples of extracts were prepared to contain an analyzed compound about 0.5–100 μg/ml. An equal volume of standard solution with the same amount of tested compound was then added. Recoveries ranged from 97.56 to 103.68%.
The identified and quantified phenolic acids were p-coumaric acid, caffeic acid, caffeic acid phenethylester, and ferulic acid (). Caffeic acid was the most abundant identified phenolic acid (0.639–4.172 mg/g) in the propolis extracts. The 80% methanol extract of West Macedonia showed the highest concentration of phenolic acids (10.622 mg/g), while the 80% methanol extract of Rhodes showed the lowest (2.363 mg/g). Flavonols (quercetin and galangin) and flavones (luteolin and apigenin) were identified and quantified in the methanol and 80% methanol propolis extracts (). Galangin was found at the highest concentration (1.317–8.551 mg/g) in the propolis extracts. The 80% methanol extract of West Macedonia showed the highest amount of flavonoids (14.497 mg/g) while the 80% methanol extract of Rhodes showed the lowest (2.507 mg/g).
Table 2 HPLC phenolic compounds (mg/g propolis)a
Our results agree with the findings of other studies on other geographical regions in relation to phenolic acids and flavonoids such as caffeic acid (3.3–32.2 mg/g), p-coumaric acid (0.92–52.2 mg/g), ferulic acid (0.51–6.42 mg/g), quercetin (nd–4.8 mg/g), apigenin (1.04–18.4 mg/g), galangin (nd–58.2 mg/g), and CAPE (8.7–29.2 mg/g).[Citation5,Citation10,Citation58,Citation59] According to CitationBankova et al. (2000), propolis from temperate climatic zones, like Europe, North America, and the non-tropical regions of Asia, originate mainly from the bud exudates of Populus species that are rich in flavonoids, phenolic acids, and their esters, such as caffeic acid (3.3 mg/g), p-coumaric acid (27.4 mg/g), 3,4-dimethoxycinnamic acid (8.6 mg/g), pinobanskin (84.8 mg/g), pinocembrin (99.7 mg/g), galangin (58.2 mg/g), caffeic acid phenethylester (29.2 mg/g), cinnamyl caffeate (20.3 mg/g), artepillin C (43.9 mg/g), apigenin (18.4 mg/g), kaempferol (10.9 mg/g), and quercetin (4.8 mg/g).[Citation6]
In the present study, no significant correlation was observed between antioxidant activity of propolis extracts and total phenols or total flavonoids. It was also observed that antioxidant activity was not significantly correlated to the phenolic compounds identified by HPLC. However, the high activity of methanol and 80% methanol propolis extracts was probably due to the higher polyphenol content and also due to the better solubility of its polyphenol constituents in the methanol and methanol/water mixtures. There were also no significant correlations between antioxidant activity and phenolic content in the propolis extracts from other geographic regions (Argentina, Portugal, and Iran).[Citation9,Citation42,Citation49,Citation60]
CONCLUSIONS
The results obtained showed that the methanol and 80% methanol propolis extracts from both geographical regions (West Macedonia, Rhodes) showed higher radical scavenging and reducing activities than the aqueous extracts. The geographical areas affected the antioxidant activity as well as the amount of the phenolic compounds more than the solvents of extraction. Propolis from West Macedonia showed higher antioxidant activities than propolis from Rhodes. Its methanol and 80% methanol extracts showed higher radical scavenging activity than the synthetic antioxidant BHT, while exhibiting the same levels of reducing activity with that of quercetin. In addition, West Macedonia propolis presented the highest amount of total phenolic compounds, especially phenolic acids and flavonoids. The presence and the levels of phenolic antioxidants in the extracts of propolis could be used as the main indicators of quality in order to standardize the raw materials and preparations of propolis. Further studies should be carried out to identify other phenolic components present in propolis and to investigate the contribution of minor phenolic compounds to the total antioxidant activity of the propolis extracts.
REFERENCES
- Burdock , G.A. 1998 . Review of the biological properties and toxicity of bee propolis . Food and Chemical Toxicology , 36 : 347 – 363 .
- Kujumgiev , A. , Tsvetkova , T. , Serkedjieva , Y. , Bankova , V. , Christov , R. and Antibacterial , Popov, S. 1999 . antifungal and antiviral activity of propolis of different geographic origin . Journal of Ethnopharmacology , 64 : 235 – 240 .
- Banskota , A.H. , Tezuka , Y. and Kadota , S. 2001 . Recent progress in pharmacological research of propolis . Phytotherapy Research , 15 : 561 – 571 .
- Isla , M.I. , Nieva Moreno , M.I. , Sampietro , A.R. and Vattuone , M.A. 2001 . Antioxidant activity of Argentine propolis extracts . Journal of Ethnopharmacology , 76 : 165 – 170 .
- Ahn , M.R. , Kunimasa , K. , Ohta , T. , Kumazawa , S. , Kamihira , M. , Kaji , K. , Uto , Y. , Hori , H. , Nagasawa , H. and Nakayama , T. 2007 . Suppression of tumor-induced angiogenesis by Brazilian propolis: Major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation . Cancer Letters , 252 : 235 – 243 .
- Bankova , V.S. , De Castro , S.L. and Marcucci , M.C. 2000 . Propolis: Recent advances in chemistry and plant origin . Apidologie , 31 : 3 – 15 .
- Moreno , N.M. , Isla , M.I. , Sampietro , A.R. and Vattuone , M. 2000 . Comparison of the free radical-scavenging activity of propolis from several regions of Argentina . Journal of Ethnopharmacology , 71 : 109 – 114 .
- Kalogeropoulos , N. , Konteles , S.J. , Troulidou , E. , Mourtzinos , I. and Karathanos , V.T. 2009 . Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus . Food Chemistry , 116 : 452 – 461 .
- Luna , L. and Fabani , M. 2009 . Schmeda-Hirschmann, G.; Podio, N.; Wunderlin, D.; Feresin, G. Main flavonoids, DPPH activity and metal content allow determination of the geographical origin of propolis from the province of San Juan (Argentina). Journal of Agricultural and Food Chemistry , 57 : 2691 – 2698 .
- Isla , M.I. , Paredes-Guzman , J.F. , Nieva-Moreno , M.I. , Koo , H. and Park , Y.K. 2005 . Some chemical composition and biological activity of Northern Argentine propolis . Journal of Agricultural and Food Chemistry , 53 : 1166 – 1172 .
- Rice-Evans , A.C. , Miller , N.J. and Papanga , G. 1996 . Structure antioxidant activity relationship of flavonoids and phenolic acids . Free Radical Biology and Medicine , 20 : 933 – 956 .
- Kroon , P.A. and Williamson , G. 1999 . Hydroxycinnamates in plants and food: Current and future perspectives . Journal of the Science of Food and Agriculture , 79 : 355 – 361 .
- Nijveldt , R.J. , Van Nood , E. , Van Hoorn , D.E.C. , Boelens , P.G. , Van Noren , K. and Van Leeuwen . 2002 . P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications . The American Journal of Clinical Nutrition , 74 : 418 – 425 .
- Chen , J.H. and Ho , C.T. 1997 . Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds . Journal of Agriculture and Food Chemistry , 41 : 2371 – 2378 .
- Wu , W.-M. , Lu , L. , Long , Y. , Wang , T. , Liu , L. , Chen , Q. and Wang , R. 2007 . Free radical scavenging and antioxidative activities of caffeic acid phenethyl ester (CAPE) and its related compounds in solution and membranes: a structure-activity insight . Food Chemistry , 105 : 107 – 115 .
- Ogiwara , T. , Satoh , K. , Kadoma , Y. , Murakami , Y. , Unten , S. and AtsumI , T. 2002 . Radical scavenging activity and cytotoxicity of ferulic acid . Anticancer Research , 22 : 2711 – 2717 .
- Srinivasan , M. , Sudheer , A.R. , Pillai , K.R. , Kumar , P.R. , Sudhakaram , P.R. and Moon , V.P. 2006 . Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes . Toxicology , 228 : 249 – 258 .
- Mandsen , H.L. and Bertelesen , G. 1995 . Spices as antioxidants . Trends of Food Science and Technology , 6 : 271 – 277 .
- Sancez-Moreno , C. , Jimenez-Escrig , A. and Saura-Calixto , F. 2000 . Study of low density lipoprotein oxidizability indexes to measure the antioxidant activity of dietary polyphenols . Nutrition Research , 20 : 941 – 953 .
- Cushnie , T.P. and Lamb , A.J. 2005 . Antimicrobial activity of flavonoids . International Journal of Antimicrobial Agents , 26 : 343 – 356 .
- Haenen , G.R.M.M. and Bast , A. 1999 . Nitric oxide radical scavenging of flavonoids . Methods in Enzymology , 301 : 490 – 503 .
- Ferry , D.R. , Smith , A. , Malkhandi , J. , Fyfe , D.W. , deTakats , P.G. Anderson , D. 1996 . Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition . Clinical Cancer Research , 2 : 659 – 668 .
- Verma , A.K. , Johnson , J.A. , Gould , M.N. and Tanner , M.A. 1988 . Inhibition of 7,12-dimethylbenz(a)anthracene and N-nitrosomethylurea induced mammary cancer by dietary flavonol quercetin . Cancer Research , 48 : 5754 – 5788 .
- Pignatelli , P. , Pulcinelli , F.M. , Celestini , A. , Lenti , L. , Ghiselli , A. Gazzaniga , P.P. 2000 . The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide . The American Journal of Clinical Nutrition , 72 : 1150 – 1155 .
- Heo , M.Y. , Lee , J.H. , Sohn , S.J. and Au , W.W. 1996 . Anticlastogenic effects of galangin against mitomycin C-induced micronuclei in reticulocytes of mice . Mutatation Research , 360 : 37 – 41 .
- Wall , M.E. , Wani , M.C. , Manikumar , G. , Abraham , P. , Taylor , H. , Hughes , T.J. , Warner , J. and McGiveney , R. 1988 . Plant antimutagenic agents . 2. Flavonoids. Journal of Natural Products , 51 : 1084 – 1091 .
- Cholbi , M.R. , Paya , M. and Alcaraz , M.J. 1991 . Inhibitory effects of phenolic compounds on CCl4-induced microsomal lipid peroxidation . Experientia , 47 : 195 – 199 .
- Cai , Q. , Rahn , R.Q. and Zhang , R. 1997 . Dietary flavonoids quercetin, luteolin and genistein reduce oxidative DNA damage and lipid peroxidation and quench free radicals . Cancer Letters , 119 : 99 – 107 .
- Mira , L. , Fernandez , M.T. , Santos , M. , Rocha , R. , Florencio , M.H. and Jennings , K.R. 2002 . Interactions of flavonoids with iron and copper ions a mechanism for their antioxidant activity . Free Radical Research , 36 : 1199 – 1208 .
- Sadik , C.D. , Sies , H. and Schewe , T. 2003 . Inhibition of 15-lipoxygenases by flavonoids: Structure activity relations and mode of action . Biochemical Pharmacology , 65 : 773 – 781 .
- McKay , D.L. and Blumberg , J.B. 2006 . A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) . Phytotherapy Research , 20 : 519 – 530 .
- Mendes da Silva , J.F. , De Souza , M.C. , Matta , S.R. , De Andrade , M.R. and Vidal , F.V.N . 2006 . Correlation analysis between phenolic levels of Brazilian propolis extracts and their antimicrobial and antioxidant activities . Food Chemistry , 99 : 431 – 435 .
- Chaillou , L.L. and Nazareno , A. 2009 . Bioactivity of propolis from Santiago del Estero, Argentina related to their chemical composition . LWT–Food Science and Technology , 42 : 1422 – 1427 .
- Gregoris , E. and Stevanato , R. 2010 . Correlations between polyphenolic composition and antioxidant activity of Venetian propolis . Food and Chemical Toxicology , 48 : 76 – 82 .
- Scheller , S. , Krol , W. , Swiacik , J. , Owczarek , S. , Gabrys , J. and Shani , J. 1989 . Antitumoral property of ethanolic extract of propolis in mice-bearing Ehrlich carcinoma, as compared to bleomycin . Zeitschrift fur Naturforschung , 44 : 1063 – 1065 .
- Tosi , E.A. , Ortega , M.E. and Cazzoli , A.F. 2007 . Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli . Food Chemistry , 104 : 1025 – 1029 .
- Chikaraishi , Y. , Izuta , H. , Mishima , S. and Hara , H. 2010 . Angiostatic effects of Brazilian green propolis and its chemical constituents . Molecular Nutrition and Food Research , 54 : 566 – 575 .
- Basnet , P. , Matsushige , K. , Hase , K. , Kadota , S. and Nanba , T. 1996 . Four di-O-caffeoyl quinic acid derivatives from propolis . Potent hepatoprotective activity in experimental liver injury models. Biological and Pharmaceutical Bulletin , 19 : 1479 – 1484 .
- Khayyal , M.T. , El-Ghazaly , M.A. and El-Khatib , A.S. 1993 . Mechanisms involved in the antiinflammatory effect of propolis extract . Drugs under Experimantal and Clinical Research , 19 : 197 – 203 .
- Nagai , T. , Inoue , R. and Suzuki , N. 2003 . Preparation and antioxidant properties of water extracts of propolis . Food Chemistry , 80 : 29 – 33 .
- Gulcin , I. , Bursal , E. , Sehitoglu , H.M. , Bilsel , M. and Goren , A.C. 2010 . Polyphenol contents and antioxidant activity of lyophilized aqueous extracts of propolis from Erzurum, Turkey . Food and Chemical Toxicology , 48 : 2227 – 2238 .
- Laskar , R.A. , R , Nayan and Begum . 2010 . N.A. Antioxidant activity of Indian propolis and its chemical constituents . Food Chemistry , 122 : 233 – 237 .
- Melliou , E. and Chinou , I. 2004 . Chemical analysis and antimicrobial activity of Greek propolis . Planta Medica , 70 : 515 – 519 .
- Velikova , M. , Bankova , V. , Sorkun , K. , Houcine , S. , Tsvetkova , I. and Kujumgiev , A. 2000 . Propolis from the Mediterranean region: Chemical composition and antimicrobial activity . Zeitschrift fur Naturforschung , 55 : 790 – 793 .
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of free radical method to evaluate antioxidant activity . Lebensmittel-Wissenschaft und- Technology , 28 : 25 – 30 .
- Benzie , I.F. and Strain , J.J. 1996 . The ferric reducing ability of plasma as a measure of “antioxidant power” the FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Slinkard , K. and Singleton , V.L. 1997 . Total phenol analysis: automation and comparison with manual methods . American Journal of Enology and Viticulture , 28 : 49 – 55 .
- Zhisen , J. 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Moreira , L. , Dias , L.G. , Pereira , J.A. and Estevinho , L. 2008 . Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal . Food Chemical and Toxicology , 46 : 3482 – 3485 .
- Lagouri , V. and Alexandri , G. 2013 . Antioxidant properties of Greek O. Dictamnus and R. officinalis methanol and aqueous extracts-HPLC determination of phenolic acids . International Journal of Food Properties , 16 (3) : 549 – 562 .
- Silva , F.A. , Borges , F. , Guimaraes , C. , Lima , J.L. , Matos , C. and Reis , S. 2000 . Phenolic acids and derivatives: Studies on the relationship among structure, radical scavenging activity, and physicochemical parameters . Journal of Agricultural and Food Chemistry , 48 : 2122 – 2126 .
- Graf , E. 1992 . Antioxidant potential of ferulic acid . Free Radical Biology and Medicine , 13 : 435 – 448 .
- Cholbi , M.R. , Pays , M. and Alcaraz , M.J. 1991 . Inhibitory effects of phenolic compounds on carbon tetrachloride induced microsomal lipid peroxidation . Experientia , 47 : 195 – 199 .
- Heijnen , C.G. , Haenen , G.R.M.M. , Oostveen , R.M. , Stalpers , E.M. and Bast , A. 2002 . Protection of flavonoids against lipid peroxidation: The structure activity relationship revisited . Free Radical Research , 36 : 575 – 581 .
- Arts , M.J.T.J. , Dallinga , J.S. , Voss , H.P. and Haenen , G.R.M.M. 2004 . A new approach to assess the total antioxidant capacity using the TEAC assay . Food Chemistry , 88 : 557 – 567 .
- Russo , A. , Longo , R. and Vanella , A. 2002 . Antioxidant activity of propolis: Role of caffeic acid phenethyl ester and galangin . Fitoterapia , 73 : 21 – 29 .
- Son , S. and Lewis , B.A. 2002 . Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: Structure–activity relationship . Journal of Agriculture and Food Chemistry , 50 : 468 – 472 .
- Kumazawa , S. , Hamasaka , T. and Nakayama , T. 2004 . Antioxidant activity of propolis of various geographic origins . Food Chemistry , 84 : 329 – 339 .
- Aliyazicioglu , R. , Sahin , H. , Erturk , O. , Ulusoy , E. and Kolayli , S. 2013 . Properties of phenolic composition and biological activity of propolis from Turkey . International Journal of Food Properties , 16 (2) : 277 – 287 .
- Mohammadzadeh , S. , Sharriatpanahi , M. , Hamedi , M. , Amanzadeh , Y. , Ebrahimi , S.E.S. and Ostad , S.N. 2007 . Antioxidant power of Iranian propolis extract . Food Chemistry , 103 : 729 – 33 .

