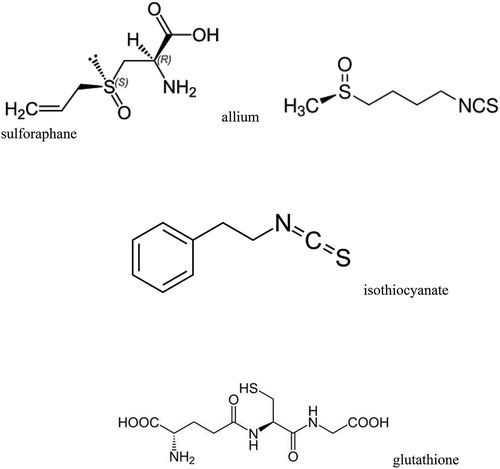
Abstract
Phytonutrients are chemicals that are derived from plants and aid in both human health as well as the prevention of chronic disease. One type of phytonutrient classification is organosulfides which includes mostly cruciferous vegetables, as well as garlic. Allium, sulforaphane, glutathione, and isothiocyanates are organosulfides that are examined in this current review of literature for their anti-carcinogenic, anti-thrombotic, anti-atherosclerotic, anti-inflammatory, anti-microbial, and anti-oxidative effects. The following review will also focus on specific research examining the effects of organosulfides on health outcomes such as prostate cancer, breast cancer, gastric cancer, colorectal cancer, diabetes, and hypertension.
INTRODUCTION
Phytonutrients are chemicals found naturally in plants. Often, these phytonutrients are the first defense mechanism for plants to protect themselves from fungi, germs, and insects. Similar to vitamins and minerals found in plants, phytonutrients have many health benefits and are known as functional foods.[Citation1] The Academy of Nutrition and Dietetics defines functional foods as “whole foods and fortified, enriched, or enhanced foods that have a potentially beneficial effect on health when consumed as part of a varied diet on a regular basis, at effective levels.” Neutraceuticals are foods that are used to prevent or treat a disease. Neutraceuticals contain essential nutrients and phytochemicals that have proven to have disease-fighting properties in the realm of nutrition therapy. Unlike functional foods, the scientific value of neutraceuticals on health has been based on thorough scientific research. Information on chemical structures, biological functions, and implications for clinical use are all associated with neutraceuticals for health.[Citation2] Both functional foods and neutraceuticals contain organosulfides, a type of phytonutrient.
Allium
Allium sativum (garlic) has been examined for its anti-carcinogenic, anti-thrombotic, anti-atherosclerotic, anti-inflammatory, anti-microbial, and anti-oxidative effects. The most active compound found in garlic is allicin (dyallyl disulfide). Although many benefits can be derived from allicin, its instability necessitates proper preparation practices for consumption. Allicin’s chemical structure shows its instability, which makes it sensitive to reactivity. Allicin only exists as a biologically active compound when garlic is crushed or cut, so that the enzyme allinase can metabolize alliin to allicin; thus, it is important to know how to prepare allicin to derive optimum benefits.[Citation3] It is suggested to wait 10 min after mincing and cutting in order to give enough time for the allicin to form. Consuming garlic raw also achieves the most optimal benefits.[Citation4] In a review of literature examining the action mechanism of garlic, it was suggested that garlic derivatives influence DNA adduct formation, mutagenesis, cell proliferation, scavenging of free radicals, angiogenesis, and differentiation.[Citation3] Additionally, garlic was found to stimulate apoptosis as well as reduce the growth rate of cancer cells with cell cycle blockage in the G2/M phase.[Citation5]
ANTI-MICROBIAL RESEARCH
Research has shown that allicin is the most effective phytonutrient found in garlic in regard to anti-microbial activity. Allicin is shown to have a broad range of effects, such as inhibiting the growth of over 300 kinds of microbes including methicillin resistant staphylococcus as well as both gram positive and gram negative bacteria.[Citation6] The anti-microbial properties of allicin are often compared to the anti-microbial properties of prescribed antibiotic drugs. At a cellular level, the anti-microbial benefit is due to the phytonutrient’s ability to be transported across the cell membrane and into the cytoplasm where it exerts its action intracellularly.[Citation7] Allicin’s potency may also contribute to its anti-microbial action, as 1 mg of allicin is equivalent to 15 international units of Penicillin. In just one clove of garlic, there can be up to 13 mg of allicin, an amount not considered miniscule.[Citation4] Allicin is what gives garlic its potent smell and when consumed, it bathes bodily systems, a plausible explanation for garlic-scented breath.
HYPERTENSION RESEARCH
Focusing on the preventative and therapeutic role of garlic on hypertension, a study examined the effects of garlic on blood pressure in patients with stage 1 essential hypertension.[Citation8] This was a 24-week, single-blind, placebo controlled study with 210 newly diagnosed patients with stage 1 hypertension. Patients were divided into seven groups each containing 30 participants. Patients in each group were either given garlic tablets in doses of 300, 600, 900, 1200, and 1500 mg per day. A separate group was given 100 mg per day of atenolol and another group was given a placebo. Results of this study showed a significant decrease in systolic blood pressure and diastolic blood pressure for all dose groups of garlic when compared to the placebo group at week 0, week 12, and week 24. Higher doses of garlic and longer duration periods were associated with a reduction in blood pressure.[Citation8] The findings in this study support the anti-hypertensive effects of garlic.[Citation9]
CANCER RESEARCH
In a series of animal experiments, allicin was injected into the tumor site of rats. Allixin’s cytotoxic and apoptotic abilities were able to destroy 97% of the tumor cells.[Citation6] In a Canadian study, garlic showed to be the most effective against human stomach, pancreas, breast, prostate, lung, kidney, and brain cancers, which was superior to that of broccoli, kale, cabbage, and brussel sprouts in eliminating 100% of growths.[Citation4] Studies have shown that allicin modifies the proteins that are necessary for replication by inhibiting proliferation and growth, and inducing apoptosis.[Citation6]
A meta-analysis looked at prospective studies that examined the association between all allium-containing vegetables (garlic, onions, and leeks), garlic supplements and colorectal cancer.[Citation9] Studies were included if they met the following inclusion criteria: The study had a prospective cohort or case cohort study design; the relationship between allium vegetables or garlic supplements and colorectal cancer was present; and, the study provided a relative risk statistically significant level.. Eight cohort studies were identified using allium vegetables and a total of 5458 patients. Five cohort studies on garlic supplements were also identified with a total of 2685 patients. A pooled analysis revealed no significant association between high allium vegetable intake and colorectal cancer; however, a subgroup analysis showed a 23% increased risk for colon cancer in women associated with low allium vegetable intake.[Citation9] A meta-analysis of two cohort and 19 case-control studies analyzed the association between allium vegetable consumption and gastric cancer.[Citation10] Studies met inclusion criteria if they used a case control or cohort study design, had data on the occurrence of gastric cancer, included consumption of allium vegetables, and used odds ratios or relative risks with confidence intervals. A total of 543,220 subjects were included in the study. The meta-analysis finds that when comparing the highest and lowest groups of allium vegetable consumption, the highest group had a reduced risk for gastric cancer. In a dose-response analysis for the case control studies, results found that an overall consumption of 20 g/day was associated with a significant 9% decreased of gastric cancer risk. While this meta-analysis suggests a preventative effect of allium vegetables on risk of gastric cancer, there are several limitations that need to be considered. Confounders, such as infection of H pylori, were not controlled for. The individual studies varied in the amounts of allium vegetables consumed in the highest and lowest consumption groups, and some of the studies were not originally designed to study the consumption of allium vegetables in relation to gastric cancer.[Citation10]
Sulforaphane
Sulforaphane, a phytonutrient that is classified in a group of plant-based biologically active compounds called isothiocyanates associated with positive health benefits.[Citation11] Sulforaphane is found in many cruciferous vegetables belonging to the family of Brassicaceae.[Citation11] This vegetable family includes broccoli, cabbage, cauliflower, and Brussels sprouts (Brassica oleracea), Chinese cabbage and turnips (Brassica rapa), and other lettuce crops including watercress (Rorrippa nasturtium-aquaticum) and arugula (Eruca sativa).[Citation11] Sulforaphane is formed indirectly when sulfur-containing molecules present in crucifers, called glucosinolates are metabolized in the body. Plant enzymes called myrosinases hydrolyze glucosinolates to form biologically active compounds called isothiocyanates that include sulforaphane.[Citation11] Sulforaphane (R-1-isothiocyanato-4-methylsulfinylbutane) structurally consists of a β-D-thioglucose group, a sulphonated oxime group, and a side chain derived from amino acids.[Citation2] It is the most hydrophilic dietary isothiocyanate with a molecular formula of C6H11NOS2.[Citation11] It is important to note that the benefits of sulforaphane are only released from plants when the food is chewed, causing the plant tissues to break down leading to the reaction between myrosinase and glucoraphanin.
CANCER RESEARCH
Sulforaphane was identified in 1992 by a group of researchers while investigating the activity of several fruit and vegetable extracts as enzymes.[Citation12] Researchers found that broccoli extract was one of the most potent inducers of anti-carcinogenic enzymes and attempted to isolate the compound responsible. An extract was successfully isolated and was found to be responsible for more than 80% of the inducer activity in broccoli. The compound that was isolated was identified as sulforaphane.[Citation12] Clinical studies of sulforaphane indicated its potential health benefits as an anti-carcinogenic compound, including its ability to act indirectly as an anti-oxident and induce phase II detoxification enzymes.[Citation13] In addition to its anti-carcinogenic properties, sulforaphane has also been implicated as a broad-spectrum anti-microbial and anti-inflammatory agent.[Citation13]
Moon et al.[Citation15] investigated the potential anti-helicobacter activity of sulforaphane and related compounds in broccoli.[Citation15] Broccoli sprouts were used to make a crude methanol extract that was extracted with hexane, chloroform, ethyl acetate, and butanol. The extractions were then inoculated with a strain of H. pylori and analyzed for the presence of inhibition zones. The chloroform extract tested positive for the isothiocyanate sulforaphane and was found to have the greatest inhibition zone (>5 cm) after exposure to the H. pylori strain. The results indicate that sulforaphane may have bactericidal activity against H. pylori and cruciferous vegetables may be excellent food sources for medicinal substances.[Citation15]
In a 2012 review article, a comparison was made between studies which used different methods for analyzing, isolating, and purifying sulforaphane in order to examine its chemopreventive properties.[Citation16] The articles that were reviewed explored the anti-inflammatory, anti-carcinogenic, cancer cell inhibiting, and anti-oxident properties of sulforaphane. Studies done on cancer cells explored the effects of sulforaphane on leukemia, prostate cancer, colon cancer, bladder cancer, breast cancer, ovarian cancer, pancreatic cancer, and melanoma. Although research has shown many positive effects from the use of sulforaphane, the authors pointed out that there are many areas that need further exploration. More clinical studies on humans must be done in order to better understand the chemopreventative effects of sulforaphane and to distinguish the differing effects of pure sulforaphane versus vegetable extracts.[Citation16]
ANTI-INFLAMMATORY RESEARCH
A study examined the anti-inflammatory properties of sulforaphane as expressed in the ovalbumin-induced murine asthma pathway.[Citation16] In the study, mice were sensitized by an exposure to ovalbumin, treated with injections of sulforaphane, and subsequently challenged with ovalbumin inhalation.[Citation16] The mice treated with the sulforaphane injections experienced lower levels of inflammatory cells, mainly eosinophils, in the bronchoalveolar fluid within the lungs as compared to the untreated mice and airway hyper-responsiveness was greatly reduced.[Citation16] Furthermore, sulforaphane was shown to reduce levels of Th2 cytokines. Th2 cytokines are inflammatory molecules that are present in chronic asthma that promote inflammation in the endothelial cells of the bronchi and epithelial cells in the airway. Sulforaphane was shown to reduce levels of ovalbumin-specific IgE, an immunoglobulin associated with allergies.[Citation18] These results indicate that sulforaphane may be therapeutic for asthma and other allergic diseases, but further research is needed.[Citation16]
There are numerous published reports on the effect of sulforaphane as an inducer of phase II enzymes, which have antioxidant and detoxification properties. These enzymes play a role in regulating the reduction of reactive oxygen species (ROS) in cells, as well as in the concentration of thiol buffers that protect cells from oxidative damage.[Citation18] A study by Angeloni et al. examined the time-dependent expression and enzyme activity of the phase II enzymes glutathione (GSH) reductase, GSH-s-transferase, GSH peroxidase, nicotinamide adenine denucleotide phosphate (NADPH): quinone oxidoreductase-1 and thioredoxin reductase in the presence of sulforaphane in the neonatal cardiomyocytes of rats.[Citation19] The cultured cardiac cells were treated with sulforaphane, exposed to hydrogen peroxide, and intracellular anti-oxident activity was subsequently measured.
There was evidence that sulforaphane helped decrease the production of intracellular ROS, increased cell viability, and promoted a drop in DNA fragmentation while at the same time inducing antioxidants and up-regulating phase II enzymes.[Citation5] These results indicate the potential cardio protective nature of sulforaphane through the induction of phase II enzymes in cardiomyocytes and the increased resistance of cardiac cell injury by way of ROS.[Citation19]
DIABETES RESEARCH
In addition to the cancer fighting effects of sulforaphane, this phytonutrient has also been studied in humans and animals for its effects in diabetes. Mirmiran et al. explored the question of whether broccoli sprouts powder loaded with high levels of sulforaphane would have an effect on inflammatory markers in diabetic patients.[Citation20] The protocol consisted of a randomized, double-blind, placebo controlled clinical trial. A total of 81 type 2 diabetic patients participated in this trial and were randomly assigned to three groups: the placebo group, the 5 g/d of broccoli sprouts powder group and 10 g/d of broccoli sprouts powder group.[Citation20] The clinical trial lasted 4 weeks during which 3-day dietary recalls were performed (at baseline and four weeks after). Weight, body mass index measures and fasting blood samples (taken after 12–14 h of overnight fasting at baseline and again 4 weeks after the intervention) were taken. At the end of the trial, data on 63 of the initial 81 participants were presented. At the conclusion, the two groups who were given the broccoli sprouts powder experienced decreases in serum hs-C-Reactive Protein concentrations, as well as decreases in serum interluekin-6 and tumor necrosis factor-alpha concentrations. These decreases in serum markers show that the high concentration of sulforaphane in broccoli sprouts powder has an effect on inflammatory markers that are present in patients with type 2 diabetes. Treatment approaches for type 2 diabetes have anti-inflammatory effects. These are important to the treatment of patients with type 2 diabetes because they allow for better management of the symptoms and can lead to better prevention of some of the vascular diseases that are associated with type 2 diabetes.[Citation20]
The connection between sulforaphane and diabetes has also been studied in rats. Souza et al. explored the effects of orally administering doses of sulforaphane on the occurrence or progression of diabetes in male Wistar rats.[Citation21] The rats were divided into five groups, including a control group, a streptozotocin group, and three different streptozotocin groups which were treated with different levels of sulforaphane concentrations. Samples of liver tissue, adipose tissue, retroperitoneal tissue, and skeletal muscle were dissected from the rats after death and serum levels of cholesterol, urea, creatinine, and activities of the enzymes alanine aminotransferase and aspartate aminotransferase.[Citation21] The study showed that all three of the levels of sulforaphane doses had a protective effect against the effects of hypoglycemia in the kidneys by lowering creatinine levels. In addition to the effects on liver function, this study also showed a protective effect against alterations in the function of glucose uptake in adipose tissue and maintenance of hepatic glycogen.[Citation21]
VASCULAR RESEARCH
Kwon et al. evaluated the effects sulforaphane on smooth muscle cells by inducing carotid artery injuries in rats and then treatings with either sulforaphane or a vehicle treatment.[Citation22] The results of the experiment revealed that sulforaphane has the potential to prevent restenosis, which is a common issue that occurs after a vascular injury. Sulforaphane is thought to regulate adhesion molecules in order to inhibit certain pathways that can result in the regulation of vascular inflammatory responses as well as the proliferation and migration of vascular smooth muscle cells after an injury. This research opens the door for further research on the possible use of sulforaphane in the treatment of certain vascular diseases.[Citation22]
In addition to the possible effects of sulforaphane on treatment of vascular disease, another publication explores the possibility of neuroprotective effects of sulforaphane in response to a spinal cord injury.[Citation23] The study found that when two doses of sulforaphane were administered to rats after a contusion spinal cord injury, there was evidence of beneficial effects—both long-term and short-term. These effects include antioxidant response at the site of the injury, lower levels of inflammatory cytokines, and increased locomotor function in the hind limbs to name a few. These results show that there is a possible use for sulforaphane as a part of spinal cord injury therapy treatment.[Citation23]
OTHER RESEARCH
Other studies on sulforaphane focus on detoxification effects. The study examined tilapia fish bred on a local fish farm in Mexico.[Citation24] The fish were separated into three different groups; one which was fed regular feed that consisted of alfalfa sprouts, another group were fed broccoli sprouts, and a third group were fed alfalfa sprouts that were mixed with sulforaphane. After 30 days of the experimental diets, results showed that those fish that were given a diet that included sources of sulforaphane showed an ability to detoxify the body of the harmful compounds known as polycyclic aromatic hydrocarbons (PAHs). These compounds are known to causes disease, alter growth patterns, alter reproductive schedules, and cause cancers and mutations in certain animals including fish. The study found that the presence of sulforaphane helps to improve the levels of CYP450, which plays an important role in the elimination of PAHs.[Citation24] With this long list of benefits of sulforaphane, scientists are interested in finding more sources of sulforpahane and determining what foods have the highest concentration of sulforaphane.[Citation25]
Isothiocynate
Wasabi japonica (wasabi) is part of the chemical group of organosulfides known as isothiocyanates. The isothiocyanates found in wasabi are derived from the hydrolysis, or breakdown, of glucosinalates, which are the sulfur-containing compounds found in the wasabi root. According to a study, the isothiocyanate content of wasabi is about 100–150 mg per 100 g.[Citation26] The plant’s medicinal uses date back to the 10th century in Japan, where it is widely believed that the chemical components in wasabi act as anti-microbials, killing off bacteria and treating infections. This also serves as an explanation as to why it was originally selected as an accompaniment for raw fish dishes such as sushi. Botanically, the wasabi root is part of the Brasssicaceae family, which is also known as cruciferous and is the same family as horseradish, mustard seed, brussel sprouts, broccoli, and cauliflower.[Citation26]
CANCER RESEARCH
Isothiocyanates have been touted as a promising phytonutrient for its role in chemoprevention, which is the use of natural or synthetic agents to reverse, inhibit, or prevent the development of a chronic-degenerative disease as well as decrease its incidence.[Citation27] More specifically, there has been much research on their cancer-prevention properties. It is thought that isothiocyanates may help to prevent cancer by promoting the elimination of potential carcinogens from the body by heightening the transcription of proteins capable of suppressing tumors.[Citation15] Another mechanism of action by which they are thought to eradicate cancer cells is by stopping their individual cell cycle and inciting apoptosis.[Citation27]
In an animal breast cancer study, progress was made in examining the chemopreventative qualities of isothiocyanates.[Citation28] Breast cancer was induced in eight week old female rats. The rats were administered an isothiocyanate compound and their tumor appearance was monitored over the course of 18 weeks. It was found that those rats given the isothiocyanate compound treatment were able to prolong their tumor-free survival and decrease their tumor incidence and duplication. This study pointed to the supplementation of isothiocyanates for prolonging the tumor-free survival as well as its chemoprevention in the rats.[Citation28] Additionally, these researchers highlighted that this was the first time it had been reported in a scientific study that an isothiocyanate compound, such as those found in cruciferous vegetables, had anti-angiogenic effects in breast cancer-induced animals. Because a compound with anti-angiogenic effects inhibits the growth of blood vessels, this was a significant finding as a method attesting to the chemopreventative activity of isothiocyanates.[Citation28]
Recent research examines the mechanisms of phenethyl isothiocyanate and its anticancer activity toward malignant melanoma cancer A375.S2 cells in humans. Results of this research found that phenethyl isothiocyanate induced G2/M phase arrest as well as apoptosis. This occurred via the endoplasmic reticulum stress-mediated mitochondria-dependant pathway. Another mechanism by which phenethyl isothiocyanate induced apoptosis was by increasing ROS levels, which can cause mitochondrial membrane depolarization leading to apoptosis. Phenethyl isothiocyanate also promoted Bax, a proapoptotic protein, and decreased Bcl-2, an antiapoptotic protein.[Citation29]
A similar study examines the role of phenethyl isothiocyanate and apoptosis in KKU-M214 cells of the cholangiocarcinoma (CCA) cell line compared to Chang cells as a reference.[Citation30] Phenethyl isothiocyanate was evaluated in relation to cellular GSH. Results found that in both KKU-M214 and Chang cells, phenethyl isothiocyanate induced apoptosis as evidenced by a decrease in Bcl-xl levels and an increase in Bax levels. The mechanism of action for phenethyl isothiocyanate proposed in this study is an increase of cellular mobilization, as well as activation of mitochondrial cell death pathway.[Citation30]
OTHER RESEARCH
Although it seems the majority of the research of isothicyanates is focused on cancer prevention, there has also been significant science conducted on the anti-inflammatory effects of wasabi due to its high isothiocyanate content. Focus was put on wasabi’s preventative measures in inflammation, specifically in its role as defense against bacterial infection. This was a key study because it was the first time it was found that the research conducted indicated that the isothiocyanate content in wasabi may target immune and inflammation-related genes in relation to anti-inflammatory function.[Citation31]
A study was published that looked at the wasabi plant in regards to rats with hypercholesterolemia.[Citation32] It was hypothesized that a condition such as hypercholesterolemia increased the production of oxygen-free radicals and animals fed a high cholesterol diet were shown to have a depressed oxidant system. Therefore, it was suggested that any positive anti-oxidant effect would potentially improve hypercholesterolemia in rats. The study aimed to prove this hypothesis and did so successfully. Results revealed that a wasabi-based diet had significant anti-hypercholesterolemia effects in rats with high cholesterol. At the same time, the results showed the high density lipoproteins cholesterol levels in those rats increase as their low density lipoproteins cholesterol levels decreased, illustrating the benefits. The study concluded that the results may provide rational for using wasabi leaf extract in certain conditions related with oxidative stress or hypercholesterolemia.[Citation32]
GSH
GSH is phytonutrient under the classification of thiols.[Citation33] Thiols are organic compounds that contain sulfur in a unique bonding structure. GSH exists in a variety of foods, with the highest concentration found in animal products. This is due to the fact that GSH is produced in animals as a natural antioxidant. The potential health benefits of GSH are wide ranging, including prevention and treatment of almost all conditions associated with oxidative stress. GSH is a water-soluble tripeptide, made up of the amino acids: cysteine, glutamate, and glycine.[Citation33] This intracellular molecule is found in either its reduced form (GSH) or its oxidized form (oxidized glutathione with a di-sulfide bridge). When GSH is oxidized, it forms a disulfide bond with free radicals or other ROS that need to be removed from the body.[Citation34] GSH is then reduced back to its original state by the enzyme NADPH-dependent GSH reductase, as the free radical is removed from the body. It is the behavior of the sulfur-containing amino acid cysteine that gives GSH its antioxidant capabilities. Because of its prevalence in the intracellular environment, GSH is responsible for much of the detoxification load in the body from drugs, carcinogens, and other environmental pollution. Additionally, GSH has been shown to play a role in nutrient metabolism, synthesis of DNA and proteins, apoptosis, and a whole host of other cellular events.[Citation35]
ANTI-INFLAMMATORY RESEARCH
The powerful antioxidant GSH is synthesized within the body.[Citation33] In fact, GSH is the most abundant, endogenously manufactured antioxidant that exists in humans. First identified by Frederick Gowland Hopkins at Cambridge University in 1929, GSH’s function in biochemistry has led to the theory of free radicals that we know today.[Citation36] This landmark discovery now informs our very understanding of disease, guiding our therapies and treatments. Because of its role in disease prevention at a systemic level, GSH has been implicated in prevention and/or treatment of almost all oxidative stress related conditions, including cancer, HIV and AIDS, Parkinson’s disease, hepatitis, autism, type 2 diabetes, drug hepatoxicity (especially acetaminophen), and even psychiatric disorders such as schizophrenia and bipolar disorder.[Citation33,Citation38,Citation39] A cross-sectional study focusing on polymorphisms in the GSH pathways and modulation in cystic fibrosis severity found that the polymorphisms in GSH pathways are associated with the severity of cystic fibrosis.[Citation39] The study was conducted between 2011 and 2012 and included 180 patients with cystic fibrosis. Polymerase chain reaction (PCR) was used to identify polymorphisms.
Cysteine is the limiting amino acid of the tripeptide for GSH synthesis, increased dietary cysteine intake has been shown to raise GSH levels. In animal trials involving rats given whey protein supplementation (a rich source of cysteine), a significant rise in plasma GSH levels was observed.[Citation40] Increasing precursors to cysteine itself, such as the amino acid methionine, has shown the same relationship with GSH levels.[Citation41] Derivatives of cysteine, primarily N-acetylcysteine, has been found to also be a GSH precursor.[Citation42] Treatment involving N-acetylcysteine supplementation has been given to individuals experiencing acetaminophen hepatoxicity because it has been found to replenish intracellular GSH.[Citation43]
In the GSH pathway that removes ROS from the body, the GSH molecule is recycled and has the ability to be used again. However, when an extreme burden of toxins is present, GSH can be depleted. Acetaminophen, the common anti-inflammatory drug has been found to deplete GSH levels.[Citation37] It is important to note that the ratio of reduced-GSH to oxidized-GSH changes as we age, skewing toward higher levels of the oxidized form of GSH over time.[Citation44] Additionally, the body’s ability to synthesize GSH to recover from depleted levels decreases with age. This has wide-ranging implications for individuals of advanced age living in areas with many environmental pollutants or those taking GSH-depleting drugs like acetaminophen regularly. The need to focus on GSH level enhancement is clear.
ANTIBACTERIAL RESEARCH
Low concentrations of GSH occur when the body is unable to properly synthesize GSH. This leads to central nervous system damage and recurring bacterial infections.[Citation41,Citation46] In contrast, a high concentration of GSH molecules within the body occur in areas of extreme oxidative stress such as cancerous tissue; research that has attempted to modulate GSH levels appropriately during cancer treatment has been found to increase cancer survivorship and effect tumor drug effectiveness.[Citation18] This shows the active, protective effect of GSH and how important healthy GSH levels are to human health.
Other sources have been identified that raise GSH-related enzymes known as GSH peroxidases.[Citation46] GSH peroxidases represents a family of eight currently identified molecules that each have their own unique tissue location in the body that they protect. The primary function of these enzymes is to locate and inactive the hydrogen peroxide that acts as a ROS in the body. GSH peroxidases, unlike GSH, contains selenium that must be obtained from the diet. Recent trials have found that organic selenium supplements increase GSH peroxidases activity in health adults.[Citation47] Silymarin, also known as milk thistle, has been found to effectively prevent and treat the characteristic GSH drops associated with ethanol-induced oxidative damage.[Citation48] Additionally, Alpha-lipoic acid has been shown to restore blood GSH levels in HIV patients, when administered at 300 mg three times daily.[Citation49] To meet this amount of alpha-lipoic acid through dietary sources would be difficult, especially for those that avoid eating meat; heart, kidney, and liver meats are highest in alpha-lipoic acid and fruit/vegetable sources contain much less.[Citation50]
CONCLUSION
Research into the beneficial health impacts of phytonutrients and neutraceuticals has been at the center of functional food creation in recent years (see ). Most commonly, phytonutrients are examined for their role as antioxidants, protecting the body from the oxidative stress caused by free radicals as well as anti-carcinogenic effects, anti-microbial effects, and anti-inflammatory effects. It is important to know how to increase the optimal benefits phytonutrients have to offer. Its medicinal value can contribute to modern medicine as a more natural and economical alternative to drugs and supplements, and can also offer a more economical and natural alternative to chemical fertilizers and anti-microbials that deplete foods of their nutrients and contribute to bacterial resistance. While often times research is in its infancy, the promise of alternatives to traditional medicine is incredibly enticing, especially to those in the field of dietetics. New research about phytonutrients and organosulfides in particular and how they affect our health are rapidly becoming a part of medical science.[Citation51–Citation54]
REFERENCES
- Linus Pauling Institute. Micronutrient Information Center: Phytonutrients. http://lpi.oregonstate.edu/infocenter/phytochemicals.html (accessed on Sept 2, 2014).
- Zhao, J. Nutraceuticals, Nutritional Therapy, Phytonutrients, and Phytotherapy for Improvement of Human Health: A Perspective on Plant Biotechnology Application. Recent Patents on Biotechnology 2007, 1, 75–97.
- Capasso, A. Anti-Oxident Action and Therapeutic Efficacy of Allium Sativum L. Molecules 2013, 18(1), 690–700.
- Robinson, J. Garlic. Eating on the Wild Side: The Missing Link to Optimum Health. Little Brown and Company: New York, NY, 2013.
- Ana, L.; Colín-González, R.A.; Santana, C.A.; Silva-Islas, M.E.; Chánez-Cárdenas, A.S.; Perla, D.M. The Anti-Oxident Mechanisms Underlying the Aged Garlic Extract- and S-Allylcysteine-Induced Protection. Oxidative Medicine and Cellular Longevity [Online] 2012, 2012, http://www.hindawi.com/journals/omcl/2012/907162/ (accessed on Sept 2, 2014).
- Ariga, T.; Fujisawa, H.; Matsufuji, H.; Origuchi, K.; Seki, T.; Suma, K.; Watanabe, K. Antibacterial Potential of Garlic Derived Allicin and Its Cancellation by Sulfhydryl Compounds. Bioscience, Biotechnology, and Biochemistry 2009, 73(9), 1948–1955.
- Hyldgaard, M.; Meyer, R.; Mygrind, T. Essential Oils in Food Preservation: Mode of Actions, Synergies and Interactions with Food Matrix Components. Frontiers in Microbiology 2012, 3, 12–16.
- Ashraf, R.; Khan, R.A.; Ashraf, I.; Qureshi, A.A. Effects of Allium Sativum (Garlic) on Systolic and Diastolic Blood Pressure in Patients with Essential Hypertension. Pakistan Journal of Pharmaceutical Sciences 2013, 26(5), 859–863.
- Zhu, B.; Zou, L.; Qi, L.; Zhong, R.; Miao, X. Allium Vegetables and Garlic Supplements Do Not Reduce Risk of Colorectal Cancer, Based on Meta-Analysis of Prospective Studies. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association 2014, 12(12), 1991–2001.
- Zhou, Y.; Zhuang, W.; Hu, W.; Liu, G.; Wu, T.; Wu, X. Consumption of Large Amounts of Allium Vegetables Reduces Risk for Gastric Cancer in a Meta-Analysis. Gastroenterology 2011, 141(1), 80–89.
- Juge, N.; Mithen, R.F.; Traka, M. Molecular Basis for Chemoprevention of Sulforaphane: A Comprehensive Review. Cellular and Molecular Life Sciences 2007, 64(9), 1105–1127.
- Zhang, Y.; Tang, L. Discovery and Development of Sulforaphane As a Cancer Chemopreventive Phytochemical. Acta Pharmacologica Sinica 2007, 28(9), 1343–1354.
- Alternative Medicine Review, LLC. Sulforaphane Glucosinolate Monograph. Alternative Medicine Review 2010, 15(4), 352–360.
- Moon, J.K.; Kim, J.R.; Ahn, Y.J.; Shibamoto, T. Analysis and Anti-Helicobacter Activity of Sulforaphane and Related Compounds Present in Broccoli (Brassicaoleracea L.) Sprouts. Journal of Agriculture and Food Chemistry 2010, 58(11), 6672– 6677.
- Liang, H.; Yuan, Q. Natural Sulforaphane As a Functional Chemopreventive Agent: Including a Review of Isolation, Purification, and Analysis Methods. Critical Reviews in Biotechnology 2012, 32(3), 218–234.
- Park, J.H.; Kim, J.W.; Lee, C.M.; Kim, Y.D.; Chung, S.W.; Jung, I.D.; Noh, K.T.; Park, J.W.; Heo, D.R.; Shin, Y.K.; Seo, J.K.; Park, Y.M. Sulforaphane Inhibits the Th2 Immune Response in Ovalbumin-Induced Asthma. BMB Reports 2012, 45(5), 311–316.
- Singh, S.; Khan, A.R.; Gupta, A.K. Role of Glutathione in Cancer Pathophysiology and Therapeutic Interventions. Journal of Experimental Therapeutics & Oncology 2012, 9(4), 303–316.
- James, D.; Devaraj, S.; Bellur, P.; Lakkanna, S.; Vicini, J.; Boddupalli, S. Novel Concepts of Broccoli Sulforaphanes and Disease: Induction of Phase II Anti-Oxident and Detoxification Enzymes by Enhanced-Glucoraphanin Broccoli. Nutrition Reviews 2012, 70(11), 654–665.
- Angeloni, C.M.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of Phase II Enzymes by Sulforaphane: Implications for Its Cardioprotective Potential. Journal of Agriculture and Food Chemistry 2009, 57(12), 5615–5622.
- Mirmiran, P.; Bahadoran, Z.; Hosseinpanah, F.; Keyzad, A.; Azizi, F. Effects of Broccoli Sprout with High Sulforaphane Concentration on Inflammatory Markers in Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Journal of Functional Foods 2012, 4(4), 390–397.
- Guerini de Souza, C.G.; Sattler, J.A.; de Assis, A.M.; Rech, A.; Santos Perry, M.L.; Souza, D.O. Metabolic Effects of Sulforaphane Oral Treatment in Streptozotocin-Diabetic Rats. Journal of Medicinal Food 2012, 15(9), 795–801.
- Kwon, J.; Joung, H.; Kim, Y.S.; Shim, Y.; Ahn, Y.; Jeong, M.H.; Kee, H.J. Sulforaphane Inhibits Restenosis by Suppressing Inflammation and the Proliferation of Vascular Smooth Muscle Cells. Atherosclerosis 2012, 225(1), 41–49.
- Benedict, L.; Mountney, A.; Hurtado, A.; Bryan, K.E.; Schnaar, R.L.; Dinkova-Kostova, A.T.; Talalay, P. Neuroprotective Effects of Sulforaphane after Contusive Spinal Cord Injury. Journal of Neurotrauma 2012, 29(16), 2576–2586.
- Villa-Cruz, V.; Davila, J.; Viana, M.T.; Vazquez-Duhalt, R. Effect of Broccoli (Brassica Oleracea) and Its Phytochemical Sulforaphane in Balanced Diets on the Detoxification Enzymes Levels of Tilapia (Oreochromis Niloticus) Exposed to a Carcinogenic and Mutagenic Pollutant. Chemosphere 2009, 74(9), 1145–1151.
- Sasaki, K.; Neyazaki, M.; Shindo, K.; Ogawa, T.; Momose, M. Quantitative Profiling of Glucosinolates by LC-MS Analysis Reveals Several Cultivars of Cabbage and Kale As Promising Sources of Sulforaphane. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences 2012, 903, 171–176.
- Imaizumi, K.; Sato, S.; Sakakibara, Y.; Mori, S.; Masaki, O.; Kawashima, Y.; Ban, T.; Sasaki, H.; Tachiyashiki, K. Allyl Isothiocyanate-Induced Changes in the Distribution of White Blood Cells in Rats. The Journal of Toxicological Sciences 2010, 35(4), 583–589.
- Hayes, J.; Kelleher, M.; Eggleston, I. The Cancer Chemopreventive Actions of Phytochemicals Derived from Glucosinolates. European Journal of Nutrition 2008, 47(2), 73–88.
- Aras, U.; Gandhi, Y.A.; Masso-Welch, P.A.; Morris, M.E. Chemopreventive and Anti-Angiogenic Effects of Dietary Phenethyl Isothiocyanate in An N-Methyl Nitrosourea-Induced Breast Cancer Animal Model. Biopharmecals and Drug Disposition 2012, 34(2), 98–106.
- Huang, S.; Hsu, M.; Hsu, S.; Yang, J.; Huang, W.; Huang, A.; Chung, J. Phenethyl Isothiocyanate Triggers Apoptosis in Human Malignant Melanoma A375.S2 Cells Through Reactive Oxygen Species and the Mitochondria-Dependent Pathways. Human & Experimental Toxicology 2014, 33(3), 270–283.
- Tusskorn, O.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, U.; Kukongviriyapan, V. Phenethyl Isothiocyanate Induces Calcium Mobilization and Mitochondrial Cell Death Pathway in Cholangiocarcinoma KKU-M214 Cells. BMC Cancer 2013, 13, 571–571.
- Chen, J.; Uto, T.; Tanigawa, S.; Yamada-Kato, T.; Hou, D. Microarray-Based Determination of Anti-Inflammatory Genes Targeted by 6-(Methylsulfinyl)Hexyl Isothiocyanate in Macrophages. Experimental Therapeutic Medicine 2010, 1(1), 33–40.
- Lee, Y.; Yang, J.; Bae, M.; Yoo, W.; Ye, S.; Xue, C.; Li, C. Anti-Oxidant and Anti-Hypercholesterolemic Activities of Wasabia Japonica. Evidence-Based Complementary and Alternative Medicine 2008, 7(4), 459–464.
- Allen, J.; Bradley, R.D. Effects of Oral Glutathione Supplementation on Systemic Oxidative Stress Biomarkers in Human Volunteers. Journal of Alternative and Complementary Medicine 2011, 17(9), 827–833.
- Monostori, P.; Wittmann, G.; Karg, E.; Turi, S. Determination of Glutathione and Glutathione Disulfide in Biological Samples: An In-Depth Review. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 2009, 877(28), 3331–3346.
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The Central Role of Glutathione in the Pathophysiology of Human Diseases. Archives of Physiology and Biochemistry 2007, 113(4–5), 234–258.
- Simoni, R.; Hill, R.; Vaughn, M. The Discovery of Glutathione by F. Gowland Hopkins and the Beginning of Biochemistry at Cambridge University. The Journal of Biological Chemistry 2002, 54, 527–563.
- Saito, C.; Zwingmann, C.; Jaeschke, H. Novel Mechanisms of Protection Against Acetaminophen Hepatotoxicity in Mice by Glutathione and N-Acetylcysteine. Hepatology 2010, 51(1), 246–254.
- Sansone, R.; Sansone, L. Getting a Knack for NAC. Innovations in Clinical Neuroscience 2011, 8(1), 10–14.
- Marson, F.A.D.L.; Bertuzzo, C.S.; Ribeiro, A.F.; Ribeiro, J.D. Polymorphisms in the Glutathione Pathway Modulate Cystic Fibrosis Severity: A Cross-Sectional Study. BMC Medical Genetics 2014, 15, 15–27.
- Ebaid, H.; Salem, A.; Sayed, A.; Metwalli, A. Whey Protein Enhances Normal Inflammatory Responses During Cutaneous Wound Healing in Diabetic Rats. Lipids in Health and Disease 2011, 10, 235–239.
- Jahoor, F. Effects of Decreased Availability of Sulfur Amino Acids in Severe Childhood Undernutrition. Nutrition Review 2012, 70(3), 176–187.
- Dean, O.M.; van den Buuse, M.; Berk, M.; Copolov, D.L.; Mavros, C.; Bush, A.I. N-Acetyl Cysteine Restores Brain Glutathione Loss in Combined 2-Cyclohexene-1-One and D-Amphetamine-Treated Rats: Relevance to Schizophrenia and Bipolar Disorder. Neuroscience Letters 2011, 499(3), 149–153.
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine—A Safe Antidote for Cysteine/Glutathione Deficiency. Current Opinion in Pharmacology 2007, 7(4), 355–359.
- Rebrin, I.; Sohal, R.S. Pro-Oxidant Shift in Glutathione Redox State During Aging. Advanced Drug Delivery Review 2008, 60(13–14), 1545–1552.
- Ristoff, E.; Larsson, A. Inborn Errors in the Metabolism of Glutathione. Orphanet Journal of Rare Diseases 2007, 2, 16–19.
- Stephensen, C.B.; Marquis, G.S.; Douglas, S.D.; Kruzich, L.A.; Wilson, C.M. Glutathione, Glutathione Peroxidase, and Selenium Status in HIV-Positive and HIV-Negative Adolescents and Young Adults. The American Journal of Clinical Nutrition 2007, 85(1), 173–181.
- Jiang, X.; Dong, J.; Wang, B.; Yin, X.; Qin, L. Effects of Organic Selenium Supplement on Glutathione Peroxidase Activities: A Meta-Analysis of Randomized Controlled Trials. Journal of Hygiene Research 2012, 41(1), 120–123.
- Das, S.K.; Mukherjee, S. Biochemical and Immunological Basis of Silymarin Effect, a Milk Thistle (Silybum Marianum) Against Ethanol-Induced Oxidative Damage. Toxicology Mechanisms and Methods 2012, 22(5), 409–413.
- Jariwalla, R.J.; Lalezari, J.; Cenko, D.; Mansour, S.E.; Kumar, A.; Gangapurkar, B. Restoration of Blood Total Glutathione Status and Lymphocyte Function Following Alpha-Lipoic Acid Supplementation in Patients with HIV Infection. Journal of Alternative and Complementary Medicine 2008, 14(2), 139–146.
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-Lipoic Acid As a Dietary Supplement: Molecular Mechanisms and Therapeutic Potential. Biochimica Et Biophysica Acta 2009, 1790(10), 1149–1160.
- Bower, A.H. Immunomodulatory Activity of Garlic. Current Immunology Reviews 2014, 10(2), 113–121.
- Arisawa, M. Synthesis of Organosulfides Using Transition-Metal-Catalyzed Substitution Reactions: To Construct Exergonic Reactions Employing Metal Inorganic and Organic Co-Substrate/Co-Product Methods. Tetrahedron Letters: International Organ for the Rapid Publication of Preliminary Communications in Organic Chemistry 2014, 55(23), 3391–3399.
- McCaskill, M.L.; Rogan, E.; Thomas, R.D. Diallyl Sulfide Inhibits Diethylstilbestrol Induced DNA Damage in Human Breast Epithelial Cells (MCF-10A). Steroids 2014, 92, 96–100.
- Secci, F.; Frongia, A.; Piras, P.P. Ammonium Salt Catalyzed Oxidation of Organosulfides to Organosulfoxydes. Tetrahedron Letters: International Organ for the Rapid Publication of Preliminary Communications in Organic Chemistry 2014, 55(3), 603–605.