ABSTRACT
The aim of this article was to study the influence of Saccharomyces cerevisiae strains on the chemical composition and sensory properties of mulberry wines. For this purpose, three Saccharomyces cerevisiae strains, YJM 681 (isolated from raspberry), ySR 127 (commercial yeast) and Y1 (isolated from kefir fermented milk) were used for the alcoholic fermentation of mulberry juice. Yeast strains had deep influences on oenological, phenolic, amino acid, volatile and sensory profiles of resulting mulberry wines. Specifically, the beverages fermented with YJM 681 were characterised by relatively high volatile acidity and high contents of total flavonols, amino acids and some phenolic acids, including protocatechuic acid, p-hydroxybenzoic acid, caffeic acid and veratric acid. Samples fermented with ySR 127 possessed higher cyanidin-3-O-glucoside content, whereas, total amino acid content was markedly lower than that of other samples. Furthermore, mulberry wines fermented with Y1 showed the lowest volatile acidity and the lowest contents of total anthocyanins and tartaric esters. The amounts of major aromatic compounds, including 1-propanol, 2-methyl-1-propanol and ethyl acetate inside were also smaller than that of samples fermented with YJM 681 and ySR 127. Besides, the sensory results revealed that mulberry wines fermented with Y1 exhibited the lowest intensity of fruity aroma, while samples fermented with ySR 127 had the strongest sour and sweet tastes and aftertaste. These findings provide fundamental knowledge about the influence of yeast strains on the quality of mulberry wine.
Introduction
Mulberry (Morus alba L.), belonging to the Morus genus and Moraceae family, is widely planted in tropical, subtropical and temperate areas of north hemisphere, as well as tropical areas of south hemisphere. [Citation1] Mulberry fruits are recognised as a kind of berry fruit with high nutritional value, due to their rich contents of bioactive compounds, such as phenolics and polysaccharides. [Citation2,Citation3] Many scientific articles have demonstrated that mulberry exhibited excellent antioxidant, anti-aging, anti-obesity, anti-inflammatory, anti-cancer, hypoglycemic and hypolipidemic properties. [Citation4,Citation5]
Although mulberry is famous for rich nutrition and succulence, it has a short harvest season and is susceptible to spoilage during storage and transportation. [Citation1] To make full use of this fruit, mulberry is usually processed into various products, including juice, jam, syrup, vinegar and alcoholic beverage. [Citation1,Citation6] There are different types of mulberry-related alcoholic beverages, such as mulberry wine [Citation6], Korean traditional alcoholic beverage Yakju enriched with mulberry [Citation7], a distillate produced by solid-state fermentation of black mulberry in Galicia. [Citation8] As a competitive fruit wine variety, the production of mulberry wine can boom the fruit wine market.
Mulberry wine is less studied compared with the popular fruit wine, namely grape wine. According to the literature review, the researches about mulberry wine are mainly concentrated on analyzing the aromatic profile, colour and antioxidant ability [Citation9,Citation10], comparing wine quality produced from different mulberry cultivars [Citation6], optimizing alcoholic fermentation process [Citation11], and studying the dynamic changes of phenolic compounds and antioxidant activity during alcoholic fermentation. [Citation1] On the other hand, the studies about various fruit wines revealed that Saccharomyces cerevisiae yeast strains used for alcoholic fermentation had a deep influence on wine colour and phenolic, aromatic compounds and amino acid profiles. [Citation12–Citation16] Yeast metabolism during fermentation contributes a lot to the final properties of wine appearance, taste and aroma. However, the influence of Saccharomyces cerevisiae strains on the physicochemical and sensory properties of mulberry wine is still unclear. To produce high-quality mulberry wines, it is of importance to investigate the effect of yeast strains on the different aspects of mulberry wine quality.
Therefore, in this study, three Saccharomyces cerevisiae strains, including one commercial yeast, one isolated from raspberry and the other one isolated from kefir fermented milk were used for mulberry winemaking. The oenological, phenolic, amino acid, volatile and sensory profiles of mulberry wines were compared. These results can enrich the knowledge about the yeast influence on mulberry wine quality, as well as provide guidance about producing high-quality mulberry wines.
Materials and methods
Yeast strains
Three Saccharomyces cerevisiae yeasts were used. Among them, one commercial yeast Saccharomyces cerevisiae ySR 127 was purchased from Chr. Hansen (Hoersholm, Denmark). Another yeast named Saccharomyces cerevisiae YJM 681 was isolated from raspberries grown in the plantation of Bilushan Agriculture Co. Ltd. (Lishui, Nanjing, China). Furthermore, one yeast named Saccharomyces cerevisiae Y1 was isolated from the homemade kefir fermented milk in Yongjing, Gansu, China. All these yeasts were kindly provided by the Laboratory of Food Microbiology and Bioengineering, Institute of Agro-product processing, Jiangsu Academy of Agricultural Sciences. Yeasts were activated in YPD broth (10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose with 20% glycerol) for 48 h before inoculation.
Vinification process
The vinification process was carried out following the procedures used by Duarte et al. [Citation13] and Juan et al. [Citation6] with some modifications. To be exact, mature mulberry fruits, cultivar Husang, were collected from the mulberry plantation belonging to Jiujiu Silk Co. Ltd. (Suqian, China). Then mulberry fruits were crushed using a domestic juice extractor (JYZ-E6, Jiuyang, Hangzhou, China). Juices were separated from pulp through centrifugation at 8000 rpm for 15 min.
Next, sucrose and potassium metabisulfite were added to mulberry juice, so as to making juice Brix value 22 and potassium metabisulfite concentration 120 mg/L. Afterwards, the pH value was adjusted to 3.5 through addition of critic acid. Mulberry juices were inoculated with yeast preculture to an initial concentration of (1–2)×106 cfu/mL. Fermentation was performed in 1-L Erlemeyer flasks under static conditions at 25°C. Once the total sugar content was lower than 5.0 g/L, mulberry wines were centrifuged at 8000 rpm for 10 min. Samples were transferred to glass bottles filled completely and stored at 10°C in darkness. The vinification processes were repeated in triplicate.
Chemical analysis
Analysis of oenological parameters
Alcohol degree, total acidity, volatile acidity, pH, total SO2 content, free SO2 content were determined using the OIV official analytical methods. [Citation17] Total sugar content was measured using the anthrone-sulfuric acid method [Citation6] and the results were expressed as g/L of glucose equivalents.
Spectrophotometric measurements of phenolic families, colour and antioxidant activity
Total phenolic content was determined using the Folin-Ciocalteu method and the results were expressed as g/L of gallic acid equivalents. [Citation18] Total anthocyanin content was assayed by a spectrophotometric method described by Ivanova et al. [Citation19] and the results were expressed as mg/L of malvidin-3-glucoside equivalents. Another spectrophotometric method described by Cliff et al. [Citation20] was used to measure the contents of tartaric esters and flavonols. The contents of tartaric esters and flavonols were expressed as mg/L of caffeic acid equivalents and quercetin equivalents, respectively. A quartz cuvette with the path length of 1 cm was used.
Monomeric, copigmented, polymeric and total anthocyanin contents were determined using the colourimetric effects of SO2 and acetaldehyde on different forms of anthocyanins. [Citation20] All the results were expressed in absorbance units (AU). The absorbance was measured in a 1 mm path-length quartz cuvette and then corrected for 1 cm cuvette. For the determination of wine colour, the absorbances at 420, 520 and 620 nm were measured with a 1 mm path-length quartz cuvette. The readings were then corrected for a path length of 1 cm. Colour intensity (A420 + A520 + A620), tint (A420/A520), and percentages of red (%Rd), yellow (%Ye) and blue (%Bl) colours were calculated. [Citation21]
Wine antioxidant activities were evaluated using both 2, 20 -azinobis-(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) and ferric reducing antioxidant power (FRAP) assays. ABTS assay was used to assess the free radical scavenging activity of wine samples [Citation22], while FRAP assay was performed to measure the reducing power of wine samples. [Citation23] For ABTS assay, Trolox was used as the standard and the results were expressed as Trolox equivalent antioxidant capacity (mmol/L). For FRAP assay, FeSO4 solution was used to make the calibration curve. The results were expressed as mmol/L of Fe2+. A quartz cuvette with the path length of 1 cm was used.
Identification of individual phenolic compounds
Individual phenolic compounds in mulberry wines were identified by high resolution mass spectrometry (HRMS). The identification process was performed using an ultrahigh performance liquid chromatography (UHPLC, Nexera X2, SHIMADZU, Japan) system coupled to a Triple-TOF-MS system (Triple TOF 4600, AB SCIEX, USA) with the DuoSprayTM ionisation source. The DuoSprayTM was composed of an electrospray (ESI) and an atmospheric pressure chemical ionisation (APCI) inlet. An Ultimate XB-C18 column (100 × 2.1 mm, 3 μm) was used for UHPLC separation. The mobile phases consisted of (A) 10 mmol/L ammonium formate solution containing 0.1% formic acid and (B) acetonitrile. A gradient program at a flow rate of 0.4 mL/min was used as follows: 0–1 min 5% B, 1–15 min 5–40% B, 15–20 min 40–70% B, 20.1 min 90% B, 20.1–25 min 90% B, 25.1 min 5% B, 25.1–30 min 5% B. The column oven temperature was 30°C. For MS/MS detection, the positive and negative ion modes were operated separately. The APCI probe of the DuoSprayTM source was employed for automatic mass calibration by the Calibrant Delivery System (CDS). A calibration solution that matched ionisation polarity was injected during CDS. Correspondingly, the mass axis of the Triple-TOF-MS system was calibrated in each scan function used (MS and MS/MS). Solutions were infused from ESI source at 10 μL/min. The working parameters were listed as follows: ion spray voltage floating, 4500 V; ion source gas 1, 55 psi; ion source gas 2, 55 psi; curtain gas, 30 psi; temperature, 550°C. Nitrogen was utilised as both curtain gas and ion source gas. Furthermore, the collision energy and collision energy spread in positive ion mode were 45 and 15 V, respectively. In negative ion mode, the collision energy and collision energy spread depended on specific compounds. Data analysis was carried out in MultiQuantTM software (AB SCIEX, USA) and PeakViewTM software with the XIC Manager.
Quantification of individual phenolic compounds
Anthocyanins were analysed by HPLC directly using the method of Cui et al. [Citation24] A HPLC system (Agilent 1200; GMI, Ramsey, MN, USA) equipped with an Agilent TC-C18 column (4.6 × 250 mm, 5 μm) was used. Samples were detected at 520 nm using UV-Vis diode-array absorbance detection and the column temperature was 30°C. Mobile phase A was deionised water with 0.5% trifluoroacetic acid, while mobile phase B was acetonitrile. A gradient program at a flow rate of 0.8 mL/min was used as follows: 0–5 min, 10–12% B; 5–14 min, 12–13%B; 14–16 min, 13–14%B; 16–18 min, 14–16%B; 18–19 min, 16–18%B; 19–22 min, 18–22%B; 22–35 min, 22–30%B. The injection volume was 20 μL.
Non-anthocyanin phenolics were extracted from mulberry wines before HPLC analysis following the method of Juan et al. [Citation6] For the extraction of phenolic acids, samples were first acidified to pH 2.0 to make free phenolates protonated. Next, the suspension was saturated using NaCl and extracted with ethyl acetate (1:1, v:v) for 12 h. The extraction process was repeated for three times to ensure the complete recovery of phenolic acids. The collected three organic layer fractions were mixed and solvents were removed by rotary evaporation. The dried residues were dissolved in methanol and then filtered for subsequent HPLC analysis.
For the extraction of flavonols, the pH of mulberry wines was first adjusted to 7.0. Next, flavonols were extracted using ethyl acetate (1:1, v:v) for 12 h. The extraction was performed in triplicates and the organic phases were mixed together. Rotary evaporation was used to remove solvents. Finally, the dried residues were suspended in methanol and filtered.
HPLC analysis of phenolic acids and flavonols was performed in the aforementioned HPLC system coupled with an Agilent Zorbax Eclipse SB-C18 column (4.6 × 250 mm, 5 μm). The mobile phases were composed of deionised water with 1% trifluoroacetic acid (A) and methanol with 1% acetic acid (B). A gradient program at a flow rate of 1 mL/min was used as follows: 0–10 min, 10–26%B; 10–25 min, 26–40%B; 25–45 min, 40–65%; 45–55 min, 65–95%B; 55–58 min, 95–10%B; 58–61 min, 10%B. Phenolic acids and taxifolin were detected at 280 nm, while other flavonols were detected at 350 nm. The injection volume was 20 μL. The quantification was made according to the calibration curves of standards of identified phenolic compounds.
Analysis of free amino acids
Contents of free amino acids in mulberry wines were determined according to the procedures of Aro et al. [Citation25] Samples were mixed with 4% trichloroacetic acid at a ratio of 1:1 (v:v) and then incubated at 37°C for 30 min. After that, samples were centrifuged at 15000 rpm for 30 min. The supernatant was collected and analysed by a fully automated amino acid analyser HITACHI L-8900 (Hitachi Ltd., Japan).
Analysis of volatile compounds
Volatile profile of mulberry wines was analysed by purge-and-trap-gas chromatography-mass spectrometry according to the method of Aznar and Arroyo [Citation26] with some modifications. To start with, volatile compounds were extracted in a purge-and-trap (P&T) sample enrichment system (US14121004, Teledyne Tekmar, USA) controlled by Atomax Teklink software. The P&T extraction conditions were: sample/water dilution (v:v) 1:4, sample volume 5 mL, purge flow 40 mL/min of N2, purge time 11.0 min (at ambient temperature), desorption time 5 min (at 250°C). To ensure the complete cleaning of the system, water sample was extracted between mulberry wine samples.
After extraction, samples were analysed by GC-MS in an Agilent 7890B (USA) gas chromatography coupled to an Agilent 5977A mass spectrometer (USA). The Agilent J&W DB-624 ultra-inert capillary column (30 m × 250 μm×1.4 μm) was used. Helium was used as the carrier gas at 1 mL/min. The temperature program was shown as: 35°C for 2 min, then raised to 120°C at 5°C/min, further increased to 220°C at 10°C/min, and lastly held at 220°C for 2 min. The detection was performed by electron impact mass spectroscopy in total ion current (TIC) mode with the ionisation energy of 70 eV. The analysis was carried out in a scan range between m/z 35–550. Volatile compounds were identified based on comparison of obtained mass spectra with the mass spectra from MS databases and literature. Linear retention index of each compound was also calculated and compared with those reported in the literature. Furthermore, the peak area of each compound in ion chromatograms was calculated to represent the relative amount of each volatile compound.
Sensory analysis
Sensory analysis was performed following the procedures described by Tao et al.. [Citation27] The sensory panel was composed of seven judges (2 males and 5 females) from Shanghai Moutai Haima Enterprise Development Co., Ltd., which is a company specialised in international wine trading. All the panelists had attended the training courses organised by the famous Wine & Sprit Education Trust (WSET). Two of them had already received the WSET Level 3 Award in Wines before joining the tasting sessions in this study. Therefore, the judges were familiar with the sensory properties of various types of fruit wines, as well as the intensity rating of sensory descriptors.
Before evaluation sessions, a round-table session was held to select the descriptors about mulberry wines under investigation. Wines were assessed in three aspects: appearance (colour, clarity, tears and overall appearance), aroma (complex, fruity, roast, alcoholic, sour, olfactory intensity and overall aroma) and taste (body, alcoholic, fruity, sweet, sour, tannic, bitter, aftertaste, texture and overall taste). These attributes were selected by consensus to describe mulberry wines. The intensity of each attribute was rated using a 10-cm unstructured scale. The left side represented the lowest intensity of each sensory attribute and the right side denoted the highest intensity. Samples were presented in ISO wine glasses covered with plastic lids, following a complete randomised design. [Citation28] In the interval of each tasting, the panelists were asked to cleaning their palates using spring water.
Statistical analysis
All the chemical and sensory analysis was carried out in duplicates. One-way analysis of variance (ANOVA) was performed in SPSS 11.5 (SPSS, Inc., USA) to compare the means of different parameters of mulberry wines. Significance was defined at p = 0.05 and the Least Significant Difference (Fisher’s LSD) test was used to separate the mean differences. Furthermore, principal component analysis (PCA) was performed in Minitab 16.1.1 (Minitab, Inc., USA) to reveal any grouping of mulberry wines produced by different Saccharomyces cerevisiae strains based on chemical data.
Results and discussion
Basic oenological parameters of mulberry wines
Mulberry juices were fermented by Saccharomyces cerevisiae YJM 681, ySR 127 and Y1 for 7 days, separately. The basic oenological parameters of mulberry juice and the resulting mulberry wines are summarised in . As can be seen, the total sugar contents in mulberry wines fermented with different Saccharomyces cerevisiae strains all decreased below 5.0 g/L on the 7th day of fermentation, indicating the high fermentative capability of yeast strains under investigation. Alcoholic fermentation of mulberry juice resulted in the increase of both total acidity and volatile acidity, suggesting that alcoholic fermentation had a profound influence on the profile of organic acids in mulberry juices and new organic acids were formed. [Citation29]
Table 1. Basic oenological parameters, contents of phenolic families, colour and antioxidant activity of mulberry juice and mulberry wines fermented with different yeast strains.
All the values of basic oenological parameters for mulberry wines under investigation stayed within the ranges acceptable to fruit wine industry. [Citation6] The alcoholic beverages fermented with Y1 isolated from kefir fermented milk were characterised by higher pH and lower volatile acidity compared with other samples. The volatile acidity of alcoholic beverages produced by Saccharomyces cerevisiae usually ranges from 0.25 to 0.50 g/L [Citation30], whereas the volatile acidity of samples fermented with Y1 was merely 0.20 ± 0.01 g/L. Volatile acidity of mulberry wines fermented with YJM 681 was the highest, being 0.39 ± 0.01 g/L. Meanwhile, mulberry wines fermented with YJM 681 possessed the lowest total SO2 content (65.57 ± 0.98 mg/L), while there was no significant (p ≥ 0.05) difference in free SO2 content among all the mulberry wine samples. Moreover, the alcoholic degree in mulberry wines fermented with YJM 681 was lower than that of other wine samples. In recently years, consumers’ demand for fruit wines with moderate alcohol degree is increasing continuously. [Citation31,Citation32] Thus, YJM 681 can be a suitable Saccharomyces strain to reduce ethanol content in mulberry wines.
Phenolic family, colour and antioxidant activity of mulberry wines
Mulberry wine is rich in various phenolic compounds with antioxidant activity. One of the important phenolic families, namely anthocyanins, provides products attractive red colour. The contents of phenolic families, colour properties and antioxidant activity of mulberry juice and mulberry wines fermented with different Saccharomyces cerevisiae strains are also shown in .
Generally, alcoholic fermentation affected the phenolic composition, colour properties and antioxidant capacity of mulberry juice significantly. After fermentation for 7 days, there was a decreasing trend about total phenolics, total anthocyanins, tartaric esters and flavonols. Especially for total anthocyanins, the original total anthocyanin content in mulberry juice was 1136.0 ± 3.0 mg/L, whereas the total anthocyanins in mulberry wines fermented by three yeasts were all below 480 mg/L. This result was reasonable since anthocyanins usually experience a degradation and conversion process during fermentation, leading to the decrease of anthocyanin content. [Citation1] Owing to the decrease of anthocyanin content, both the colour intensity and percentage of red colour declined after fermentation. Furthermore, the antioxidant capacities of the three mulberry wines were all higher than that of mulberry juice. Similar results were also found after alcoholic fermentation of mulberry juice and strawberry purée. [Citation1,Citation33] It was supposed that bio-conversion of phenolics during fermentation may result in the formation of phenolic derivatives with high antioxidant capacity. [Citation34,Citation35]
On the other hand, the contents of phenolic families, colour parameters and antioxidant capacity of mulberry wine were dependent on the yeast strains used for alcoholic fermentation. Although all the mulberry wine samples had similar total phenolic contents, samples fermented with ySR 127 had the highest total anthocyanin content, being 470.1 ± 7.4 mg/L. Similarly, contents of monomeric and polymeric anthocyanins in samples fermented with ySR 127 were also significantly (p < 0.05) higher than that of other samples, implying that anthocyanins were better preserved during fermentation by ySR 127. Due to the relatively high anthocyanin content in samples fermented with ySR 127, they were characterised by highest values of colour intensity and percentage of red colour, being 25.51 ± 0.09 and 49.75 ± 0.17, respectively. On the other hand, anthocyanin content, colour intensity, percentage of red colour for mulberry wines produced by Y1 were the lowest. Similar results were reported by Berenguer et al. [Citation16] that Saccharomyces cerevisiae yeast strains affected the anthocyanin contents in pomegranate wines. During alcoholic fermentation, yeasts can interact with anthocyanins to remove polar compounds, resulting in the decrease of anthocyanin content. [Citation36] Meanwhile, enzymes produced by yeasts can also affect anthocyanin stability negatively. [Citation37] Thus, the low anthocyanin content in samples fermented with Y1 may be due to the strong interaction between Y1 and anthocyanins, as well as the high sensitivity of anthocyanins to enzymes produced by Y1.
Furthermore, the contents of flavonols and tartaric esters in mulberry wines fermented with Y1 were the lowest among all the samples, being 349.1 ± 13.2 and 405.0 ± 7.8 mg/L, respectively. At the same time, flavonol content in samples fermented with YJM 681 was significantly higher than that of other samples, being 443.6 ± 15.2 mg/L. Regarding antioxidant activities of the three mulberry wines, the ability of samples fermented with Y1 to scavenge ABTS˙+ radical was significantly lower than that of other samples, while the reducing powers of all the samples were close to each other. Overall, the contents of different phenolic families and antioxidant activity of mulberry wines fermented with Saccharomyces cerevisiae isolated from kefir fermented milk were relatively low compared with samples fermented with the commercial yeast and yeast isolated from raspberry.
Phenolic composition of mulberry wines
To gain the insight about the differences in phenolic composition among samples fermented with different Saccharomyces cerevisiae strains, the individual phenolic compounds in mulberry wines were identified by HRMS and then quantified by HPLC. 12 phenolic compounds, including 2 anthocyanins, 5 phenolic acids and 5 flavonols were identified tentatively by published data and standard chemicals (). It was found that cyaniding-3-O-glucoside and cyaniding-3-O-rutinoside were the major anthocyanins in mulberry wines under investigation, which was in agreement with the studies of Wang et al. [Citation1] and Yu et al. [Citation38] Meanwhile, most of phenolic acids and flavonols in , including protocatechuic acid, p-hydroxybenzoic acid, caffeic acid, p-hydroxycinnamic acid, rutin, myricetin, quercetin and kaempferol. These compounds have also been previously reported in mulberry-based products. [Citation6,Citation38] It should be mentioned that the types of individual phenolic compounds identified in each mulberry wine sample were the same regardless of yeast strains used for fermentation.
Table 2. Phenolic compounds identified in mulberry wines by high resolution mass spectrometry.
The contents of aforementioned individual phenolic compounds were then determined by HPLC and the results are presented in . As can be seen, cyanidin-3-O-rutinoside, protocatechuic acid and taxifolin were the most abundant anthocyanin, phenolic acid and flavonol, respectively. It can also be found that Saccharomyces cerevisiae strains affected the contents of individual phenolic compounds significantly. For anthocyanins, the contents of cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside in mulberry wines fermented with ySR 127 were higher than that in other samples, although the difference in cyanidin-3-O-rutinoside content among all the samples was statistically insignificant (p ≥ 0.05). This result was consistent with the founding exhibited in . Since monomeric anthocyanins can interact with other substances, such as phenolic acids, yeast metabolites, etc., to form pyran anthocyanins and polymeric pigments during fermentation [Citation39], more studies are needed to analyse anthocyanin-derived pigments in mulberry wines fermented with different yeast strains.
Table 3. Contents (mg/L) of individual phenolic compounds in mulberry juice and mulberry wines fermented with different yeast strains.
Regarding phenolic acids, the contents of all the identified phenolic acids, except protocatechuic acid, in samples fermented with Y1 were lower than that in other wine samples. To be exact, the contents of p-hydroxybenzoic acid, caffeic acid, p-hydroxycinnamic acid and veratric acid in samples fermented with Y1 were 6.12 ± 0.66, 4.23 ± 0.40, 3.69 ± 0.33 and 2.57 ± 0.12 mg/L, respectively. Samples fermented with YJM 681 possessed the highest contents of protocatechuic acid (35.19 ± 1.55 mg/L), p-hydroxybenzoic acid (10.18 ± 0.58 mg/L), caffeic acid (8.33 ± 0.41) and veratric acid (5.70 ± 0.18) among all the mulberry wines. It was noteworthy that the contents of protocatechuic acid, caffeic acid and vatic acid in the three mulberry wines were all higher than that in mulberry juice before fermentation. The study of Faria et al. [Citation34] pointed out that anthocyanins can be converted to phenolic acids through the cleavage of 3-glycosidic linkage under bacterial metabolism. Thus, the enrichment of aforementioned phenolic acids after alcoholic fermentation may be associated with the yeast metabolism of anthocyanins. However, the details about the bio-transformation of anthocyanins into phenolic acids by yeast need to be clarified in future studies. As for flavonols, rutin content in samples fermented with Y1 was the lowest, while samples fermented with ySR 127 had the lowest content of taxifolin among all the investigated samples. Besides, the contents of other flavonols in all the mulberry wine samples were very low. Phenolics are a group of bioactive compounds with a series of health-beneficial properties, such as antioxidant, anti-cancer and anti-inflammation functions. [Citation40] The aforementioned results suggested that Saccharomyces cerevisiae YJM 681 from raspberry can be a suitable yeast strain to produce mulberry wines with considerably high amounts of phenolic compounds.
Free amino acid profile of mulberry wines
The contents of 16 free amino acids in mulberry juice and resulting mulberry wines are shown in . Considering that amino acids were the major nitrogen source for the growth of Saccharomyces cerevisiae, the total amino acid contents in all the mulberry wines were markedly lower than that in mulberry juice. However, the profile of individual amino acids of mulberry wines was significantly dependent on the yeast strains used.
Table 4. Contents (mg/L) of free amino acids in mulberry juice and mulberry wines fermented with different yeast strains.
It can be seen that there was a great difference in free amino acid contents between samples. The contents of total free amino acids in samples fermented with YJM 681, ySR 127 and Y1 were 554.97 ± 4.52, 253.57 ± 3.11 and 373.97 ± 4.61 mg/L, respectively. Wines produced by yeast from raspberry possessed one-fold higher content of total free amino acids than the beverages produced by commercial yeast. According to the literature, yeast strains used for fermentation had a profound influence on the free amino acids contents of fruit alcoholic beverages. [Citation12,Citation41] Pro, Glu, Ala and Asp were the major amino acids in samples fermented with YJM 681 with their contents higher than 50 mg/L, while Pro, Glu, Phe and Ala were the major amino acids in samples fermented with Y1 with the corresponding contents exceeding 30 mg/L. Regarding samples fermented with ySR 127, only three amino acids, namely Pro, Glu and Phe, had the contents greater than 10 mg/L. All the mulberry wine samples presented relatively high contents of Glu and Pro, indicating that these two amino acids could not be easily assimilated by yeasts. Similar results were also reported by Ribéreau-Gayon et al. [Citation42] and Gutiérrez-Gamboa et al. [Citation43], who found that Glu and Pro had high concentrations in grape wines. On the other hand, some amino acids, such as Leu, Ile, Val and Phe, are the precursors of aromatic compounds including high alcohols and esters. [Citation44] The contents of the aforementioned four amino acids in wines fermented with Y1 were significantly higher than that in other wine samples, indicating that Y1 had the weakest ability to metabolise amino acids for the production of aromatic compounds.
Besides, the contents of His and Lys in mulberry wines fermented with Y1 were significantly higher than that in other wine samples, while the wines fermented with YJM 681 had the highest content of Tyr among the three wine samples. His, Lys and Tyr are the precursors of toxic biogenic amines, which have high risk to human health. [Citation44] Thus, extra attention should be paid to the potential formation of biogenic amines if mulberry wines were further subjected to malolactic fermentation, especially for wines fermented with Y1 and YJM 681.
Volatile profile of mulberry wines
The volatile profile of mulberry wines fermented with different Saccharomyces cerevisiae strains was determined using the purge-and-trap-gas chromatography-mass spectrometry. Compared with traditional solid-phase micro-extraction method, purge-and-trap method can extract more quantity of volatile compounds from raw materials, except volatiles with high molecular mass. [Citation26] This technology has been previously used for the analysis of volatile profile of Spanish red and white wines. [Citation26]
A total of 32 individual volatile compounds were identified in mulberry wines, which belonging to different families including alcohols, esters, aldehydes, acetals, ketones, sulphur compounds, alkanes and others. According to a comprehensive literature search, all these compounds have been previously reported in various types of fruit wines. For the quantitative purpose, the peak areas of identified compounds in extracted ion chromatograms were calculated [Citation45] and summarised in .
Table 5. Relative amounts of volatile compounds identified by GC-MS in mulberry wines.
Alcohols are the products of yeast metabolism during alcoholic fermentation. 1-propanol (1), 2-methyl-1-propanol (3), 3-methyl-1-butanol (4) and 2-octanol (6) were the dominant alcohols in mulberry wines under investigation, the amounts of which were markedly higher than that of other alcohols. 2-methyl-1-propanol and 3-methyl-1-butanol were also the major alcohols presented in raspberry wines in the study of Duarte et al. [Citation13], which provided wines a malty smell. Esters are another important group of aromatic compounds in fruit-based alcoholic beverages. For esters, ethyl acetate (9) was the major acetate ester in all the mulberry wine samples, while octanoic acid ethyl ester (15) and decanoic acid ethyl ester (17) were the most two abundant ethyl esters. Furthermore, mulberry wines had considerably high amounts of acetaldehyde (20) and 1,1-diethoxy-ethane (23). The former one can be involved in the reactions about anthocyanin copigmentation, while the latter one can provide alcoholic beverages buttery and creamy odors. [Citation20,Citation46]
Regarding the influence of Saccharomyces cerevisiae strains on volatile profile of mulberry wines, there was no significant difference in the contents of several major aromatic compounds, including 3-methyl-1butanol, 2-octanol and octanoic acid ethyl ester. However, wine samples fermented with YJM 681 had the highest contents of 1-propanol and acetaldehyde. Samples fermented with ySR 127 were characterised by the highest contents of 2-methyl-1-propanol and the lowest content of decanoic acid ethyl ester. Furthermore, samples fermented with Y1 had the lowest contents of 1-propanol, 2-methyl-1-propanol and ethyl acetate. The relatively low contents of these two high alcohols can be explained by the high retention of Leu in samples fermented with Y1 mentioned previously, since Leu was the precursor of 2-methyl-1-propanol. [Citation44] In addition, yeast strains can affect the enzymatic and chemical esterification of alcohols and acids during fermentation, resulting in the difference in ester profiles among mulberry wine samples. [Citation13,Citation45] All the mulberry wines had relatively a small quantity of alkane.
Principal component analysis
PCA was used to analyse the data presented in and –, so as to provide a more simplified view about the effect of Saccharomyces cerevisiae strains on the properties of mulberry wines. In , the data about oenological, phenolic and amino acid profile were processed by PCA. The first principal component (PC1) and PC2 accounted for 66.4% and 33.6% of total variation, respectively. Mulberry wines can be easily separated according to Saccharomyces cerevisiae strains used. Y1 was distributed on the negative side of PC1, while the other two samples located on the positive side of PC1. Meanwhile, ySR 127 and YJM 681 were projected on different sides of PC2. Blue% (19), Cys (39), Val (40), Met (41), Ile (42), Leu (43), Phe (45), Lys (46), His (47), Arg (48) had high negative values on PC1, indicating the high values of these parameters in Y1. Meanwhile, alcoholic degree (1), total sugar (7), antioxidant activity assayed by FRAP assay (21) and cyanidin-3-O-glucoside (22) had similar positive values on PC2 to ySR 127, implying that ySR 127 was characterised by relatively high alcoholic degree, sugar content, reducing Fe3+ power and cyanidin-3-O-glucoside content. Moreover, YJM 681 had higher contents of flavonols (11), Asp (34), Glu (36) and Tyr (44) due their negative values on PC2.
Figure 1. Bi-plots of principal component analysis of mulberry wines fermented with different Saccharomyces cerevisiae strains. a: data in , and about oenological, phenolic and amino acid profile were analyzed; b: data in about volatile profile were analyzed. Numbers in Fig. 1a were referred in , and , while numbers in Fig. 1b were referred in .
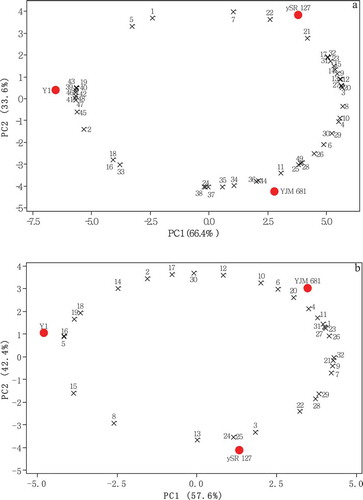
On the other hand, the data about volatile profile of mulberry wines were involved in . PC1 and PC2 explained 100% of the total variance of mulberry wine samples. Volatile data can also be used for the discrimination of mulberry wines fermented with different Saccharomyces cerevisiae strains. Similar to the position in , Y1 was projected on the negative side of PC1, while ySR 126 and YJM 681 were distributed on the positive side of PC1. The parameters responsible for the separation of Y1 from other samples included 2-methyl-1-butanol (5), nonanoic acid, ethyl ester (16), dodecanoic acid, ethyl ester (18), ethyl 9-decenoate (19). The contents of these volatile compounds in Y1 were higher than that in other samples. YJM 681 was projected on the positive side of PC2 and ySR 127 located on the negative side of PC2. Acetaldehyde (20), benzothiazole (24) and undecane (25) were the responsible components for the separation of these two samples.
Descriptive sensory analysis
Mulberry wines were further assessed by descriptive sensory analysis. The organoleptic characteristics were assessed in terms of visual, olfactory and gustative attributes. The average scores of each sensory attribute are plotted in . It can be seen that there were no significant differences in visual descriptors, including colour, clarity and tears.
Figure 2. Descriptive sensory analysis of colour (a), aroma (b), taste (c), and global attributes (d) of mulberry wines fermented with different Saccharomyces cerevisiae strains. Data were expressed as mean value ± standard deviation. Different letters indicated significant differences (p < 0.05).
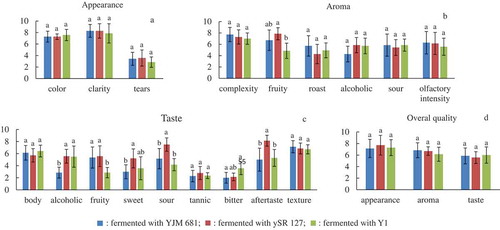
Regarding olfactory attributes, samples fermented with Y1 presented a significantly (p < 0.05) lower scent of fruity aroma in comparison with samples fermented with ySR 127. This result was probably due to the lower content of ethyl acetate, which was the dominant ester in mulberry wines responsible for the fruity aroma. [Citation9] Meanwhile, all the samples exhibited similar intensities of roast and sour smells. Moreover, the differences in olfactory complexity and intensity among samples were statistically insignificant (p ≥ 0.05).
More differences were found in gustative attributes among mulberry wines. The perception of alcoholic taste for YJM 681 was weaker than that of other samples, probably due to the lower alcoholic degree. The intensities of sweet and sour tastes for ySR 127 were higher compared with other samples, which may be ascribed to the higher total acidity and sugar content. Y1 exhibited higher intensity of bitter taste, which could be explained by its higher contents of Ile and Phe. Ile and Phe are classified into bitter-tasting amino acids according to the study of Toko. [Citation47] Furthermore, the score of fruity taste of Y1 was lower than that of other samples, while the score of aftertaste of ySR 127 was the highest among all the samples.
Despite of the aforementioned differences in olfactory and gustative attributes, the overall quality in terms of appearance, aroma and taste for all the mulberry wine samples were regarded to be equal to each other. This result was probably because of the high perception thresholds of molecules showing differences among mulberry wine samples fermented selected yeast strains. Besides, ordinary wine consumers should be involved for the sensory evaluation of mulberry wines fermented with different strains in the future, so as to get a general idea about consumers’ acceptance of studied mulberry wines.
Conclusion
This research demonstrated that phenolic, amino acid, volatile and sensory profiles of mulberry wine were affected profoundly by Saccharomyces cerevisiae strains. Mulberry wines fermented with Saccharomyces cerevisiae YJM 681 isolated from raspberry possessed higher contents of total flavonols, amino acids, 1-propanol, and some phenolic acids, including protocatechuic acid, p-hydroxybenzoic acid, caffeic acid and veratric acid in comparison with other samples. Meanwhile, mulberry wines fermented with commercial yeast Saccharomyces cerevisiae ySR 127 had higher contents of anthocyanins and 2-methyl-1-propanol. Furthermore, yeast strain isolated from kefir fermented milk named Saccharomyces cerevisiae Y1 was first applied for the alcoholic fermentation of mulberry juice. The resulting mulberry wines had lower contents of total anthocyanins and tartaric esters, as well as lower amounts of 1-propanol, 2-methyl-1-propanol and ethyl acetate. Y1 also had the weaker ability to produce aromatic compounds through the metabolism of amino acids. The differences in wine physicochemical properties led to the diversity of wine sensory properties. The lowest scent of fruity aroma was perceived in mulberry wines fermented with Y1. Samples fermented with ySR 127 were characterised by highest intensities of sour and sweet tastes and aftertaste. Phenolic and aromatic compounds are the key contributors to high-quality alcoholic beverages. Taking these factors into consideration, Saccharomyces cerevisiae Y1 was not the suitable Saccharomyces cerevisiae strain to produce high-quality mulberry wine. Saccharomyces cerevisiae YJM 681 can be used to enrich phenolic acids and flavonols in mulberry wine. Moreover, Saccharomyces cerevisiae ySR 127 can be considered for the preservation of anthocyanins and red colour during the alcoholic fermentation of mulberry juice. In conclusion, this study revealed detailed influence of Saccharomyces cerevisiae strains on mulberry wine quality, which can provide useful information to fruit wine industry.
Additional information
Funding
References
- Wang, L.; Sun, X.; Li, F.; Yu, D.; Liu, X.; Huang, W.; Zhan, J. Dynamic Changes in Phenolic Compounds, Colour and Antioxidant Activity of Mulberry Wine during Alcoholic Fermentation. Journal of Functional Foods 2015, 18, 254–265.
- Chen, C.; You, L. J.; Abbasi, A. M.; Fu, X.; Liu, R. H. Optimization for Ultrasound Extraction of Polysaccharides from Mulberry Fruits with Antioxidant and Hyperglycemic Activity in Vitro. Carbohydrate Polymers 2015, 130, 122–132.
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C. G.; Barbero, G. F. Optimization of the Ultrasound-Assisted Extraction of Anthocyanins and Total Phenolic Compounds in Mulberry (Morus Nigra) Pulp. Food Chemsitry 2017, 219, 23–32.
- Choi, J. W.; Synytsya, A.; Capek, P.; Bleha, R.; Pohl, R.; Park, Y. I. Structural Analysis and Anti-Obesity Effect of a Pectic Polysaccharide Isolated from Korean Mulberry Fruit Oddi (Morus Alba L.). Carbohydrate Polymers 2016, 146, 187–196.
- Liu, C. J.; Lin, J. Y. Anti-Inflammatory and Anti-Apoptotic Effects of Strawberry and Mulberry Fruit Polysaccharides on Lipopolysaccharide-Stimulated Macrophages through Modulating Pro-/Anti-Inflammatory Cytokines Secretion and Bcl-2/Bak Protein Ratio. Food and Chemical Toxicology 2012, 50, 3032–3039.
- Juan, C.; Jianquan, K.; Junni, T.; Zijian, C.; Ji, L. The Profile in Polyphenols and Volatile Compounds in Alcoholic Beverages from Different Cultivars of Mulberry. Journal of Food Science 2012, 77, C430–C436.
- Kwak, E. J.; Lee, J. Y.; Choi, I. S. Physicochemical Properties and Antioxidant Activities of Korean Traditional Alcoholic Beverage, Yakju, Enriched with Mulberry. Journal of Food Science 2012, 77, C752–C758.
- González, E. A.; Agrasar, A. T.; Castro, L. M. P.; Ferńandez, I. O.; Guerra, N. P. Production and Characterization of Distilled Alcoholic Beverages Obtained by Solid-State Fermentation of Black Mulberry (Morus Nigra L.) And Black Currant (Ribes Nigrum L.). Journal of Agricultural and Food Chemistry 2010, 58, 2529–2535.
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W.; Chowtivannakul, S. HS-SPME-GC-MS Analysis of Volatile Aromatic Compounds in Alcohol Related Beverages Made with Mulberry Fruits. Food Science and Technology 2011, 20, 1021–1032.
- Kalkan, Y. H.;. Evaluation of Colour Parameters and Antioxidant Activities of Fruit Wines. International Journal of Food Sciences and Nutrition 2006, 57, 47–63.
- Wang, C. Y.; Liu, Y. W.; Jia, J. Q.; Sivakumar, T. R.; Fan, T.; Gui, Z. Z. Optimization of Fermentation Process for Preparation of Mulberry Fruit Wine by Response Surface Methodology. African Journal of Microbiology Research 2013, 7, 227–236.
- Arrieta, M. P.; Prats-Moya, M. S. Free Amino Acids and Biogenic Amines in Alicante Monastrell Wines. Food Chemistry 2012, 135, 1511–1519.
- Duarte, W. F.; Dias, D. R.; Oliveira, J. M.; Vilanova, M.; Teixeria, J. A.; Silva, J. B. A.; Schwan, R. F. Raspberry (Rubus Idaeus L.) Wine: Yeast Selection, Sensory Evaluation and Instrumental Analysis of Volatile and Other Compounds. Food Research International 2010, 43, 2303–2314.
- Martínez-Rodríguez, A. J.; Carrascosa, A. V.; Martín-Álvarez, P. J.; Moreno-Arribas, V.; Polo, M. C. Influence of the Yeast Strain on the Changes of the Amino Acids, Peptides and Proteins during Sparkling Wine Production by the Traditional Method. Journal of Industrial Microbiology and Biotechnology 2002, 29, 314–322.
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Evaluation of Different Saccharomyces Cerevisiae Strains for Red Winemaking. Influence on the Anthocyanin, Pyranoanthocyanin and Non-Anthocyanin Phenolic Content and Colour Characteristics of Wines. Food Chemistry 2007, 104, 814–823.
- Berenguer, M.; Vegara, S.; Barrajón, E.; Saura, D.; Valero, M.; Martí, N. Physicochemical Characterization of Pomegranate Wines Fermented with Three Different Saccharomyces Cerevisiae Yeast Strains. Food Chemistry 2016, 190, 848–855.
- OIV. Compendium of International Methods of Wine and Must Analysis; Paris: OIV, 2012.
- Singleton, V. L.; Rossi, J. A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Ivanova, V.; Dörnyei, Á.; Márk, L.; Vojnoski, B.; Stafilov, T.; Stefova, M.; Kilár, F. Polyphenolic Content of Vranec Wines Produced by Different Vinification Conditions. Food Chemistry 2011, 124, 316–325.
- Cliff, M. A.; King, M. C.; Schlosser, J. Anthocyanin, Phenolic Composition, Color Measurement and Sensory Analysis of BC Commercial Red Wines. Food Research International 2007, 40, 92–100.
- Glories, Y.;. La Coleur Des Vins Rouges 1ère Partie: Les Equilibres Des Anthocyanes Et Des Tannins. Connaisance De La Vigne Et Du Vin 1984, 18, 195–217.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Benzie, I. F. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Analytical Biotechnology 1996, 239, 70–76.
- Cui, C.; Zhang, S.; You, L.; Ren, J.; Luo, W.; Chen, W.; Zhao, M. Antioxidant Capacity of Anthocyanins from Rhodomyrtus Tomentosa (Ait.) And Identification of the Major Anthocyanins. Food Chemistry 2013, 139, 1–8.
- Aro, J. M. A.; Nyam-Osor, P.; Tsuji, K.; Shimada, K.; Fukushima, M.; Sekikawa, M. The Effect of Starter Cultures on Proteolytic Changes and Amino Acid Content in Fermented Sausages. Food Chemistry 2010, 119, 279–285.
- Aznar, M.; Arroyo, T. Analysis of Wine Volatile Profile by Purge-And-Trap–Gas Chromatography-Mass Spectrometry. Application to the Analysis of Red and White Wines from Different Spanish Regions. Journal of Chromatography A 2007, 1165, 151–157.
- Tao, Y.; Sun, D. W.; Górecki, A.; Błaszczak, W.; Lamparski, G.; Amarowicz, R.; Fornal, J.; Jelińki, T. A Preliminary Study about the Influence of High Hydrostatic Pressure Processing in Parallel with Oak Chip Maceration on the Physicochemical and Sensory Properties of A Young Red Wine. Food Chemistry 2016, 194, 545–554.
- Casassa, L. F.; Bolcato, E. A.; Sari, S. E. Chemical, Chromatic, and Sensory Attributes of 6 Red Wines Produced with Prefermentative Cold Soak. Food Chemistry 2015, 174, 110–118.
- Ordoudi, S. A.; Mantzouridou, F.; Daftsiou, E.; Malo, C.; Hatzidimitriou, E.; Nenadis, N.; Tsimidou, M. Z. Pomegranate Juice Functional Constituents after Alcoholic and Acetic Acid Fermentation. Journal of Functional Foods 2014, 8, 161–168.
- Bely, M.; Stoeckle, P.; Masneuf-Pomarede, I.; Dubourdieu, D. Impact of Mixed Torulaspora delbrueckii-Saccharomyces Cerevisiae Culture on High-Sugar Fermentation. International Journal of Food Microbiology 2008, 122, 312–320.
- Röcker, J.; Strub, S.; Ebert, K.; Grossmann, M. Usage of Different Aerobic non-Saccharomyces Yeasts and Experimental Conditions as a Tool for Reducing the Potential Ethanol Content in Wines. European Food Research and Technology 2016, 242, 2051–2070.
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The Use of Glucose Oxidase and Catalase for the Enzymatic Reduction of the Potential Ethanol Content in Wine. Food Chemistry 2016, 210, 660–670.
- Hornedo-Ortega, R.; Krisa, S.; García-Parrilla, M. C.; Richard, T. Effects of Gluconic and Alcoholic Fermentation on Anthocyanin Composition and Antioxidant Activity of Beverages Made from Strawberry. LWT - Food Science and Technology 2016, 69, 382–389.
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between Anthocyanins and Gut Microbiota. Journal of Agricultural and Food Chemistry 2014, 62, 6898–6902.
- Hao, X.; Hn, Z.; Li, Y.; Li, C.; Wang, X.; Zhang, X.; Yang, Q.; Ma, B.; Zhu, C. Synthesis and Structure–Activity Relationship Studies of Phenolic Hydroxyl Derivatives Based on Quinoxalinone as Aldose Reductase Inhibitors with Antioxidant Activity. Bioorganic & Medicinal Chemistry Letters 2017, 27, 887–892.
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Yeast Interactions with Anthocyanins during Red Wine Fermentation. American Journal of Enology and Viticulture 2005, 56, 104–109.
- Romero-Cascales, I.; Fernández-Fernández, J. I.; López-Roca, J. M.; Gómez-Plaza, E. The Maceration Process during Winemaking Extraction of Anthocyanins from Grape Skins into Wine. European Food Research and Technology 2005, 221, 163–167.
- Yu, Y.; Xu, Y.; Wu, J.; Xiao, G.; Fu, M.; Zhang, Y. Effect of Ultra-High Pressure Homogenisation Processing on Phenolic Compounds, Antioxidant Capacity and Anti-Glucosidase of Mulberry Juice. Food Chemistry 2014, 153, 114–120.
- Tao, Y.; García, J. F.; Sun, D. W. Advances in Wine Aging Technologies for Enhancing Wine Quality and Accelerating Wine Aging Process. Critical Reviews in Food Science and Nutrition 2014, 54, 817–835.
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. Journal of Functional Foods 2015, 18, 820–897.
- Benito, Á.; Calderón, F.; Benito, S. Combined Use of S. Pombe and L. Thermotolerans in Winemaking. Beneficial Effects Determined through the Study of Wines’ Analytical Characteristics. Molecules 2016, 21, 1744.
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. . Handbook of Enology Volume 2: The Chemistry of Wine Stabilization and Treatments, 2nd ed. Press: John Wiley & Sons Ltd: UK, 2006; 115 pp.
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Portu, J.; Moreno-Simunovic, Y.; Martínez-Gil, A. M. Foliar Nitrogen Application in Cabernet Sauvignon Vines: Effects on Wine Flavonoid and Amino Acid Content. Food Research International 2017, 96, 46–53.
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on Quality and Composition of Riesling Wines Fermented by Sequential Inoculation with non-Saccharomyces and Saccharomyces Cerevisiae. European Food Research and Technology 2015, 241, 707–717.
- Petropulos, V. I.; Bogeva, E.; Stafilov, T.; Stefova, M.; Siegmund, B.; Pabi, N.; Lankmayr, E. Study of the Influence of Maceration Time and Oenological Practices on the Aroma Profile of Vranec Wines. Food Chemistry 2014, 165, 506–514.
- Lee, S. J.; Noble, A. C. Characterization of Odor-Active Compounds in Californian Chardonnay Wines Using GC-Olfactometry and GC-Mass Spectrometry. Journal of Agricultural and Food Chemistry 2003, 51, 8036–8044.
- Toko, K.;. Taste Sensor. Sensors and Actuators B: Chemical 2000, 64, 205–215.

