ABSTRACT
Leaf protein concentrate of edible fern Diplazium esculentum was extracted using sonication and non-sonication methods and its functional properties were evaluated. The leaf protein concentrate contained 34.28 and 9.89% protein for sonicated and non-sonicated extractions, respectively. Sonication yielded (36.36%) better emulsion activity over non-sonication (31.10%). Foaming capacity was also found better in sonication (7.27%) over non-sonication (6.99%), and oil absorption capacity also improved in sonication (7.55 g of oil/g) over its counterpart (7.41 g of oil/g). Contact angle experiments with the leaf protein concentrate evidenced the enhancement of more hydrophilicity of the sonicated sample. Thermal properties of the leaf protein concentrate with sonication evidenced more stability (55.58 and 95.95°C) over the non-sonicated (46.75 and 75.60°C). Fourier transform infrared spectral analysis with the leaf protein concentrate of Diplazium esculentum was used to assess the secondary structural data such as α-helix and β-sheets. The various percentages of secondary structures of protein in sonicated and non-sonicated samples were 49.06 and 69.16% for α-helix, 33.59% and 17.02% for β-sheet and 42.02 and 35.68% for turn, respectively. The results of the present investigation showed credible evidence to support that sonication method of extraction of leaf protein concentrate was better in terms of protein yield and functional properties as compared to the non-sonication. The sonicated extracted leaf protein concentrate has the potential for developing value added products.
Introduction
Green leafy vegetables are acknowledged to be the cheapest and the most potential source of protein because of its capability to form amino acid from readily available sources of primary material such as sunlight, carbon dioxide, and atmospheric nitrogen.[Citation1–Citation3] Leafy vegetables are reported to be rich sources of proteins (20–30%), minerals (micro and macro) and vitamins.[Citation3–Citation5] Diplazium esculentum, is perhaps the most edible fern throughout Asia and Oceania.[Citation6]
In Assam it is known as Dhekia, in Bengal Dhenkir shaak, and Linguda in Northern India, which also refers to the curled fronds. It is well-established that proteins are important biomolecules required for the sustenance of health, but unfortunately it is deprived in the diets of the people of the developing countries.[Citation1] Currently, it has been recognized in the developing world that there is a serious gap between the demand and supply of food. Today, research has been focused in narrowing this gap by introducing lesser known edible plant that can uplift the nutritional security of underprivileged people.[Citation7]
In Northeast India and particularly in Assam, the socio-economic and nutritional status are not very satisfactory, compared to the national standards.[Citation8] Moreover, due to the lack of scientific knowledge, this green leafy vegetable is mostly wasted and is a great loss in terms of food security and nutrition. The aim of this study was to extract leaf protein concentrate (LPC) from Diplazium esculentum following sonication and non-sonication extraction procedures and to compare its functional, thermal, and structural properties.
Materials and Methods
Diplazium esculentum was procured from the local markets of Tezpur, Sonitpur district of Assam, India and thereafter brought to laboratory where the vegetable stalks were removed and the leaves were thoroughly washed with tap water followed by rinsing with distilled water. The leaves were allowed to drain the excess amount of water. Thereafter, it was used for extraction of LPC and the functional, thermal, and structural properties were studied.
LPC Production
The leaves were washed, weighed, and pulped in Posho mill, followed by pressing using a screw-press[Citation9] and illustrated in . The extracted leaf juice was heated in batches at 80–90°C for about 10 min to coagulate and pasteurize the leaf protein. The protein coagulum obtained was separated by filtering through pillow cases followed by pressing with a screw-press.[Citation10] The LPC was washed with water and repressed. The product was oven-dried at 40°C and pulverized thereafter. The same procedure was applied with slight modification for ultrasonic extraction.
Figure 1. Flowchart of leaf protein concentrate (LPC) production by A: sonicated and B: non-sonicated Fellows[Citation9] methods.
![Figure 1. Flowchart of leaf protein concentrate (LPC) production by A: sonicated and B: non-sonicated Fellows[Citation9] methods.](/cms/asset/7359a145-4460-481e-befa-cef2761add83/ljfp_a_1199034_f0001_b.gif)
Determination of Total Protein Content
The nitrogen content was determined by micro-Kjeldahl method[Citation11] using Kjeldahl apparatus (Model Kel plus-kes, 201, Pelican Equipment, Chennai, India). The percentage of nitrogen was converted to crude protein by multiplying with a factor of 6.25.
Water Absorption Capacity (WAC)
The WAC was determined according to the method of Rodriguez et al.,[Citation12] with some modifications. A sample (1.0 g) was dissolved in 10 mL distilled water and after mixing, it was allowed to stand for 30 min and protein was centrifuged at 3000 rpm for 20 min. The supernatant was decanted and weighed the amount of sediment present in tubes. The WAC (g of water/g of LPC) was calculated by using the following formula:
where = weight of the dry sample (g),
weight of the tube + dry sample (g), and
weight of the tube + sediment (g)
Oil Absorption Capacity (OAC)
Using the method of Linn et al.,[Citation13] a sample (1.0 g) was taken in a pre-weighed centrifuge tube and thoroughly mixed with 5 mL of sunflower oil.[Citation47–Citation50] The mixture was then centrifuged at 3000 rpm for 30 min at 25°C. The supernatant was removed and tubes were weighed. The OAC (g of oil per g of protein) was calculated as follows:
where = weight of the dry sample (g), F1 = weight of the tube + dry sample (g), and F2 = weight of the tube + sediment (g).
Emulsifying Properties
Emulsifying activity and stability were determined as described by Pedroche et al.,[Citation14] with slight modification where 1.0 g sample was added with 30 mL distilled water and homogenized at 10,000 rpm for 5 min. The protein solution was mixed with 30 mL of sunflower oil and homogenized at 10,000 rpm for 5 min. Finally, the emulsion was centrifuged at 2000 rpm for 5 min and emulsion activity was calculated as follows:
The emulsion stability (ES) was determined by heating of emulsion for 30 min at 85°C,[Citation29,Citation51] followed by re-centrifugation at 3000 rpm for 10 min. The ES was expressed as follows:
Foaming Properties
The foaming capacity and stability were determined based on the methods described by Adebiyi and Aluko[Citation15] with slight modification where 5.0 g sample was dispersed in 50 mL of distilled water. The solution was stirred at 10,000 rpm for 5 min and the blend was immediately transferred to a 100 mL measuring cylinder. The foaming capacity was expressed in volume percentage due to stirring. Foaming stability was determined by measuring the foam volume after the standing condition of 30 min of storage.[Citation29,Citation51] The foaming capacity and stability were calculated as follows:
Differential Scanning Calorimetry (DSC) and Thermal Gravimetric Analysis (TGA)
Thermal denaturation of LPC (from Diplazium esculentum) was performed in (DSC-60 Thermal Analyzer Shimadzu, Japan), according to the methods of Guerrero et al.,[Citation16] with some modification. Sample (2 mg) was weighed into aluminum pans and heated from 30–200°C at a rate of 10°C/min. The denaturation temperature (Td), and enthalpy (were measured from thermograms by TA-60WS software (Shimadzu). Thermal stability was studied using thermogravimetric analyzer (TGA, Perkin Elmer STA 6000, USA). TGA measurements were performed in the range 50–900°C with nitrogen atmosphere (nitrogen flow rate of 20 mL/min) using heating rate of 20°C min−1.
Contact Angle
Static contact angle measurements were carried out using the sessile drop technique on gradients formed on flat silicon wafers. A small droplet (approximately 100 µL) of high-purity Milli-Q water (supplied by Elga UHQ water system), was deposited on a given position of the surface using a motorized syringe. The silhouette of the droplet was captured and imaged with a progressive scan CCD (Charge Couple Device) camera attached in the contact angle measurement set up (Kruss easydrop, OCA 15EC). The contact angle was determined by drawing a tangent close to the edge of the droplet at the three-phase point (using Image J software, Drop-Snake). Experiments were conducted at 22°C in a class-100 clean room.
Fourier Transform Infrared (FTIR) Spectroscopy
LPC was grounded with KBr at a ratio (1:100) and was pressed at a high pressure into KBr pellet. The spectral analysis was carried out using a FTIR spectrometer (Model-100 Perkin Elmer Spectrum, USA). The FTIR spectra of the sample were recorded in the range of 4000–400 cm−1 region at room temperature.
Results and Discussion
Total Protein Content
The total protein content of LPC of Diplazium esculentum extracted through sonication and non-sonication was found to be 34.28 and 9.89%, respectively (). The enhancement in extractability of protein yield for sonicated extraction might be attributed to the propagation of ultrasonic pressure resulting in cavitation phenomena. The energy released during the collapse of cavitation bubbles might have promoted higher penetration of the solvent into the cellular material which, in turn, improved the mass transfer to and from the interfaces.[Citation17–Citation19]
WAC
The ability of a protein matrix, such as protein particles, protein gels, or muscle, to imbibe and retain water against gravity is known as water holding capacity (WHC). In food systems, interactions with water and proteins are very important because it affects the flavor and texture of foods.[Citation20] WAC is dependent on various parameters such as size, configuration, conformational characteristics, hydrophobic and hydrophilic balance of the protein.[Citation21] WHC is an important processing parameter and has implications for viscosity, bulking, and consistency of products, as well as in baking applications. The WAC was found to be 8.36 and 7.65g water/g of protein concentrate for sonicated and non-sonicated LPC of Diplazium esculentum, respectively, and results () are comparatively higher than[Citation22,Citation23] Arthropteris orientalis (2.39 g water/g of protein concentrate), Nephrolepis biserrata (2.13 g water/g protein), and Kappaphycus alvarezii (Doty) an edible seaweed (2.22 g water/g of protein). According to Kinsella,[Citation24] an increase in the WHC is because of the ability of a protein to isolate, swell, and unfold, and thereby exposing additional binding sites, whereas the carbohydrate and other components of the protein concentrate may impair it.[Citation25] The pH of a system influences the WAC considerably due to changes in the surface charges of protein. When pH is altered from isoelectric point it results in increase of WAC by creating charge imbalance.
OAC
OAC was recorded to be 7.55 and 7.41 g of oil/g of protein with sonication and non-sonicated extraction, respectively (). The results of the present study are comparatively high[Citation22] with two ferns viz. Arthropteris orientalis (2.93 mL/g of protein) and Nephrolepis biserrata (2.73 mL/g of protein). However, it was relatively lower[Citation1,Citation5] to the chemical composition of common leafy vegetables and oil absoption capacity, in plants viz. Vernonia amygdalina, Solanum africana, Amaranthus hybridus, Telfaria occidentalis, Talinum triangulare, and Amaranthus cruentus. The plausible reasons of the increase in OAC of the LPC extracted by sonication may be attributed to a decrease in droplet size which increased the percentage of adsorbed proteins with the ultrasound treatment[Citation26] and it might have favored the adsorption of proteins and formation of aggregates.[Citation27]
Emulsion Activity and ES
The emulsion activity of sonicated and non-sonicated LPC of Diplazium esculentum was found to be 36.36 and 31.10%, respectively (), and are comparable[Citation1] with four plant species viz. Vernonia amygdalina, Solanum africana, Amaranthus hybridus, and Telfaria occidentalis, but higher than wheat flour.[Citation28] The ES of the present study was recorded to be 30.0% for sonicated and 25.0% for non-sonicated LPC. The increase in the emulsion activity of the sonicated sample was due to the influence of turbulent behavior produced by the ultrasound that resulted in the aggregation of oil bubbles in the emulsion.[Citation29] Overall, the results are comparatively lower than some common vegetables.[Citation1] The present study revealed that LPC of Diplazium esculentum can be used as an alternative for the stabilization of emulsions.
Foaming Properties
Foams are formed because of rapid diffusion of molecules in the interface followed by the molecular rearrangement which allows these films to entrap air.[Citation22] The foaming capacity of LPC of Diplazium esculentum was recorded 7.27 and 6.99% for sonicated and non-sonicated, respectively. The increase in foaming capacity might be ascribed to the homogenizing effect of ultrasound.[Citation30] The foaming stability after 30 min was 5.21 and 4.26% for sonicated and non-sonicated, respectively (), and the results are lower than the lima bean.[Citation31] Hence, the LPC of Diplazium esculentum may not be a prudent choice for whipping products, cakes, etc., where foaming is an important characteristic.[Citation24]
Thermal Properties
DSC properties
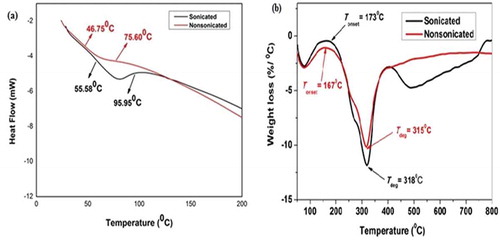
The protein sample was heated from 30 to 200°C at a rate of 10°C/min. As illustrated in , the LPC showed two observable denaturation temperatures at about 55.58 and 95.95°C for sonicated and 46.75 and 75.60°C for non-sonicated. The enthalpy change reflects the status of ordered confirmations of food proteins.[Citation32] Therefore, the net enthalpy change (ΔH) indicates cumulative effects of endothermic events like breakdown of hydrogen bonds and exothermic phenomenon such as aggregation of food proteins.[Citation33] The DSC of the present study revealed that the sonicated LPC shows more stability compared to non-sonicated, and is presumably afforded to prolonged sonication which increased the enthalpy of denaturation due to protein aggregation.[Citation34]
TGA properties
The TGA of both sonicated and non-sonicated LPC are elucidated in . The initial and final degradation temperatures were 173 and 167°C, and 318 and 315°C for sonicated and non-sonicated LPC, respectively. The decomposition temperature of LPC can be correlated with the TGA of soy protein whose initial and final decomposition temperatures were 260.86 (9.69% weight loss) and 356.68°C.[Citation35] The percentage weight loss observed was 6% at temperatures between 167 to173°C, and can be attributed to the loss of moisture with the degradation of major protein component.[Citation36] This observation clearly shows that sonicated LPC has higher thermal stability compared to the non-sonicated.
Contact angle measurements
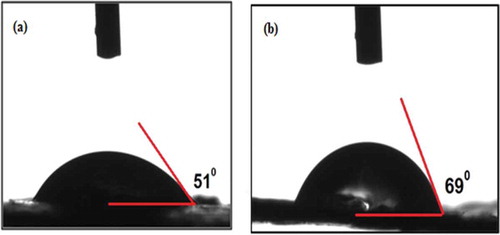
Contact angle measurements revealed the surface hydrophilicity or hydrophobicity. The measured values of contact angles of the samples before and after sonication exhibited that both the angles were less than 90° () which proved that the samples are hydrophilic in nature. It is emphasized that the system becomes conventionally hydrophilic, if the contact angle is less than 90°.[Citation37] From the results it was noted that ultrasound increased the surface area which in turn increased the wettability and as a result there was increase in the other physicochemical behavior such as WHC and foaming property.[Citation29]
FTIR Analysis
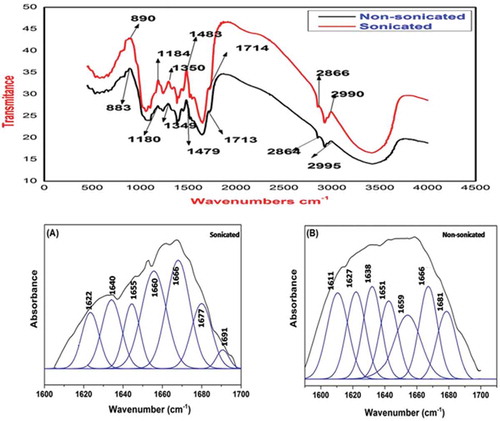
FTIR analysis was performed with and without sonication in LPC of Diplazium esculentum and the measurements was recorded between 4000 to 400 cm−1. Determination of secondary structure of protein is one of the major applications of the FTIR methods.[Citation38] The IR spectral bands are assigned and described in . Proteins are generally known to have different fraction of structural components (α-helix, β-sheet, random coil, etc.); however, the secondary structural composition is most important information for the structural elucidation of unknown protein. The spectrum of sonicated and non-sonicated LPC of Diplazium esculentum presented in showed the bands at 2995, 2990, 2864, and 2866 cm−1, which could be due to CH2 and CH3 asymmetric stretching and are found in aliphatic side chain of the protein. The five major peaks at 1654, 1637, 1525, 1565, 1292, and 1655, 1636, 1522, 1554, 1288 cm−1 are assigned to be as amide I, amide II, and amide III,[Citation38] respectively, that are found both in the sonicated and non-sonicated LPC. These three peaks correspond to C=O stretching, C-N stretching, NH-bending and CH3 bending vibration of the protein. Similar bands were also reported in soy protein using aqueous extraction methods.[Citation39] The bands at 1350, 1391, 1637, 1655, and 1714 cm−1 for sonicated LPC has shifted to 1349, 1390, 1636, 1654, and 1714 cm−1 for non-sonicated LPC and there was no major difference in their position. Apart from that, the bands at 890, 1090, 1184, 1292, 1434, 1484, 1525, 2866, and 2995 cm−1 for sonicated LPC had shifted to lower frequency of 883, 1087, 1180, 1288, 1427, 1479, 1422, 2864, and 2990 cm−1 for non-sonicated LPC. The shifting might be due to out of plane/N-H bending, C-O stretch of α-anomer, OH and CO group stretching vibration, CN stretching, NH bending, and CH2-CH3 asymmetric stretching of proteins. The results have the credence to support that, there was hydrostatic and electrostatic interactions in sonicated and non-sonicated LPC[Citation40] and which affects the structure of the protein.
Table 1. Functional properties parameters of sonicated and non-sonicated LPC of Diplazium esculentum.
Table 2. FTIR analysis of sonicated and non-sonicated LPC of Diplazium esculentum.
The deconvoluted spectra of Diplazium esculentum protein concentrate using sonication and non-sonication methods are presented in and . In the present study, the spectral assignment and calculation have been performed by second derivative infrared spectra (peak fit method). The frequencies of seven bands in non-sonication shifted when LPC was extracted under sonication. The lower or higher wave numbers reflect the secondary structure of the protein, which may be attributed to the stronger interaction with alkali or aqueous medium of the protein molecule. This, in turn, increased the effects of H-bonds, and decreased the frequency of amide I band by shifting of bands, which affected the strong or weak H-bonds, easily formed between C=O and N-H stretch of amide I bands. If the shifting of bands takes place toward the higher side, the hydrogen bonds get weakened.[Citation41–Citation43]
According to Byler[Citation44] the bands at 1650–1660 cm−1 are assigned to α-helix. The bands at 1655, 1660 cm−1 and 1651, 1659 cm−1 were found in both the extraction methods. The shifting of higher wave numbers may be due to addition of H-bond formed in sonicated LPC. The frequency regions of 1610–1640 and 1670–1680 cm−1 have been assigned to β-sheet structure.[Citation44] Three bands were observed ( and ) in both the extraction processes. In sonicated LPC of Diplazium esculentum the bands at 1622, 1640, 1677 cm−1 had shifted toward higher frequency than non-sonicated one. Increment in band intensity may be due to protein unfolding, which increased the protein-protein interaction.[Citation45] Similarly, in turn a structure (1660–1700 cm−1) band of sonicated LPC was also in higher frequency end compared to the non-sonicated. Interestingly, the bands at 1666 cm−1 did not change its position in both the extraction methods ( and ) and no unordered structure was found in both the cases, which may be due to changes in the H-bon.[Citation46]
Using second-derivative analysis of infrared-standard deviation (IR-SD) direct quantitative analysis of the secondary structure of the Diplazium esculentum proteins has been obtained. The areas assigned as amide I bands in second derivative spectra ( and ) correspond to the amount of different types of secondary structure in the protein. Quantitative analysis of secondary structure of protein is summarized in . Comparison of the structure of LPC of Diplazium esculentum proteins extracted with non-sonicated and sonicated methods revealed that the percentage of α-helix decreased on sonication (49.06%) while the percentage of β-sheet (33.59%) and turn (42.02%) structures increased. The changes of secondary structure of Diplazium esculentum protein viz. α-helix, β-sheet, and turn might affect the structural conformation and functional properties of Diplazium esculentum protein.[Citation43]
Table 3. Quantification of secondary structure of protein of Diplazium esculentum.
Conclusion
Sonication exhibited a considerable influence on extraction of protein content from Diplazium esculentum and various functional properties viz. WHC, oil holding capacity, foaming property, and emulsifying property. A deconvoluted FTIR graph revealed improvement in secondary structures, coupled with the enhancement in functional properties of sonicated LPC over non-sonicated. Contact angle experiments affirmed more increase in hydrophilicity of sonicated LPC compared to its counterpart. Thermal behavior of the sonicated extraction LPC proved better, compared to non-sonication. Results of the present investigation has credible evidence to support that sonicated extraction of LPC of Diplazium esculentum phenomenally improved the yield of protein content and functional and thermal behavior over its counterpart.
Funding
Financial support was provided by Ministry of Food Processing Industries (MOFPI) through Science & Engineering Research Board (SERB), Department of Science & Technology (Govt. of India), New Delhi, and has been duly acknowledged.
Additional information
Funding
References
- Aletor, O.; Oshodi, A.A.; Ipinmoroti, K. Chemical Composition of Common Leafy Vegetables and Functional Properties of their Leaf Protein Concentrates. Food Chemistry 2002, 78(1), 63–68.
- Agbede, J. O.; Adegbenro, M.; Aletor, O.; Mohammed, A. Evaluation of the Nutrition Value of Vernonia Amygdalina Leaf Protein Concentrates for Infant Weaning Foods. Acta Alimentaria 2007, 36(3), 387–393.
- Aletor, O. Nutritive and Physico-Chemical Characteristics of Some Plants and Animal Based Protein Concentrates. International Journal of Chemical Sciences Nassarawa 2010, 2, 155–165.
- Aletor, V.A.; Adeogun, O.A. Nutrient and Antinutrient Components of Some Tropical Leafy Vegetables. Food Chemistry 1995, 53(4), 375–379.
- Fasuyi, A.O. Bio-Nutritional Evaluations of Three Tropical Leafy Vegetables (Telfairia Occidentalis, Amaranthus Cruentus and Talinum Triangulare) as Sole Dietary Protein Sources in Rat Assay. Food Chemistry 2007, 103(3), 757–765.
- Copeland, E.B. Edible Ferns. American Fern Journal. 1942, 32(4), 121–126.
- Getachew, A.G.; Asfaw, Z.; Singh, V.; Woldu, Z.; Baidu-Forson, J.J.; Bhattacharya, S. Dietary Values of Wild and Semi-Wild Edible Plants in Southern Ethiopia. African Journal of Food, Agriculture, Nutrition and Development 2013, 13(2), 7485–7503.
- Dhar, S. Socio-Economic and Demographic Status of Assam: A Comparative Analysis of Assam with India. International Journal of Humanities & Social Science Studies 2014, 1(3), 108–117.
- Fellows, P. Village-Scale Leaf Fractionation in Ghana. Tropical Science 1987, 27, 77–84.
- Aletor, V.A. Cyanide in Garri. 1. Distribution of Total, Bound and Free Hydrocyanic acid in Commercial Garri, and the Effect of Fermentation Time on Residual Cyanide Content. International Journal of Food Sciences and Nutrition 1993, 44(4), 281–287.
- Wathelet, B. Nutritional Analyses of Proteins and Amino Acids in Beans (Phaseolus sp.) Biotechnology, Agronomy, Society and Environment 1999, 3, 197–200.
- Rodriguez-Ambriz, S.L.; Martinez-Ayala, A.L.; Millan, F.; Davila-Ortiz, G. Composition and Functional Properties of Lupinus Campestris Protein Isolates. Plant Foods for Human Nutrition 2005, 60(3), 99–107.
- Lin, C.S.; Zayas, J.F. Functionality of Defatted Corn Germ Proteins in a Model System Fat Binding Capacity and Water Retention. Journal of Food Science 1987, 52(5), 1308–1311.
- Pedroche, J.; Yust, M.M.; Lqari, H.; Giron-Calle, J.; Alaiz, M.; Vioque, J.; Millan, F. Brassica Carinata Protein Isolates: Chemical Composition, Protein Characterization and Improvement of Functional Properties by Protein Hydrolysis. Food Chemistry 2004, 88(3), 337–346.
- Adebiyi, A.P.; Aluko, R.E. Functional Properties of Protein Fractions Obtained from Commercial Yellow Field Pea (Pisum Sativum L.) Seed Protein Isolate. Food Chemistry 2011, 128(4), 902–908.
- Guerrero, P.; Retegi, A.; Gabilondo, N.; De la Caba, K. Mechanical and Thermal Properties of Soy Protein Films Processed by Casting and Compression. Journal of Food Engineering 2010, 100(1), 145–151.
- Knorr, D. Impact of Non-Thermal Processing on Plant Metabolites. Journal of Food Engineering 2003, 56(2), 131–134.
- Li, H.; Pordesimo, L.; Weiss, J. High Intensity Ultrasound-Assisted Extraction of Oil from Soybeans. Food Research International 2004, 37(7), 731–738.
- Vinatoru, M. An Overview of the Ultrasonically Assisted Extraction of Bioactive Principles from Herbs. Ultrasonics Sonochemistry 2001, 8(3), 303–313.
- Kanu, P.J.; Kerui, Z.; Ming, Z.H.; Haifeng, Q.; Kanu, J.B. Kexue, Z. Functional Properties of Sesame (Sesamum Indicum L.) Protein Isolate as Influenced by pH, Temperature, Time and Ratio of Flour to Water During Its Production. Asian Journal of Biochemistry 2007, 2(5), 289–301.
- Chavan, U.D.; McKenzie, D.B. Shahidi, F. Protein Classification of Beach Pea. In Influence of pH on the Extraction Yield and Functional Properties of Macadamia (Macadamia Integrofolia) Protein Isolates. Food Science and Technology 2001, 10, 263–267.
- Essuman, E.K. Thesis on Protein Extraction from Fern and its Physicochemical Properties. Kwame Nkrumah University of Science and Technology, College of Science, Accra Road, Kumasi, Ghana, 2013; 85.
- Kumar, K.S.; Ganesan, K.; Selvaraj, K.; Rao, P.S. Studies on the Functional Properties of Protein Concentrate of Kappaphycus Alvarezii (Doty) Doty–Edible Seaweed. Food Chemistry 2014, 153, 353–360.
- Kinsella, J.E. Functional Properties of Soy Proteins. Journal of the American Oil Chemists Society 1979, 56(3), 242–258.
- Bandyopadhyay, K.; Ghosh, S. Preparation and Characterization of Papain-Modified Sesame (Sesamum Indicum L.) Protein Isolates. Journal of Agricultural and Food Chemistry 2002, 50(23), 6854–6857.
- Caessens, P.W.; De Jongh, H.H.; Norde, W.; Gruppen, H. The Adsorption Induced Secondary Structure of β-Casein and of Distinct Parts of Its Sequence in Relation to Foam and Emulsion Properties. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology 1999, 1430(1), 73–83.
- Hailing, P.J.; Walstra, P. Protein‐Stabilized Foams and Emulsions. Critical Reviews in Food Science and Nutrition 1981, 15(2), 155–203.
- Lin, M.J.; Humbert, E.S.; Sosulski, F.W. Certain Functional Properties of Sunflower Meal Products. Journal of Food Science 1974, 39(2), 368–370.
- Anet, R.J.; Vesna, L.; Timothy, J.M.; Greta, K.; Marija, B. Physical Properties of Ultrasound Treated Soy Proteins. Journal of Food Engineering 2009, 93, 386–393.
- Lomakina, K.; Mikova, K. A Study of the Factors Affecting the Foaming Properties of Egg White: A Review. Czech Journal of Food Science 2006, 24(3), 110–118.
- Oshodi, A.A.; Adeladun, M.O.A. Proximate Composition, Some Nutritionally Valuable Minerals and Functional Properties of Three Varieties of Lima Bean (Phaseolus Lunatus L.) Flour. International Journal of Food Sciences and Nutrition 1993, 43(4), 181–186.
- Koshiyama, I.; Hamano, M.; Fukushima, D. A Heat Denaturation Study of the 11S Globulin in Soybean Seeds. Food Chemistry 1981, 6(4), 309–322.
- Murray, E.D.; Arntfield, S.D.; Ismond, M.A.H. The Influence of Processing Parameters on Food Protein Functionality II. Factors Affecting Thermal Properties as Analyzed by Differential Scanning Calorimetry. Canadian Institute of Food Science and Technology Journal 1985, 18(2), 158–162.
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashok, K.M. Effects of Ultrasound on the Thermal and Structural Characteristics of Proteins in Reconstituted Whey Protein Concentrate. Ultrasonics Sonochemistry 2011, 18(5), 951–957.
- Kaur, I.; Gautam, N. Synthesis and Characterization of Soy Protein Grafted Polyethylene: Effect of Reaction Parameters. Malaysian Polymer Journal 2010, 5(1), 39–54.
- Suhaimibin, Y.M.D. Thesis on Role of Protein Cross Linking in Soy Food Texture, University of Canterbury, Christchurch, New Zealand, 2005, 167.
- Forch, R.; Schonherr, H.; Jenkins, A.T.A. Surface Design: Applications in Bioscience and Nanotechnology. John Wiley and Sons: Weinheim, Germany, 2009.
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochimica et Biophysica Sinica 2007, 39(8), 549–559.
- Chen, X.; Ru, Y.; Chen, F.; Wang, X.; Zhao, X.; Ao, Q. FT-IR Spectroscopic Characterization of Soy proteins Obtained through AOT Reverse Micelles. Food Hydrocolloids 2013, 31(2), 435–437.
- Correa, N.M.; Durantini, E.N.; Silber, J.J. Binding of Nitrodiphenylamines to Reverse Micelles of AOT in-Hexane and Carbon Tetrachloride: Solvent and Substituent Effects. Journal of Colloid and Interface Science 1998, 208(1), 96–103.
- Tcholakova, S.; Denkov, N.D.; Ivanov, I.B.; Campbell, B. Coalescence Stability of Emulsions Containing Globular Milk Proteins. Advances in Colloid and Interface Science 2006, 123, 259–293.
- Zhao, X.; Chen, F.; Chen, J.; Gai, G.; Xue, W.; Li, L. Effects of AOT Reverse Micelle on Properties of Soy Globulins. Food Chemistry 2008, 111(3), 599–605.
- Zhao, X.; Ao, Q.; Chen, F.; Xue, W.; Li, L.; Liu, J. Effect of Reverse Micelle on Conformation of Soy Globulins: A Raman Study. Food Chemistry 2009, 116(1), 176–182.
- Byler, D.M.; Susi, H. Examination of the Secondary Structure of Proteins by Deconvolved FT-IR Spectra. Biopolymers 1986, 25(3), 469–487.
- Fragoso, A.; Pacheco, R.; Karmali, A. Investigation of Structural Effects and Behaviour of Pseudomonas Aeruginosa Amidase Encapsulated in Reversed Micelles. Process Biochemistry 2012, 47(2), 264–272.
- Bu, G.; Yang, Y.; Chen, F.; Liao, Z.; Gao, Y.; Yang, H.; Zhao, J. Extraction and Physicochemical Properties of Soya Bean Protein and Oil by a New Reverse Micelle System Compared with Other Extraction Methods. International Journal of Food Science and Technology 2014, 49(4), 1079–1089.
- Pan, L.G.; Tomas.; M.C. Anon. Oil-in-Water Emulsions Formulated with Sunflower Lecithins: Vesicle Formation and Stability. Journal of the American Oil Chemists’ Society 2004, 81(3), 241–244.
- Souza, C.J.F.D.; Rojas, E.E.G. Emulsion of Systems Containing Egg Yolk, Polysaccharides and Vegetable Oil. Ciencia e Agrotecnologia 2012, 36(5), 543–550.
- Farr, W.E. Refining of Fats and Oils. Introduction to Fats and Oils Technology, 2nd ed.; AOCS: Champaign, IL, 2000; 136–157.
- Chowdhury, K.; Banu, L.A.; Khan, S.; Latif, A. Studies on the Fatty Acid Composition of Edible Oil. Bangladesh Journal of Scientific and Industrial Research 2007, 4(3), 311–316.
- Yasumatsu, K.; Sawada, K.; Moritaka, S.; Misaki, M.; Toda, J.; Wada, T.; Ishii, K. Whipping and Emulsifying Properties of Soybean Products. Agricultural and Biological Chemistry 1972, 36(5), 719–727.