Abstract
The present investigation was carried out to evaluate the antifungal activities of Phyllanthus polyphyllus L. leaf extract, to isolate its active constituent 4-o-methyl gallic acid (4-o-MGA), and to determine the antioxidant and antiaflatoxigenic properties. The bioassay-guided fractionation of methanol extract led to the isolation of active compound 4-o-methylgallic acid. The methanol extract showed the highest amount of phenolic content (290 mg GAE/g dry extract), which indicated the involvement of phenolic compounds in the radical scavenging activity observed by the methanol extract. The antioxidant capacity of 4-o-MGA was greater than the standard butylated hydroxytoluene, followed by methanol extract. The concentration-dependent growth inhibitory activity was observed against the tested fungi, in which the field fungi were susceptible, while the storage fungi were found to be more resistant including aflatoxigenic A. flavus. A correlation was observed between fungal biomass and aflatoxin production in control and treatment, there was a decrease in biomass of A. flavus and aflatoxin production with increasing concentration. The aflatoxin production was completely inhibited in vitro by methanol extract at 1 mg/mL and 4-o-MGA at 2 mg/mL, but the mycelial growth was not inhibited completely. The inhibition of aflatoxin production was relatively higher than the mycelial growth inhibition of A. flavus, such behaviors might have been determined by the presence of hydrolysable tannin 4-o-MGA in the extract, which is known to inhibit the aflatoxin biosynthesis. The significant antioxidant and aflatoxin inhibitory activities of P. polyphyllus could be exploited for its application in preventing oxidative deterioration and fungal spoilage of food products.
INTRODUCTION
Moulds are responsible for off-flavor formation, production of allergenic compounds, and mycotoxin contamination, which are a risk for human and animal health. Mycotoxins are produced in grains in the field, during transport, and storage where conditions are suitable for their production.[Citation1] Many fungi produce mycotoxins such as aflatoxins, ochratoxins, and fumonisins, which are mutagenic, teratogenic, and hepatotoxic secondary metabolites.[Citation2] The fungi cause food spoilage, which causes reduction in quality and quantity. Food quality has been guaranteed by controlling fungi that produce mycotoxins.[Citation3] Fungi have also unfavorable effects on quality, safety, and preservation of food. Synthetic chemicals are widely used for control, however, these chemicals may cause toxic residues in treated products.[Citation4] Several strategies are used at controlling fungal growth and the mycotoxin synthesis in stored grains by chemical treatments. According to Wu and Khlangwiset,[Citation5] interventions to reduce mycotoxin-induced illness can be grouped into three categories; agricultural, dietary, and clinical. Agricultural interventions are methods that can be applied either in the field (pre-harvest) or in drying, storage, and transportation (post-harvest) to reduce mycotoxin levels in food. The dietary and clinical interventions are considered as secondary interventions by which the aflatoxin related illness can be reduced.
Aspergillus flavus is an important mycotoxigenic mould that colonizes food grains and their by-products, it is an important aflatoxin B1 (AFB1) producer and can infect food grains during pre- and post-harvest; aflatoxin contaminations can occur mainly due to the poor management of drying and storage phases. It is estimated that around a 25% of world’s crops are annually affected by mycotoxins, mainly those produced by the genera Aspergillus, Fusarium, and Penicillium.[Citation1,Citation6] Furthermore, the reactive oxygen species (ROS) molecules cause oxidative stress by damaging the proteins, lipids, nucleic acids and also stimulate aflatoxin biosynthesis. Lipid peroxidation is one of the major causes of deterioration of food products during processing and storage, which alter taste and aroma, and cause undesirable effects on the human health.[Citation7] Use of synthetic chemicals to control biodeterioration is concerned to the risk of toxicity and negative impact on human health. Furthermore, synthetic preservatives can induce generation of ROS, leading to oxidative damage of metabolites and enhancing aflatoxin biosynthesis.[Citation8] Synthetic antioxidants are mainly used to prolong the storage stability of foods, however, some synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and propyl gallate have been revealed to be toxic, carcinogenic, and other side effects.[Citation7,Citation9−Citation11] Hence, interest has been increased for finding antioxidants and antifungal compounds of natural source, which are suitable for use in food and/or medicine.
A large number of synthetic chemicals are used to control of fungal contamination, however, these synthetic chemicals are restricted, due to the their possible carcinogenicity, teratogenicity, high and acute toxicity, long degradation periods, environmental pollution, and their effects on human beings.[Citation4,Citation7,Citation12] Therefore, there is an increased interest in finding natural antifungals of plant origin alternative to synthetic chemicals. Phytocompounds are expected to be safer than synthetic pesticides, as they are easily decomposable, not environmental pollutants, and possess no residual or phytotoxic properties.[Citation13]
Plant extracts are potentially useful additives for food preservation as they are likely to prolong shelf life and improve the quality of stored food products.[Citation14] Plant extracts contain various antioxidant compounds such as polyphenols, phenols, flavonoids, etc. which could be the bioactive basis of their antimicrobial properties. Phenolic compounds have been shown to have an inhibitory effect on growth and toxin production in Aspergillus spp. by their antioxidant potencies.[Citation15−Citation17] Plant extracts with antifungal properties could potentially be used to control mycotoxigenic fungi in foods and feeds. Due to the environmental effects of synthetic pesticides and their side-effects associated with health risks, natural pesticides of plant origin are being evaluated in order to control fungal spoilage and mycotoxins production in different storage grains.[Citation6] In this way, researchers have focused on the potentiality of plants and their metabolites to inhibit toxigenic fungus growth and/or toxin production as a useful tool for controlling mycotoxin contamination of crops and agricultural commodities.
Phyllanthus polyphyllus L. (Euphorbiaceae) is a deciduous shrub widely distributed in tropical and subtropical regions in India and Srilanka.[Citation18] In India, the raw drugs of Phyllanthus species have been extensively used for treating liver disorders, intestinal infections, jaundice, and diabetes in traditional medicine.[Citation19] The genus Phyllanthus contains highly valued medicinal plants which produce a variety of secondary metabolites including flavonoids, phenolic acids, alkaloids, lignans, and tannins, have long been extensively used in folk medicine all over the world.[Citation20] P. polyphyllus has been investigated for their various bioactive properties such as anti-inflammatory,[Citation18] anti-tumour,[Citation21] cytotoxic,[Citation21,Citation22] and hepatoprotective activities.[Citation23,Citation24] Species of these plant genera are used in folk medicine and consumed as healthy ingredients, they could also be useful in preventing mould growth and mycotoxin contamination due to their antifungal properties. To the best of the authors knowledge, the antifungal and antiaflatoxigenic activities of P. polyphyllus have not been evaluated so far. Therefore, the present study was aimed at the investigation of antifungal and antiaflatoxigenic, antioxidant activities of P. polyphyllus, and isolation of the active constituents responsible for such activities.
MATERIALS AND METHODS
Chemicals and Reagents
All the culture media were purchased from Hi-Media, Mumbai (India). All solvents, reagents, BHT and iodo-nitro-tetrazolium (INT) were procured from SRL (Mumbai, India). All chemicals and solvents used were of analytical grade. Microtiter-plates (96-well) were purchased from Axiva, New Delhi (India). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was obtained from Sigma-Aldrich (Steinheim, Germany) and thin layer chromatography (TLC, Silica gel 60) plates from Merck (Dermhadt, Germany).
Collection of Plant Materials
Fresh disease free leaves of P. polyphyllus were collected from the Jnanabharathi Campus, Bangalore University, Bangalore (India) in the month of July, 2011. The collected plant material was washed with distilled water and shade-dried at room temperature. The dried leaves were ground well and stored in air-tight containers. An authenticated voucher specimen (Voch.No.MB&BT.DCM.59) of the plant was deposited in the Herbarium of Department of Microbiology and Biotechnology, Bangalore University, Bangalore, India.
Preparation of Plant Extracts
Fifty grams of the powdered plant sample was successively extracted with 200 mL of petroleum ether (60°C), toluene (95°C), chloroform (60°C), methanol (70°C), and ethanol (70°C) using a Soxhlet apparatus. The solvents were used sequentially with their increasing polarity. All the solvent extracts were concentrated separately under reduced pressure using a rotary flash evaporator and stored in air tight glass tubes.[Citation25]
Estimation of Total Phenolic Contents
The amount of phenolic content present in the solvent extracts was estimated by following Folin-Ciocalteu method with minor modifications.[Citation26] Briefly, 1 mL of each extract (1 mg/mL) was added to 0.1 mL of Folin-Ciocalteu reagent and kept for 5 min at room temperature in dark conditions. After incubation, 2 mL of 15% sodium carbonate was added and diluted to 10 mL by adding distilled water, then incubated at room temperature for 90 min in dark conditions. Absorbance was measured at 720 nm using double beam UV-VIS spectrophotometer (Elico, India). Total phenolic content was expressed as gallic acid equivalent (mg GAE/g dry extract) based on the calibration curve.
Isolation and Identification of the Bioactive Compound
The methanol extract (ME) which showed highest antimicrobial activity was fractionated into four different fractions viz., acidic, phenolic, basic, and neutral.[Citation27] The acidic fraction which showed highest antifungal activity was subjected to further purification by column chromatography (CC). The column of silica mesh 60-120 (SRL, Mumbai, India) was eluted with solvents of increasing polarity using a gradient of CHCl3 and MeOH (10:0, 9:1 → 0:10, v/v). The eluates were collected in different fractions and concentrated. Based on antifungal activity and TLC analysis, the CC fractions of similar profile were pooled together. The active compound was obtained as crystals, then analyzed for purity of the active compound following TLC analysis and tested with ferric chloride (FeCl3). The compound was identified based on the comparison of infrared (IR), electrospray ionization-mass spectrometry (ESI-MS), 1H & 13C NMR data with the published values.[Citation18,Citation28]
Antifungal Activity
Test microorganisms
A total of 15 seed-borne pathogenic fungi viz., Alternaria brassicola, A. geophila, Aspergillus flavus, A. tamari, A. terreus, A. fumigatus, Curvularia tetramera, Fusarium equiseti, F. lateratium, F. udum, Penicillium chrysogenum, and P. citrinum, which were isolated from food grains in previous studies. Fusarium moniliforme and F. oxysporum were procured from National Collection of Industrial Microorganisms (NCIM, Pune, India). Sauboraud dextrose agar (SDA) was used as the media for fungal cultures.
Poisoned food technique
Antifungal activity of the solvent extracts and 4-o-MGA was determined by the poison food technique as described by Mohana et al.[Citation25] All the test samples were dissolved in methanol and incorporated into SDA to achieve the media of requisite concentrations. The control media without test sample was added by the methanol, the solvent which was used for dissolving the samples. The prepared media were autoclaved, poured into Petri dishes (20 mL), and allowed to cool. Five millimeter discs of 7-day-old culture of test fungi were placed at the center of the Petri dishes. The inoculated plates were incubated at 28 ± 1°C for 7 days. Triplicates were maintained for each concentration and control. The fungitoxicity of test samples in terms of percentage of mycelia inhibition was calculated as follows:
Minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC)
The microbroth dilution method was employed to determine the MIC and MFC of ME and 4-o-MGA following standard procedures.[Citation9,Citation29] Two-fold serial dilutions of test samples with different concentrations (0.062 to 8.0 mg/mL) were prepared in 96-well microtitre plate with sauboraud dextrose broth (SDB). The overnight incubated test fungi in broth culture were adjusted to concentrations of 104 cfu mL−1. Each well of 96-well microtitre plate was containing 200 μL of two-fold diluted broth of different concentrations and inoculated with 15 μL of test organisms. The inoculated microtitre plate was sealed with parafilm, then agitated with a microtitre plate shaker and incubated at 30°C for 72 h. The inoculated plates were observed for the presence or absence of fungal growth. After macroscopic observation, a 10 μL of treated broths were streaked radially onto the SDS plates and incubated at 30°C for 72 h. After the incubation period, the lowest concentration at which the growth was inhibited that value was recorded as MIC. The complete absence of growth on the agar surface in the lowest concentration of the sample was defined as MFC.[Citation9] After streaking on to agar plates, 50 μL of INT (0.2 mg/mL) was added to each well and incubated at 37°C for 30 min. The pale yellow-colored INT was reduced to a pink color which indicated the presence of viable microbial cells, while the yellow color remained the same where the fungal growth was inhibited.[Citation30]
Comparison with some synthetic fungicides
The fungitoxic activities of ME and 4-o-MGA were compared with of some synthetic fungicides viz., copper oxychloride 50% WP (Fungicop-50, Karnatak Agrochemicals Pvt. Ltd., Bangalore, India) and zinc ethylene bisthiocarbamate 75% WP (Indifil Z-75, Indofil Chemicals Company, Mumbai, India) by recording the MIC and MFC following the micro-broth dilution method as described above.
Anti-Aflatoxigenic Activity
In vitro assay
Sucrose-magnesium sulphate-potassium sulphate-yeast extract (SMKY) liquid medium was used to determine the efficacy of ME and 4-o-MGA against AFB1 production.[Citation14] SMKY medium (25 mL) was taken in 100 mL flasks, to which requisite amount of test samples were added to get 0.125, 0.25, 0.50, 1.0, and 2.0 mg/mL concentrations. The flasks were aseptically inoculated with suspension of toxigenic isolates of A. flavus (106 spores/mL, 100 μL/flask) and incubated at 28°C for 10 days. The flask containing 0.5 mL of dimethyl sulphoxide (DMSO) was maintained as control. The broth cultures were filtered through Whatman No. 1 filter paper, mycelia dried at 100°C for 12 h and mycelial dry weight (MDW) was recorded. The MDW of ME and 4-o-MGA treated samples and control were compared for their MDW losses. The filtrate was used for the isolation of AFB1 by adding equal volume of CHCl3 (20 mL) and shaken well in a separating funnel. The CHCl3 layer was passed through anhydrous sodium sulphate (Na2SO4) and evaporated in dark conditions at room temperature. The residue was re-dissolved 1 mL of CHCl3 and 10 μL of sample was spotted onto the TLC plate adjacent to AFB1 standard. The plate was developed in CHCl3-acetone (96:4, v/v) solvent system, air-dried, and visualized under ultra-violet (365 nm) light (UV-cabinet, Labline, India). Qualitative identification of AFB1 content was done by visual comparison of intensity of fluorescence of the samples with standard spots. For quantitative estimation, the fluorescent spots were scraped off the plates, dissolved in 5 mL cold CH3OH, and centrifuged at 3000 rpm for 5 min. The absorbance of supernatant was measured at 265 nm using a spectrophotometer (Systronics, India) and the amount of AFB1 content was calculated following formula:
In vivo assay
The efficacy of ME and 4-o-MGA on aflatoxin production in viable (in vivo) maize was determined by following the procedure of Garcia et al.[Citation1,Citation31] and Probst and Cotty[Citation32] with slight modifications. The maize seeds were treated with different concentrations of ME and 4-o-MGA ranging from 0.125 to 4.0 mg per gram of maize seeds. The maize seeds without sample treatment were maintained as control. The sample-treated and untreated maizes were inoculated with 100 μL spore suspension of A. flavus toxigenic strain containing 104 spores/mL. The water activity (aw 0.95) of maize was adjusted by aseptically adding sterile distilled water to maize kernels in sterile container as described by Garcia et al.[Citation1] The inoculated maizes were kept for incubation at room temperature for 15 days. After incubation, the maize seeds (5 g) were milled and extracted with 15 mL of acetonitrile + water (60 + 40, v/v) and shaken for 10 min. The extract was filtered through Whatman No. 1 filter paper and the filtrate was extracted with equal volume of CHCl3. Further, the extracted AFB1 was estimated qualitatively and quantitatively as described above.
DPPH Radical Scavenging Activity
The DPPH radical scavenging activity of ME and 4-o-MGA was determined by using the method described by Ebrahimabadi.[Citation26] Briefly, plant extract of different concentrations ranging from 156 to 2000 μg/mL were prepared by using two-fold dilution method. A solution of 0.1 mM 2,2-DPPH was prepared in methanol. The reaction was containing 1 mL of sample and 3 mL of 0.1 mM 2,2-DPPH, allowed for incubation in dark conditions for 30 min (28°C). Methanol was used as negative control and BHT as standard. The absorbance of the reaction mixture was measured at 517 nm by using double beam UV-VIS spectrophotometer (Elico, India). The ability of the plant sample to scavenge 2,2-DPPH was calculated as:
Statistical Analysis
Experiments were performed in triplicate and data given were analysed by SPSS 20 (SPSS Inc., IBM, USA). Differences between means were determined using Tukey’s univariate comparisons (p ≤ 0.05).
RESULTS
The plant materials were sequentially extracted using a Soxhlet apparatus, all the solvent extracts were concentrated in vacuo and tested for their antifungal activities. The ME was obtained as brown-gummy residue (yield: 0.48%/100 g of dry plant material, w/w), which showed the highest antifungal activity. The phenolic compounds were found to be present in the ME and the total phenolic content was estimated as 290 mg GAE/g. The ME of P. polyphyllus was subjected to fractionation to give four different fractions viz., acidic, phenolic, basic, and neutral. The active acidic fraction showed antifungal activity, which was further purified by CC with a gradient elution of CHCl3 and MeOH (10:0, 9:1→0:10, v/v) to afford 8 fractions. The third and fourth fractions of CC showed similar chromatographic profile, which were pooled together, and the FeCl3 test confirmed that the compound belongs to phenolic group. In the negative ion mode [M + H]− of ESI – MS, the isolated active compound showed a molecular ion (m/z) peak at 184.3 corresponding to the molecular formula C8H8O5. The 1H-NMR [δ 7.03 (2H, s, H-2, H-6), 4.86 (br s, OH), 3.80 (3H, s, OCH3)], 13C-NMR [δ 121.47 (C-1), 110.05 (C-2), 146.49 (C-3), 139.74 (C-4), 146.49 (C-5), 110.05 (C-6), 52.24 (OCH3), 169.02 (C = O)], and IR spectral analysis revealed that the compound is 4-o-methylgallic acid (IUPAC name: 4-methoxy-3, 5-dihydroxybenzoic acid) based on the comparison of its spectral data with the reported values in literature.
The growth inhibitory effects of ME and 4-o-MGA were screened against a panel of 15 fungal pathogens containing storage and field fungi are given in . The percentage of growth inhibition by the ME and 4-o-MGA was estimated by measuring the growth diameter of the colony grown in the medium with the treatment and control. Most of the treated field fungi were susceptible to 2 mg/mL concentration of ME and 4-o-MGA with inhibition of spore germination. Among the fungi tested, A. tamari, A. flavus, and P. citrinum were found to be most resistant, with the mycelial growth inhibition 18, 12, and 0%, respectively. Moderate inhibitory activity was observed against the field fungi such as F. moniliforme (51.72%), C. tetramera (39.32%), and F. equiseti (36.68%), with inhibition of spore germination at a concentration (2 mg/mL). In micro-broth dilution assay, the ME showed significant inhibitory activity against most of the pathogenic fungi tested with lower MIC and MFC than that of 4-o-MGA and synthetic copper oxychloride. Zinc ethylene bisthiocarbamate showed highest inhibitory activity against wide range of fungi. On comparative evaluation with synthetic fungicides, ME and 4-o-MGA showed varying degrees of MIC and MFC values against different fungi tested. The results demonstrated that the ME has remarkable antifungal activities than its active constituent 4-o-MGA.
TABLE 1 Antifungal activities of methanol extract of P. polyphyllus, 4-o-MGA, and synthetic fungicides against seed-borne pathogenic fungi
Antiaflatoxigenic activities of ME and 4-o-MGA were tested in vitro and in vivo against an aflatoxigenic strain of A. flavus. The concentration-dependent antiaflatoxigenic activities of ME and MGA are presented in . The ME completely inhibited the AFB1 production at 1 mg/mL in vitro, while the 4-o-MGA showed moderate inhibition at 2 mg/mL in vitro, but failed to inhibit completely. Fungal biomass and AFB1 production in treatment sets were found to be decreased with increasing concentrations of ME and 4-o-MGA, whereas, the control set showed the highest AFB1 (1500 μg/L) content with highest biomass (MDW). The ME treated set showed moderate inhibition at 4 mg/mL with 1000 μg/Kg of AFB1 content in vivo, while the 4-o-MGA treated set showed 1500 μg/Kg of AFB1 content at 4 mg/mL. In vivo assay demonstrated that the ME is having moderate AFB1 inhibitory activity than that of 4-o-MGA, and highest AFB1 (2500 μg/Kg) content was detected in control set.
TABLE 2 In vitro and in vivo aflatoxin B1 inhibitory activity of methanol extract of P. polyphyllus and 4-o-MGA
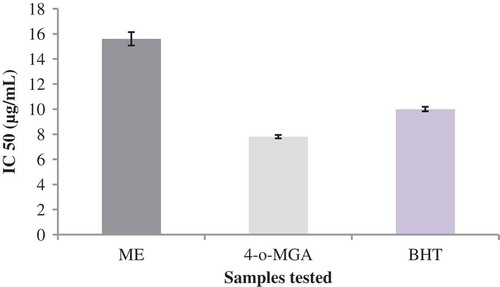
In the DPPH radical scavenging assay, the IC50 values of ME and 4-o-MGA were found to be 15.6 and 7.8 μg/mL, respectively (). The antioxidant activity of the synthetic antioxidant BHT (10.0 μg/mL) was also determined and compared with the IC50 values of ME and 4-o-MGA. A lower IC50 value indicates the greater antioxidant activity. The 4-o-MGA exhibited higher antioxidant activity with lower IC50 value than the BHT, and ME showed moderate activity with higher IC50 value. The order of antioxidant activity was MGA > BHT > ME.
DISCUSSION
Recently, several phytocompounds have been reported for their antifungal and aflatoxin inhibitory properties, especially the aflatoxin inhibitory activities are associated with the antioxidant potential of phytocompounds.[Citation15,Citation17,Citation33] Hence, the antioxidants have become an essential part of contemporary food preservation technology. In order to evaluate the various bioactive properties of natural products, it is necessary to investigate the major phytoconstituents, which are involved in such activities. In this context, the P. polyphyllus extract and its active constituent 4-o-MGA were investigated for their antifungal, antiaflatoxigenic and antioxidant activities.
The interesting biological activities and the no reports on antifungal activities of P. polyphyllus encouraged the authors to pursue an investigation to evaluate its antifungal properties, to isolate the bioactive antifungal component(s) and to determine the antiaflatoxigenic properties. In this article, the potential antifungal activity of P. polyphyllus and inhibitory activity towards aflatoxin biosynthesis is discussed, with special reference to its bioactive component. Several prior studies have highlighted the P. polyphyllus for its various bioactivities such as anti-inflammatory,[Citation18] antitumour,[Citation21] cytotoxic,[Citation21,Citation22] and hepatoprotective activities.[Citation23,Citation24] The 4-o-MGA has been well reported as active constituent for such bioactivities. Oliveira et al.[Citation34] reported the potential anti-atherogenic effect of standard 4-o-MGA on foam cell formation and showed that it was most effective among the standard gallic acid derivatives used. The 4-o-MGA has been reported in the plant Rhus glabra (Anacardiaceae) as antimicrobial constituent against bacteria by Saxena et al. (1994).[Citation28] To the best of the authors knowledge, antifungal and antimycotoxigenic activities of P. polyphyllus and its active constituent 4-o-MGA against A. flavus growth and aflatoxin production are not evaluated so far. In this investigation, the bioassay-guided fractionation of ME ion leaded to the isolation of active compound from acidic fraction. The active crystalline compound was identified as benzenoid compound 4-o-methylgallic acid (IUPAC name: 4-methoxy-3,5-dihydroxybenzoic acid) belongs to the phenolic group.[Citation18,Citation28]
The concentration-dependent growth inhibitory activity was observed against the tested fungi, in which the field fungi were susceptible, while the storage fungi were found to be more resistant. The MIC of ME was comparatively lower than that of the synthetic fungicides tested, thereby, its potency as antifungal agent of plant origin would be economical. In AFB1 inhibitory assay, a correlation was found between fungal biomass and AFB1 production in control and treatment sets comprising different concentrations of ME and 4-o-MGA. At higher concentrations, there was a decrease in biomass of the toxigenic A. flavus with decrease in AFB1 production. However, the AFB1 production was completely inhibited in vitro by ME at 1 mg/mL, while it was moderately inhibited by 4-o-MGA, but not completely. Hence, the ME was found to be more efficacious in inhibition of AFB1 production than its constituent 4-o-MGA. Even though, the ME showed moderate inhibitory activity than that of 4-o-MGA in vivo assay, both ME and 4-o-MGA failed to inhibit toxin production completely, this may be due to diffusion of the samples treated. The ME showed remarkable activity in suppression of AFB1 than the 4-o-MGA, it is evident from the results that this may be due to synergetic effect of other phytochemicals present in the extract.
On comparative evaluation, both ME and 4-o-MGA inhibited AFB1 production in vitro, but they did not show higher mycelial growth inhibitory activity. The maximum mycelial growth inhibition observed was only 57.24% in poisoned food technique, with moderate inhibition against field fungi and lower activity against storage fungi. The ME and 4-o-MGA did not show any significant activity against A. flavus aflatoxigenic strain (12%) and the colony diameters did not present a significant difference relative to controls. It is interesting to note that the inhibition of AFB1 production was relatively higher than the mycelial growth inhibition of A. flavus. Such behaviors might have been determined by the presence of hydrolysable tannin 4-o-MGA in the extract, which is known to inhibit the aflatoxin biosynthesis in the growth medium.[Citation35]
Mahoney et al.[Citation17] demonstrated the involvement of oxidative stress in stimulating aflatoxin production by Aspergillus sp. and overcome of such stress by natural antioxidants suppresses or eliminates aflatoxin formation. He reported that the effect of tannins in reducing oxidative-induced aflatoxin formation was due to their antioxidant properties, thereby over-riding the stimulative effect on aflatoxigenes. It is reported that the ochratoxin A production by Aspergillus spp. was inhibited by gallic acid derived phenolic compounds having antioxidant potencies, with no inhibition of fungal growth.[Citation33] Recent research suggests potential use of polyphenolics in food processing for improving quality, safety, and stability of food products, with human health benefits.[Citation36] Several prior reports stated that the antioxidant phenolic and polyphenolic compounds are responsible for the inhibition of aflatoxin biosynthesis in A. flavus. Such considerations have guided us to hypothesize that the inhibitory activity of P. polyphyllus extract against AFB1 production by A. flavus is due to presence of polyphenolic 4-o-MGA, which is able to relieve oxidative stress thereby suppress or eliminate aflatoxin biosynthesis.
The ME showed the highest amount of phenolic content (290 mg GAE/g), which indicate the involvement of phenolic compounds in the radical scavenging activity observed by the ME. The antioxidant capacity of 4-o-MGA was greater than the standard BHT, however, the activity of ME was close to the BHT with regardless of the concentration conditions. Rajkapoor et al.[Citation21] reported the hepatoprotective and antioxidant activity of P. polyphyllus in acetaminophen-induced hepatotoxicity in rat model. Srirama et al.[Citation22] demonstrated the antioxidant activity of crude extract of P. polyphyllus, but the active constituent responsible for such activities are not reported. P. polyphyllus has earlier been reported for its antioxidant properties based on preliminary investigations of crude extract. However, this is the first report on P. polyphyllus showing inhibitory effect on AFB1 synthesis, in checking fungal deterioration of viable maize (in vivo) and its constituent 4-o-MGA as an antioxidant.
CONCLUSION
The results of this study indicate the interesting bioactive properties of polyphenolic compounds of P. polyphyllus. The high contents of polyphenolic composition made P. polyphyllus as a promising and inexpensive source of biologically active polyphenolics. The high polyphenolic potential, and significant antioxidant and aflatoxin inhibitory activities of P. polyphyllus leaf, offer possibilities for its application in preventing oxidative deterioration and fungal spoilage of food products. Further investigations are necessary to resolve the question of whether the atoxigenicity of aflatoxigenic fungus when exposed to hydrolyzable tannins occurs permanently or not.
FUNDING
The authors are grateful to the Department of Science and Technology (No. SR/FT/LS-141/2008), Government of India and University Grant Commission (F.No. 37-82/2009 (SR)), New Delhi for the financial support.
REFERENCES
- Garcia, D.; Ramos, A.J.; Sanchis, V.; Marin, S. Effect of Equisetum arvense and Stevia rebaudiana extracts on growth and mycotoxin production by Aspergillus flavus and Fusarium verticillioides in maize seeds as affected by water activity. International Journal of Food Microbiology 2012, 153, 21–27.
- Quiroga, E.N.; Sampietro, D.A.; Sgariglia, M.A.; Soberon, J.R.; Vattuone, M.A. Antimycotic activity of 5′-prenylisoflavanones of the plant Geoffroea decorticans, against Aspergillus species. International Journal of Food Microbiology 2009, 132, 42–46.
- Irkin, R.; Korukluoglu, M. Control of some filamentous fungi and yeasts by dehydrated Allium extracts. Journal für Verbraucherschutz und Lebensmittelsicherheit 2009, 4, 3–6.
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic, and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol, and p-cymene. Bioresource Technology 2008, 99, 8788–8795.
- Wu, F.; Khlangwiset, P. Health economic impacts and cost-effectiveness of aflatoxin reduction strategies in Africa: Case studies in biocontrol and postharvest interventions. Food Additives and Contaminants 2010, 27, 496–509.
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Additives and Contaminants 2010, 27, 651–657.
- Kumar, R.; Mishra, A.K.; Dubey, N.K.; Tripathi, Y.B. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic, and antioxidant activity. International Journal of Food Microbiology 2007, 115, 159–164.
- Mishra, P.K.; Shukla, R.; Singh, P.; Prakash, B.; Kedia, A.; Dubey, N.K. Antifungal, anti-aflatoxigenic, and antioxidant efficacy of Jamrosa essential oil for preservation of herbal raw materials. International Biodeterioration Biodegradation 2012, 74, 11–16.
- Dung, N.T.; Kim, J.M.; Kang, S.C. Chemical composition, antimicrobial, and antioxidant activities of the essential oil and the ethanol extract of Cleistocalyx operculatus (Roxb.) Merr and Perry buds. Food and Chemical Toxicology 2008, 46, 3632–3639.
- Gu, L.; Wu, T.; Wang, Z. TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var. acuta. LWT-Food Science and Technology 2009, 42, 131–136.
- Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Nazarian, S.; Ahmadi, F.; Batooli, H. Chemical composition, antioxidant, and antibacterial activities of the essential oil and methanol extracts of Eucalyptus oleosa Leaves. International Journal of Food Properties 2013, 16, 1080–1091.
- Baris, D.; Kizil, M.; Aytekin, C.; Kizil, G.; Yavuz, M.; Ceken, B; Ertekin, A.S. In vitro antimicrobial and antioxidant activity of ethanol extract of three Hypericum and three Achillea species from Turkey. International Journal of Food Properties 2011, 14, 339–355.
- Boyraz, N.; Ozcan, M. Inhibition of phytopathogenic fungi by essential oil, hydrosol, ground material, and extract of summer savory (Satureja hortensis L.) growing wild in Turkey. International Journal of Food Microbiology 2006, 107, 238–24.
- Shukla, R.; Kumar, A.; Prasad, C.S.; Srivastava, B.; Dubey, N.K. Antimycotic and antiaflatoxigenic potency of Adenocalymma alliaceum Miers. on fungi causing biodeterioration of food commodities and raw herbal drugs. International Biodeterioration and Biodegradation 2008, 62, 348–351.
- Molyneux, R.J.; Mahoney, N.; Kim, J.H.; Campbell, B.C. Mycotoxins in edible tree nuts. International Journal of Food Microbiology 2007, 119, 72–78.
- Oliveira, M.S.; Furlong, E.B. Screening of antifungal and antimycotoxigenic activity of plant phenolic extracts. World Mycotoxin Journal 2008, 1, 139–146.
- Mahoney, N.; Molyneux, R.J.; Kim, J.H.; Campbell, B.C.; Waiss, A.C.; Hagerman, A.E. Aflatoxigenesis induced in Aspergillus flavus by oxidative stress and reduction by phenolic antioxidants from tree nuts. World Mycotoxin Journal 2010, 3, 49–57.
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Anti-inflammatory activities of constituents isolated from Phyllanthus polyphyllus. Journal of Ethnopharmacology 2006, 103, 181–186.
- Srirama, R.; Senthilkumar, U.; Sreejayan, N.; Ravikanth, G.; Gurumurthy, B.R.; Shivanna, M.B.; Sanjappa, M.; Ganeshaiah, K.N.; Shaanker, R.U. Assessing species admixtures in raw drug trade of Phyllanthus, a hepato-protective plant using molecular tools. Journal of Ethnopharmacology 2010, 130, 208–215.
- Priya, O.S.; Viswanathan, M.B.G.; Balakrishna, K.; Venkatesan, M. Chemical constituents and in vitro antioxidant activity of Phyllanthus wightianus. Natural Product Research 2011, 25, 949–958.
- Rajkapoor, B.; Sankari, M.; Sumithra, M.; Anbu, J.; Harikrishnan, N.; Gobinath, M.; Suba, V.; Balaji, R. Antitumour and cytotoxic effects of Phyllanthus polyphyllus on erlich ascites carcinoma and human cancer cell lines. Bioscience, Biotechnology, and Biochemistry 2007, 71, 2177–2183.
- Youkwan, J.; Srisomphot, P.; Sutthivaiyakit, S. Bioactive constituents of the leaves of Phyllanthus polyphyllus var. siamensis. Journal of Natural Products 2005, 68, 1006–1009.
- Rajkapoor, B.; Venugopal, Y.; Anbu, J.; Harikrishnan, N.; Gobinath, M.; Ravichandran, V. Protective effect of Phyllanthus polyphyllus on acetaminophen induced hepatotoxicity in rats. Pakistan Journal of Pharmaceutical Sciences 2008, 21, 57–62.
- Srirama, R.; Deepak, H.B.; Senthilkumar, U.; Ravikanth, G.; Gurumurthy, B.R.; Shivanna, M.B.; Chandrasekaran, C.V.; Agarwal, A.; Shaanker, R.U. Hepatoprotective activity of Indian Phyllanthus. Pharmaceutical Biology 2012, 50, 948–953.
- Mohana, D.C.; Raveesha, K.A.; Rai, K.M.L. Herbal remedies for the management of seed-borne fungal pathogens by an edible plant Decalepis hamiltonii (Wight & Arn). Archives Phytopathology and Plant Protection 2008, 41, 38–49.
- Ebrahimabadi, A.H.; Ebrahimabadi, E.H.; Djafari-Bidgoli, Z.; Kashi, F.J.; Mazoochi, A.; Batooli, H. Composition and antioxidant and antimicrobial activity of the essential oil and extracts of Stachys inflata Benth from Iran. Food Chemistry 2010, 119, 452–458.
- Roberts, R.M.; Gilbert, J.C.; Rodewald, L.B.; Wingrove, A.S. Modern Experimental Organic Chemistry, 3rd Ed; Saunders Golden Sunburst Series: Philadelphia, Holt-Saunders, Japan, Tokyo, 1981; pp 495–506.
- Saxena, G.; McCutcheon, A.R.; Farmer, S.; Towers, G.H.N.; Hancock, R.E.W. Antimicrobial constituents of Rhus glabra. Journal of Ethnopharmacology 1994, 42, 95–99.
- Hajji, M.; Jarraya, R.; Lassoued, I.; Masmoudi, O.; Damak, M.; Nasri, M. GC/MS and LC/MS analysis, and antioxidant and antimicrobial activities of various solvent extracts from Mirabilis jalapa tubers. Process Biochemistry 2010, 45, 1486–1493.
- Teke, G.N.; Lunga, P.K.; Wabo, H.K.; Kuiate, J.R.; Vilarem, G.; Giacinti, G.; Kikuchi, H.; Oshima, Y. Antimicrobial and antioxidant properties of methanol extract, fractions, and compounds from the stem bark of Entada abyssinica Stend ex A. Satabie. BMC Complementary and Alternative Medicine 2011, 11 (57), 1–8.
- Garcia, D.; Ramos, A.J.; Sanchis, V.; Marin, S. Modelling the effect of temperature and water activity in the growth boundaries of Aspergillus ochraceus and Aspergillus parasiticus. Food Microbiology 2011, 28, 406–417.
- Probst, C.; Cotty, P.J. Relationships between in vivo and in vitro aflatoxin production: Reliable prediction of fungal ability to contaminate maize with aflatoxins. Fungal Biology 2012, 116, 503–510.
- Palumbo, J.D.; O’Keeffe, T.L.; Mahoney, N.E. Inhibition of ochratoxin A production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathologia 2007, 164, 241–248
- Oliveira, M.V.B.; Badia, E.; Carbonneau, M.A.; Grimaldi, P.; Fouret, G.; Lauret, C.; Leger, C.L. Potential anti-atherogenic cell action of the naturally occurring 4-O-methyl derivative of gallic acid on Ang II-treated macrophages. FEBS Letters 2004, 577, 239–244.
- Mahoney, N.; Molyneux, R.J. Phytochemical inhibition of aflatoxigenicity in Aspergillus flavus by constituents of walnut (Juglans regia). Journal of Agricultural and Food Chemistry 2004, 52, 1882–1889.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. International Journal of Food Properties 2013, 16, 45–60.