ABSTRACT
The operations applied in plum processing and in the production of plum wine can significantly affect the content and activity of biologically active compounds extracted from the raw material. The aim of this study was to assess the composition of phenolic compounds, chromatic characteristics and antiradical activity of plum wines produced from three plum varieties (Čačanska rana, Čačanska lepotica and Požegača) commonly grown in Serbia. Čačanska lepotica wine was characterised by the highest content of total phenols, total anthocyanins and flavan-3-ols, the highest colour intensity (CI) and the strongest antiradical activity against DPPH free radicals. A significant positive linear correlation (r = 0.828–0.905) between the antiradical activity and the content of total phenolics and total anthocyanins was determined. Peonidin-3-glucoside, cyanidin-3-rutinoside, peonidin-3-rutinoside, chlorogenic and caffeic acids and rutin were identified as the main polyphenols in plum wines.
Introduction
Plums are raw materials rich in biologically active substances and also the carriers of antioxidant properties. Antioxidant activity is especially correlated with a high content of phenolic compounds. A number of studies have confirmed the positive health effects as a result of plum consumption. There have been examples of blood lipid profile improvement, bile acid content reduction, glucose and fats metabolism improvement, osteoporosis prevention and so on.[Citation1] The phenolic content of plums can vary greatly depending on several factors (variety, climate, soil, applied analytical methods, etc.), and according to the study of Chun et al.,[Citation2] it was in the range 125–685 mg/100 g of fresh fruit, expressed as gallic acid equivalent (GAE), whereas the total flavonoids content was in the range 60–360 mg/100 g of fresh fruit, expressed as catechin equivalent. Kim et al.[Citation3] studied six plum varieties, including Čačanska najbolja variety and Stanley variety, and obtained similar results for total phenols content (174–385 mg GAE/100 g of fresh fruit). The determination of total phenolic compounds content in three plum varieties (Bistrica, Top and Elena) was carried out in the study of Voća et al.[Citation4] The values obtained were in the range 157–344 mg GAE/100 g of fresh fruit. The content of phenolic acids in plums can also vary within a wide range, for example, neochlorogenic acid can vary from 85 to 1300 mg/kg, chlorogenic from 13 to 430 mg/kg and cryptochlorogenic acid can vary from 9 to 56 mg/kg of dry matter.[Citation5–Citation8]
Quercetin-3-glucoside and quercetin-3-rutinoside prevail among the flavonols in plum,[Citation8] whereas the main representatives of flavanols are mainly epicatechin and catechin as well as proanthocyanidins (their dimers and trimers).[Citation6,Citation8] The study that dealt with the determination of catechin content in 27 types of fruit[Citation9] reported that the highest content of this compound, expressed through the weight of fresh fruit, was recorded in plums (49 mg/100 g), followed by the content in apples (10–43 mg/100 g) and berries (5–20 mg/100 g). Cyanidin-3-glucoside, cyanidin-3-rutinoside and peonidin-3-rutinosid are the most common anthocyanins in plums.[Citation1] The total anthocyanins content ranged from 18 to 125 mg/100 g of fresh plums.[Citation3,Citation5,Citation6] The concentration of these phenolic compounds is higher in the skin than in the flesh (up to three to four times), but in most studies their content is defined through the whole mass of chopped pitted fruit.[Citation1]
Furthermore, this fruit has high antiradical activity expressed through the ORAC value of 950/100 g (ORAC – oxygen radical absorbance capacity). In terms of comparison, the same amounts of red grapes, blueberries and blackberries generated ORAC values of 739, 2400 and 2036, respectively.[Citation10] Phenolic compounds in plums have a significant scavenging ability against free oxygen radicals such as hydroxyl and peroxyl radicals.[Citation11] Kim et al.[Citation3] showed that the antiradical activity of plum extracts, expressed as vitamin C equivalent, was also significantly higher (up to three times) relative to the activity of apple extracts. In another study, Wang et al.[Citation12] pointed out 4.4 times greater overall antiradical activity of plums compared with apples, which represent one of the most commonly consumed fruits in the human diet. Voća et al.[Citation4] tried to determine the antiradical activity of plum ethanolic extracts (Bistrica, Top and Elena varieties) against DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals and reported approximately the same values for all three varieties (3.10 mmol Trolox eq/kg fresh plum) despite significant differences in the content of total phenolic compounds present in the varieties mentioned above. Studies have shown a high correlation (r = 0.96) between antiradical activity and the total content of phenolic compounds in different plum varieties.[Citation13]
The primary processing of plums as well as the specifics of the wine production process can significantly affect the extraction of biologically active compounds and their content in the finished wine. A small number of scientific studies dealing with the production of plum wine are available and therefore data on the phenolic compounds composition and chromatic characteristics of this fruit wine is limited. Dietrich[Citation14] examined the impact of the production process of juice obtained from several domestic plum varieties on stability, colour and biologically active compounds. The content of phenolic compounds was high, 1465–2590 mg/L, with neochlorogenic and chlorogenic acid, (+) - catechin and (-) – epicatechin as the most dominant polyphenols identified in the plum juice. Total anthocyanins were found in the amounts of 43–168 mg/L, mostly in the form of cyanidin-3-glucoside, cyanidin-3-rutinoside, peonidin-3-glucoside and peonidin-3-rutinoside. A significant decrease in the concentration of anthocyanins was observed during the first six months of storage at 20°C, which led to a drastic change in the red colour intensity (CI).
Heinonen et al.[Citation15] compared the contents of phenolic compounds (in GAE) and the antioxidant potential (expressed as a degree of oxidation inhibition of added methyl linoleate) in 33 different fruit wines (apple, aronia, raspberry, blueberry, cranberry, strawberry, etc.) and a few conventional red and white wines. A low level of positive correlation between the content of total phenolic compounds and the antioxidant potential of fruit wines (r = 0.32–0.47) was determined, in contrast to the conventional wines (r = 0.89–0.94). On the other hand, Kalkan Yildirim[Citation16] found a significant positive correlation between the antioxidant potential (DPPH test) and the content of total phenolic compounds in the tested fruit wines made from apple, apricot, blueberry, blackberry, quince, strawberry and cherry. The differences in the obtained values may be a result of the application of different methods for the quantification of antioxidant potential in wines. The results of a study that examined the differences in the antiradical activity mechanism in juice and wine made from pomegranate also indicated a high positive correlation between the total phenols content and the antioxidant potential against DPPH radicals.[Citation17] Yuan[Citation18] found that plum wine possesses a significant antioxidant potential, primarily against superoxide anion radicals (O2•). Plum wine, despite having a slightly lower content of total phenols, had a higher antioxidant potential compared with most of the fruit wines that were investigated.[Citation18]
Since characterisation and evaluation of the functional properties of plum wines have still not been sufficiently examined in the scientific literature, the aim of this study was to assess the composition of phenolic compounds, chromatic characteristics and antiradical activity of this fruit wine produced from three plum varieties commonly grown in Serbia.
Material and methods
Plum wine vinification
The experiments were carried out with Čačanska rana, Čačanska lepotica and Požegača (Prunus domestica L.) plum varieties as raw materials for the fruit wine production. These plum varieties grown in Serbia are characterised by different fruit-ripening times. Čačanska rana is an early, Čačanska lepotica mid-early and Požegača late-ripening variety. The fruit, which was at technological maturity, was purchased from a local market in Novi Sad, Serbia, during the period of August–September 2014. Plums from several locations (marked as I, II and III) were used in different parts of the study. They were subjected to crushing after being halved and having their stones removed. The obtained pomace was treated with K2S2O5 (the SO2 level was set to 50 mg SO2/kg pomace) and pectolytic enzyme Lallzyme EX-V (Lallemand S.A., St. Simon, France, 0.02 g/kg of pomace). An amount of 3 kg of crushed fruit per individual microvinification was used. Fermentations were conducted at the temperature of 25°C and pH 3.5., with 0.25 g/kg of commercial wine yeast Saccharomyces cerevisiae (Spiriferm, Erbslöh, Geisenheim, Germany). The plum wine was passed through a cheesecloth when the fermentation was over, and then kept in full 1.5 L glass bottles at 10°C to remove the coarse precipitate. After three days, the wine was racked off, the free SO2 level was adjusted to 30 mg/L and the wine was poured into 0.5 L bottles, closed with screw caps and kept at 10°C in the absence of light until the analyses.
Analyses
Chromatic parameters
The colour of wine consists of three components: yellow (λ = 420 nm), red (λ = 520 nm) and blue (λ = 620 nm). Spectrophotometric measurements of the absorbance at these three wavelengths were used for the calculation of six parameters: CI, hue (H), the share of yellow (A420 (%)), red (A520 (%)) and blue (A620 (%)) colour in the CI and the colour brilliance (dA (%)).[Citation19] Characteristics of the wine originating from the co-pigmentation reactions were determined on the basis of spectroscopic methods described by Boulton et al.[Citation20] and can be expressed through the following parameters:
colour fraction that comes from anthocyanins co-pigmentation (%CA):
%CA (%) = (Aacet – A20)/Aacet • 100
colour fraction consisting of free anthocyanins (%FA):
%FA (%) = (A20 – ASO2)/Aacet • 100
chemical age of the wine (%HS), expressed as a share of polymer pigments in total pigments:
%HS (%) = (ASO2/AHCl) • 100
Parameters Aacet, ASO2, AHCl and A20 represent absorbances of the wine samples previously subjected to defined treatments.[Citation20]
Phenolic compounds
The content of total phenolic compounds in the wine was determined by the Folin–Ciocalteu (FC) method using gallic acid as a standard.[Citation21] Prior to the analysis, all wine samples were filtered through a membrane filter (0.45 μm). The results were expressed as mg of chlorogenic acid equivalents (mg GAE/L). Total flavan-3-ols content was analysed by the Vanillic method[Citation22] using (+)-catechin as a standard. Total anthocyanins were determined by spectrophotometry at 520 nm, according to the ability of anthocyanins to discolour following the addition of SO2 (in the K2S2O5 form) at pH 1.[Citation23] Monomeric anthocyanins content in the plum wine samples was determined by the pH differential method.[Citation24] The concentration of monomeric anthocyanins is expressed as cyanidin-3-glucoside equivalent.
HPLC analyses
Resveratol >99%, piceid >99%, rutin hydrate 95%, caffeic acid, quercetin dihydrate 98%, chlorogenic acid 95%, kaempferol ≥97%, p-coumaric acid, vanilic acid, syringic acid ≥95%, naringenin 95%, hesperetin ≥95%, (+)-catechin ≥90%, trans-cinnamic acid 99+% and 4-hydroxy benzoic acid were purchased from Sigma-Aldrich (Steinheim, Germany); gallic acid from Alpha Aesar (Heysham Lancaster, UK) and benzoic acid from Lach-Ner (Nerastovice, Czech Republic).
Anthocyanins, malvidin-3-glucoside (oenin chloride), cyanin-3-glucoside chloride (kuromanine chloride) and delphinidin-3-glucoside chloride (delphinin, myrtillin) were obtained from AppliChem (Darmstadt, Germany); and petunidin-3-glucoside chloride and peonidin 3-glucoside chloride from Phytolab (Vestenbergsgreuth, Germany). The solvents were of analytical grade; methanol and acetonitrile were obtained from Promochem LGC (Wesel, Germany), formic acid from Lach-Ner (Neratovice, Czech Republic), acetic acid 99% from Fluka Sigma Aldrich (Steinheim, Germany) and hydrochloric acid 35% from Roth (Karlsruhe, Germany).
For the purpose of all analyses, the wine samples were prepared by filtration through a membrane filter (0.45 μm; Sartorius, USA). HPLC analyses were carried out using Agilent 1100 series liquid chromatograph (USA) consisting of a quaternary gradient pump, an auto-sampler with the injection system (10–200 μl), a column heater, UV–Vis and fluorescence detectors and a software package.
Resveratrol and piceid
: Determination was performed by injection of 10 μl of the filtered wine on a reversed-phase Zorbax SB-C18 column (4.6 mm × 150 mm, 5 μm; Agilent, USA) at 25°C, gradient elution using 0.5 ml/min flow rate of mobile phase consisting of 50 mM formic acid (A) and methanol (B) (time (min), %B: 0, 20; 25, 80; 30, 80; 31, 100; 36, 100; 41, 20; 50, 20), and fluorescence detection (λex 330 nm, λem 374 nm).[Citation25]
Anthocyanins
: Chromatographic separation of five major anthocyanins in 25 μl of the sample was carried out on a reverse-phase Poroshell 120 EC-C18 column (4.6 × 100 mm, 2.7 μm; Agilent, USA) at 40°C with solvents consisting of water/formic acid/acetonitrile mixtures (A – 87:10:3, B – 40:10:50; flow rate 0.8 ml/min; run-time 50 min) and gradient elution (0 min 94% A and 6% B; 15 min 70% A and 30% B; 30 min 50% A and 50% B; 35 min 40% A and 60% B; 41 min 94% A and 6% B), followed by detection at 518 nm.[Citation26]
Phenolic acids and flavonoids
: Determination was performed by the direct separation of phenols in 5 μl of the sample by HPLC using a reverse-phase Poroshell 120 EC-C18 column (4.6 × 100 mm, 2.7 μm; Agilent, USA) at 25°C with gradient elution (time (min), %B: 0, 8; 3.25, 10; 8, 12; 15, 25; 15.8, 30; 24, 50; 25, 100; 27, 100; 28, 8; 34, 8) by 0.1% acetic acid (A – in water; B – in acetonitrile; flow rate 1 ml/min) and with detection at the following wavelengths (nm): 225 (vanilic acid, benzoic acid), 280 (gallic acid, 4-hydroxy benzoic acid, catechin, syringic acid, trans-cinnamic acid, hesperetin, naringenin), 305 (p-coumaric acid), 330 (chlorogenic acid, caffeic acid), and 360 (rutin, quercetin, kaempferol). Quantification of the compounds of interest was performed using a calibration curve obtained by the injection of calibration solutions.
Antiradical activity
Antiradical activity of the produced plum wines was evaluated spectrophotometrically using the DPPH method.[Citation27] All wine samples were filtered through a nylon filter (0.45 µm) and used in further work. A certain amount of wine was mixed with 95% methanol to different final concentrations of wine (0.05, 0.1, 0.2, 0.4, and 0.8 ml/ml) and to a total volume of 3 ml. Around 1 ml of a freshly prepared working solution of DPPH• radicals in 96% ethanol (90 µM) was added to the prepared wine samples. The control sample was prepared by adding 1 ml of the working DPPH• solution to 3 ml of 95% methanol. After leaving it for 1 h in the dark and at room temperature, its absorbance was read on a spectrophotometer (Jenway 6405 UV/Vis, Essex, UK) at a wavelength of 515 nm, with a 95% methanol solution as a reference. All tests were carried out in triplicate. The antiradical activity of wine on DPPH• is expressed through radical scavenging capacity (RSC), based on the following equation:
RSC (%) = 100 - (As • 100/Acont) (%)
where As is absorbance of the sample and Acont is absorbance of the control. A curve representing the dependence between RSC (%) and the wine sample concentration was made to determine the IC50 values (ml/ml). The IC50 value is defined as the concentration of wine at which 50% DPPH• radicals are neutralised in accordance with the terms defined by the method and obtained from the linear regression equation.
Statistical analysis
Statistical analyses in the present study were performed using STATISTICA 12.0 (Statsoft, 2013). The statistical difference between the mean values of the parameters was estimated by analyses of variance (ANOVAs), at the 95% confidence level. Values detected as significantly different were marked with different letters (a, b, c …). All analyses were carried out in triplicate.
Results and discussion
Phenolic compounds and chromatic characteristics of plum wines
Phenolic compounds are among the most important components of wine that are directly linked not only to the wine organoleptic characteristics (colour, astringency and bitterness) but also to a number of physiological properties that have a positive impact on human health (reducing the risk of cardiovascular and neurodegenerative diseases, diabetes, etc.). These compounds are considered to be the strongest and most active antioxidants found in wine.[Citation19] The most important factors that influence the amount of phenolic compounds in wine are their contents in the raw material (grapes, fruits), the selected method of vinification and the changes (transformations) that occur during the ageing of wine.[Citation28,Citation29] Free (monomeric) anthocyanins extracted from fruit skins are responsible for the colour of young red wines. Considering that the free anthocyanins are not stable, co-pigmentation reactions are of great importance to the colour quality and stability. The colour stability is particularly influenced by the condensation reaction with flavan-3-ols and proanthocyanidins as well as by the formation of piranoanthocyanins.[Citation19] The fruit wines obtained from cherries, blueberries, blackberries or blackcurrants are often characterised by a higher content of total phenolics and anthocyanins compared with red wine.[Citation15,Citation16,Citation30] The available scientific literature provides poor data on the chromatic characteristics of fruit wines. Colour parameters, antiradical activity and the content of the most important groups of phenolic compounds in the produced plum wines are shown in .
Table 1. Content of phenolic compounds, chromatic characteristics and antiradical activity of the produced plum wines.
The content of total phenols and flavan-3-ol in the wines differed significantly (p < 0.05) in relation to the plum variety used. The results shown in indicate a high content of total phenolics (1.65–2.18 g/L) expressed as chlorogenic acid. Čačanska lepotica wine was characterised by the highest total phenols content. The use of chlorogenic acid for expressing the content of total phenolic compounds has been proposed in the literature,[Citation31] considering that this component is one of the most abundant in plums. On the other hand, the expression of total phenols in GAE is generally accepted in the wines made from grapes and many other fruits. To facilitate the comparison of total phenols content of the plum wines obtained from the aforementioned plum varieties with the literature data on the content of these compounds in other wines, the results obtained in this study are presented as equivalents of both of these acids. Heinonen et al.[Citation15] pointed out that the content of total phenols in fruit wines, produced from black currants, blueberries, cranberries, strawberries and apples and expressed as the equivalent of gallic acid, was in the range 260–1800 mg/L. Comparing the quality parameters of grape wine with other fruit wines available on the Canadian market, Rupasinghe and Clegg[Citation32] found that the total phenols content in plum wine is significantly lower compared with that of red wine (Cabernet Sauvignon) and to that of wines produced from blueberries, raspberries, blackcurrants and cranberries, while being higher than those determined in wines produced from peaches, pears, apples and the white wine varieties of Chardonnay and Riesling. The content of total phenolic compounds in the plum wine analysed in the work of Rupasinghe and Clegg[Citation32] (555 mg/L, as GAE) was significantly lower compared with the results that were obtained for the plum wines in our study (870–1160 mg/L), as shown in . The observed disparity of the results may be due to the use of different plum varieties as well as different vinification procedures, given that fruit wine production often includes the dilution of pomace with water during the primary processing phase.
Čačanska lepotica wine contained the largest amount of total anthocyanins (187.2–194.6 mg/L) and flavan-3-ols (198.8 mg/L) among the produced wines. The largest share of monomeric in total anthocyanin (65%) and the highest content of this anthocyanin fraction (111.7 mg/L) were determined in the wine made from the Požegača variety. For comparison, the content of total anthocyanins in blackberry wine was 135–165 mg/L[Citation33] and in sparkling plum wine it was 110–120 mg/L.[Citation34] When comparing two different growing sites, it can be seen that the influence of plum growing conditions on the content of major polyphenol parameters of the wines obtained from the same variety was statistically significant only in terms of total phenolics of Požegača wine and total flavan-3-ols of Čačanska lepotica wine. The plum wine CI was the most prominent in Čačanska lepotica wine, whereas Požegača wine was characterised by the highest value of colour hue. The wine obtained from the Čačanska rana variety was characterised by the lowest values of CI and hue as well as the largest share of the colour red (47.3%). The CI of the conventional red wines ranges from 0.3 to 1.8, depending on the variety used and the style of the wine produced. The colour hue in the young red wines is in the range 0.5–0.7, while ageing leads to an increase of this parameter (1.2–1.3).[Citation19]
Van Balen[Citation35] stated that the extraction of pigments from grape skins before, during and after wine fermentation is only partial (on average about 30–40%). It can be seen that yellow tones predominate in the Čačanska lepotica and Požegača varieties (from 43.9 to 47.7%), whereas the Čačanska rana variety had the highest share of the red tones recorded. Brilliance - dA (93–95%) and the share of blue (12.7–13.5%) did not differ significantly among the produced plum wines. The high value of the brilliance parameter indicates a dominance of red colour in a young wine.[Citation19] The fraction of wine colour originating from co-pigmentation reactions differed significantly depending on the plum variety used. The biggest influence of co-pigmentation on colour was found in the wine obtained from Požegača variety. Contrary to that, a distinct contribution of a high share of free anthocyanins to the colour (46.6%) characterised Čačanska lepotica wine. Furthermore, the chemical age of this wine (11.3%) was significantly less pronounced in the wines obtained from the other two plum cultivars (16.8–17.6%). The values of the chemical age index for young wines are usually in the range 2–20%. The rapid increase in the value of this chromatic parameter during ageing suggests that wine in general does not have a long ageing potential. Kalkan Yildirim[Citation16] compared, in his paper, the chromatic characteristics of the fruit wines obtained from blueberries, blackberries, black mulberries, raspberries, strawberries, cherries, quinces, apples and apricots, and found that the blueberry wine was characterised by the highest CI, the highest proportion of the yellow, red and blue colour tones and the highest share of polymeric pigments.
The characterisation of the wines obtained from the local plum varieties was also carried out by determining the qualitative and quantitative compositions of the main phenolic compounds using high-performance liquid chromatography (HPLC). In this part of the research, to assess the impact of various environmental factors (microclimate, soil) on the composition and content of individual polyphenols, Požegača plums from two additional growing sites were used for the wine production. The content of the identified components is shown in .
Table 2. HPLC analysis of individual phenolic compounds in the produced plum wines, (mg/L).
HPLC analysis of the wine obtained from the local plum varieties, conducted using the available standards, determined the presence of 13 polyphenols (eight flavonoids and five phenolic acids). The concentrations of gallic, syringic i t-cinnamic acid, resveratrol, piceid, naringenin and hesperetin in the produced plum wines were below the detection limit of the method applied. The presence of peonidin-3-glucoside, cyanidin-3-rutinoside, peonidin-3-rutinoside, chlorogenic, caffeic and benzoic acids and rutin was detected in all the tested wines, whereas the presence of other phenolic compounds characterised certain varieties or growing sites. Two anthocyanins, peonidin-3-glucoside and cyanidin-3-rutinosid, were present in high amounts (up to 97 mg/L) in the plum wines. This is in accordance with the work of Walkowiak-Tomczak,[Citation1] who pointed out that cyanidin-3-glucoside, cyanidin-3-rutinosid and peonidin-3-glucoside are the most abundant anthocyanins in plums. Malvidin-3-glucoside, petunidin-3-glucoside and delphinidin-3-glucoside were not detected in the plum wines produced in this study. Significant amounts of some phenolic acids, namely vanillic, chlorogenic, caffeic, benzoic and p-coumaric, were determined in the produced plum wines. The content of these polyphenols was in the range 1–16 mg/L, depending on the variety and the growing site. Furthermore, low concentrations (< 1.5 mg/L) of quercetin and kaempferol were determined in all analysed plum wines.
Čačanska rana wine was characterised by a relatively high concentration of cyanidin-3-rutinoside (97.2 mg/L), a low concentration of peonidin-3-glucoside (0.22 mg/L) and benzoic acid (0.74 mg/L) and the absence of catechin. The content of peonidin-3-rutinoside, certain phenolic acids (chlorogenic, vanillic and caffeic) and rutin was significantly higher in Čačanska lepotica wine compared with the wines obtained from the other two plum varieties. The most important characteristics of Požegača wine were the high contents of peonidin-3-glucoside (24.44 mg/L) and catechin (14.91 mg/L), as well as the absence of vanillic acid and quercetin.
The composition of the individual polyphenols in Požegača wine differed significantly depending on the growing location of the plums used. The most notable differences were observed in terms of peonidin-3-glucoside, cyanidin-3-rutinoside, benzoic acid and rutin contents. The plums from sites I and II provided wines with the smallest differences in the composition of polyphenols. More pronounced differences between the two wines were present only in the contents of benzoic and p-coumaric acids. Požegača wine, produced from plums from site III, was characterised by the highest content of peonidin-3-glucoside (61.6 mg/L), benzoic acid (11.35 mg/L) and catechin (19.65 mg/L) and the lowest content of cyanidin-3-rutinoside (9.89 mg/L).
The composition of polyphenols in the produced plum wines () is compared with the results for the grape and cherry wine investigated in the study of Pantelić et al.[Citation36] The obtained plum wines contain higher amounts of chlorogenic acid, catechin and rutin compared with the previously mentioned types of wine. The composition of anthocyanins in the plum wines is much more similar to the cherry wine than to the grape wine. The only anthocyanin detected in the wines produced from all three types of fruit is cyanidin-3-glucoside. The most abundant anthocyanin in the plum and cherry wine is cyanidin-3-rutinosid. Moreover, both types of wine are characterised by the presence of peonidin-3-glucoside and peonidin-3-rutinoside.
Antiradical activity of plum wines
Antiradical activity of the produced plum wines on the stable DPPH• radicals was evaluated using the DPPH spectrophotometric method. The values of free radicals scavenging capacity (RSC, %) were used to determine the IC50 values (mL/mL) of the wines, and therefore are not directly presented in the paper. All analysed plum wines showed significant antiradical activity (IC50 from 0.1 to 0.23 mL/mL) (). Free RSC (%) of the tested wines was maximal (100%), and high values of this parameter were also recorded for the analysed wines, which were initially diluted twice (RSC 80–93%). Two different types of Čačanska lepotica wine, produced from the plums from both growing sites, were characterised by the highest content of total phenolic compounds, anthocyanins and flavan-3-ol, and the lowest IC50 value (i.e. the highest antiradical activity). Significantly (p < 0.05) lower activity against DPPH• radicals was found in the wines obtained from the other two plum varieties. The wines obtained from Čačanska rana variety and Požegača variety did not show differences in antioxidant activity, despite significant differences in the content of total phenols and flavan-3-ols. Furthermore, differences in the content of total phenolics in the wines made from the same variety, but from different growing sites, did not cause differences in antiradical activity, as can be seen in the case of Požegača wine (). The scientific literature also reports cases where the wines whose total phenols content does not differ significantly are characterised by different antiradical activities.[Citation37] The antiradical activity of phenolic compounds greatly depends on their structure (the number and position of hydroxyl groups).
The correlation analysis showed a significant (p < 0.05) negative linear correlation (r = −0.828) between the content of total phenolic compounds and IC50 values () in the produced plum wines. The negative slope in the obtained regression model indicates that the increase in the concentration of total phenols leads to IC50 value decrease, i.e. the antiradical activity of the wine is increasing. A significant positive linear correlation between the antioxidant potential and total phenolic content was also previously determined for the fruit wines obtained from plums, pears, apples, apricots, raspberries, blueberries, blackberries, quinces, strawberries and cherries.[Citation16,Citation17,Citation32] Determination of the dependence between the content of the total anthocyanins and IC50 values demonstrated an even higher degree of correlation among the variables from the one manifested in the case of the total phenols content. The value of the linear correlation coefficient r = −0.905 indicated that there was a strong negative correlation between the content of total anthocyanins and the IC50 value. This means that the volumetric concentrations of wine necessary for 50% of DPPH• radicals neutralisation were lower when the content of total anthocyanins was higher. Radovanović et al.[Citation38] also confirmed a significant correlation between the content of anthocyanins and the neutralisation capacity for DPPH• radicals. On the other hand, a very weak degree of linear correlation (r = −0.323) was determined between the contents of flavan-3-ols and the IC50 value, and hence the wines with a higher content of these compounds were not characterised by a higher antioxidant potential. In the work of Puškaš et al.,[Citation39] a high antioxidant potential of the wines produced with additional amounts of grape seeds was explained by the increased content of phenolic compounds (primarily flavan-3-ols), compared with the wines made without the additional seeds. Therefore, when comparing plum wines from our study to the conventional ones, a weak contribution of flavan-3-ols to the antiradical activity of the plum wines can be explained by the different composition and content of these compounds in the raw material. Based on the results of the correlation analysis, the plum wines with a higher content of total phenolics and anthocyanins can be regarded as better antioxidants.
Figure 1. Correlation between the antiradical activity of wine and a) total phenolics, b) total anthocyanins and c) total flavan-3-ols.
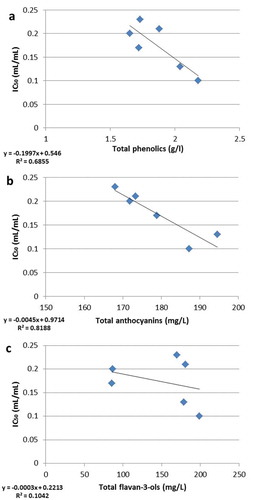
Differences in the antioxidant potential of wine depend more on the concentration of individual phenolic compounds than on the content of total phenols. The antioxidants in wine exhibit higher antiradical activities working together (synergistic effect) rather than working individually.[Citation40] The content of some phenolic compounds correlates differently with antiradical activity and therefore their contribution to the antioxidant potential of wines varies. The most prominent activity in terms of free radicals scavenging are gallic, caffeic and ferulic acid, quercetin, rutin, and some anthocyanins.[Citation41,Citation42] Significant concentrations of some of these compounds (caffeic acid, quercetin, rutin and cyanidin-3-rutinosid) characterise the plum wines produced in our study (). The results are in favour of the research, which emphasises a greater scavenging activity of plum wine compared with any other fruit wines (apple, pear, peach, cherry, raspberry).[Citation32]
Conclusion
The content of total phenols, flavan-3-ols and anthocyanins in wines differs significantly (p < 0.05) depending on the plum variety. Čačanska lepotica wine was characterised by the highest content of total phenols (2.18 g/L), total anthocyanins (187.2 mg/L) and flavan-3-ols (198.8 mg/L) and the highest wine CI (0.73). The largest share of monomeric in total anthocyanin (65%), as well as the strongest influence of co-pigmentation reactions on wine colour was determined in the plum wine obtained from the Požegača variety. The presence of peonidin-3-glucoside, cyanidin-3-rutinoside, peonidin-3-rutinoside, chlorogenic and caffeic acids and rutin was detected in all tested wines, whereas other determined phenolic compounds were characteristic for certain varieties or growing sites. The composition of the polyphenols in Požegača wine varied significantly depending on the location where the plums were grown. The produced plum wines, especially the ones obtained from Čačanska lepotica variety, exhibited significant scavenging activity (IC50 0.1–0.23 ml/mL) against DPPH free radicals. Finally, a significant positive linear correlation (r = 0.828–0.905) between the antiradical activity and the content of total phenolics and total anthocyanins was determined.
Additional information
Funding
References
- Walkowiak-Tomczak, D.;. Characteristics of Plums as a Raw Material with Valuable Nutritive and Dietary Properties—a Review. Polish Journal of Food and Nutrition Sciences 2008, 58(4), 401–405.
- Chun, O. K.; Kim, D.-O.; Moon, H. Y.; Kang, H. G.; Lee, C. Y. Contribution of Individual Polyphenolics to Total Antioxidant Capacity of Plums. Journal of Agricultural and Food Chemistry 2003, 51(25), 7240–7245.
- Kim, D.-O.; Jeong, S. W.; Lee, C. Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chemistry 2003, 81(3), 321–326.
- Voća, S.; Galić, A.; Šindrak, Z.; Dobričević, N.; Pliestić, S.; Družić, J. Chemical Composition and Antioxidant Capacity of Three Plum Cultivars. Agriculturae Conspectus Scientificus 2009, 74(3), 273–276.
- Donovan, J. L.; Meyer, A. S.; Waterhouse, A. L. Phenolic Composition and Antioxidant Activity of Prunes and Prune Juice (Prunus Domestica). Journal of Agricultural and Food Chemistry 1998, 46(4), 1247–1252.
- Łoś, J.; Wilska-Jeszka, J.; Pawlak, M. Polyphenolic Compounds of Plums (Prunus Domestica). Polish Journal of Food and Nutrition Sciences 2000, 50(1), 35–38.
- Nakatani, N.; Kayano, S.-I.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, Quantitative Determination, and Antioxidative Activities of Chlorogenic Acid Isomers in Prune (Prunus Domestica L.). Journal of Agricultural and Food Chemistry 2000, 48(11), 5512–5516.
- Tomás-Barberán, F. A.; Gil, M. I.; Cremin, P.; Waterhouse, A. L.; Hess-Pierce, B.; Kader, A. A. HPLC−DAD−ESIMS Analysis of Phenolic Compounds in Nectarines, Peaches, and Plums. Journal of Agricultural and Food Chemistry 2001, 49(10), 4748–4760.
- Auger, C.; Al-Awwadi, N.; Bornet, A.; Rouanet, J.-M.; Gasc, F.; Cros, G.; Teissedre, P.-L. Catechins and Procyanidins in Mediterranean Diets. Food Research International 2004, 37(3), 233–245.
- Siddiq, M. Plums and Prunes. In Handbook of Fruits and Fruit Processing; Yh, H.; Eds.; Blackwell Publishing Ltd: Oxford, UK, 2006; 553–564.
- Murcia, M. A.; Jiménez, A. M.; Martínez-Tomé, M. Evaluation of the Antioxidant Properties of Mediterranean and Tropical Fruits Compared with Common Food Additives. Journal of Food Protection 2001, 64, 2037–2046.
- Wang, H.; Cao, G.; Prior, R. L. Total Antioxidant Capacity of Fruits. Journal of Agricultural and Food Chemistry 1996, 44(3), 701–705.
- Rupasinghe, H. P. V.; Jayasankar, S.; Lay, W. Variation in Total Phenolics and Antioxidant Capacity among European Plum Genotypes. Scientia Horticulturae 2006, 108(3), 243–246.
- Will, F.; Dietrich, H. Optimised Processing Technique for Colour- and Cloud-Stable Plum Juices and Stability of Bioactive Substances. European Food Research and Technology 2006, 223(3), 419–425.
- Heinonen, I. M.; Lehtonen, P. J.; Hopia, A. I. Antioxidant Activity of Berry and Fruit Wines and Liquors. Journal of Agricultural and Food Chemistry 1998, 46(1), 25–31.
- Kalkan Yildirim, H.;. Evaluation of Colour Parameters and Antioxidant Activities of Fruit Wines. International Journal of Food Sciences and Nutrition 2006, 57(1–2), 47–63.
- Zhuang, H.; Du, J.; Wang, Y. Antioxidant Capacity Changes of 3 Cultivar Chinese Pomegranate (Punica Granatum L.) Juices and Corresponding Wines. Journal of Food Science 2011, 76(4), C606–C611.
- Yuan, Z. L.;. Comparison of Antioxidant Activity in Five Fruit Wine of Guangxi in China. Advanced Materials Research 2013, 641-642, 875–881.
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic Compounds. In Handbook of Enology; Eds.; John Wiley & Sons, Ltd: Chichester, England, 2006; 141–203.
- Boulton, R.; Neri, R.; Levengood, J.; Vaadia, M. Copigmentation of Anthocyanins in Cabemet Sauvignon and Merlot Wines from the Napa Valley of California. In 6th Symposium International d’CEnologie, Paris, France, 1999. 35–38.
- Singleton, V. L.; Orhofer, R.; Lamuela-Raventos, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods in Enzymology 1999, 299, 152–178.
- Revilla, E.; Alonso, E.; Burzeix, M.; Heredia, N. Dosage Des Catéchines Et Des Proanthocyanidols Dans Les Vins. Bulletin De L’ OIV 1991, 829.
- Ribéreau-Gayon, P.; Stonestreet, E. Le Dosage Des Anthocyanes Dans Le Vin Rouge. Bulletin De La Societe Chimique De France 1965, 9, 2649–2652.
- Fuleki, T.; Francis, F. J. Quantitative Determination of Anthocyanins—2. Determination of Total Anthocyanin and Degradation Index for Cranberry Juice. Journal of Food Science 1968, 33, 78–83.
- Pezet, R.; Pont, V.; Cuenat, P. Method to Determine Resveratrol and Pterostilbene in Grape Berries and Wines Using High-Performance Liquid Chromatography and Highly Sensitive Fluorimetric Detection. Journal of Chromatography A 1994, 663(2), 191–197.
- OIV. 2013. Compendium of International Methods of Wine and Must Analysis; Office International del la Vigne et du Vin: Paris, France.
- Espín, J. C.; Soler-Rivas, C.; Wichers, H. J. Characterization of the Total Free Radical Scavenger Capacity of Vegetable Oils and Oil Fractions Using 2,2-Diphenyl-1-Picrylhydrazyl Radical. Journal of Agricultural and Food Chemistry 2000, 48(3), 648–656.
- Castillo-Sánchez, J. J.; Mejuto, J. C.; Garrido, J.; García-Falcón, S. Influence of Wine-Making Protocol and Fining Agents on the Evolution of the Anthocyanin Content, Colour and General Organoleptic Quality of Vinhão Wines. Food Chemistry 2006, 97(1), 130–136.
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J. M.; Martínez, A. Color and Phenolic Compounds of a Young Red Wine. Influence of Wine-Making Techniques, Storage Temperature, and Length of Storage Time. Journal of Agricultural and Food Chemistry 2000, 48(3), 736–741.
- Buglass, A. J.;. Fruit Wines and Other Nongrape Wines. In Handbook of Alcoholic Beverages; Eds.; John Wiley & Sons, Ltd: Chichester, England, 2010; 419–435.
- Kyoung Chun, O.; Kim, D.-O. Consideration on Equivalent Chemicals in Total Phenolic Assay of Chlorogenic Acid-Rich Plums. Food Research International 2004, 37(4), 337–342.
- Rupasinghe, H. P. V.; Clegg, S. Total Antioxidant Capacity, Total Phenolic Content, Mineral Elements, and Histamine Concentrations in Wines of Different Fruit Sources. Journal of Food Composition and Analysis 2007, 20(2), 133–137.
- Mudnić, I.; Budimir, D.; Modun, D.; Gunjača, G.; Generalić, I.; Skroza, D.; Katalinić, V.; Ljubenkov, I.; Boban, M. Antioxidant and Vasodilatory Effects of Blackberry and Grape Wine. Journal of Medicinal Food 2012, 15(3), 315–321.
- Joshi, V. K.; Sharma, S. K.; Goyal, R. K.; Thakur, N. S. Effect of Method of Secondary Fermentation and Type of Base Wine on Physico-Chemical and Sensory Qualities of Sparkling Plum Wine. Brazilian Archives of Biology and Technology 1999, 42(3), 315–322.
- Van Balen, J.;. 1984. Recovery of Anthocyanins and Other Phenols from Converting Grapes into Wine; University of California, Davis.
- Pantelić, M.; Dabić, D.; Matijašević, S.; Davidović, S.; Dojčinović, B.; Milojković-Opsenica, D.; Tešić, Ž.; Natić, M. Chemical Characterization of Fruit Wine Made from Oblačinska Sour Cherry. Scientific World Journal 2014, 2014, 1–9.
- Atanacković, M.; Petrović, A.; Jović, S.; Bukarica, L. G.; Bursać, M.; Cvejić, J. Influence of Winemaking Techniques on the Resveratrol Content, Total Phenolic Content and Antioxidant Potential of Red Wines. Food Chemistry 2012, 131(2), 513–518.
- Radovanović, A.; Radovanović, B.; Jovančićević, B. Free Radical Scavenging and Antibacterial Activities of Southern Serbian Red Wines. Food Chemistry 2009, 117(2), 326–331.
- Puškaš, V.; Jović, S.; Antov, M.; Tumbas, V. Antioxidative Activity of Red Wine with the Increased Share of Phenolic Compounds from Solid Parts of Grape. Chemical Industry & Chemical Engineering Quarterly 2010, 16(1), 65–71.
- Meyer, A. S.; Yi, O.-S.; Pearson, D. A.; Waterhouse, A. L.; Frankel, E. N. Inhibition of Human Low-Density Lipoprotein Oxidation in Relation to Composition of Phenolic Antioxidants in Grapes (Vitis Vinifera). Journal of Agricultural and Food Chemistry 1997, 45(5), 1638–1643.
- Saint-Cricq De Gaulejac, N.; Glories, Y.; Vivas, N. Free Radical Scavenging Effect of Anthocyanins in Red Wines. Food Research International 1999, 32(5), 327–333.
- Sanchez-Moreno, C.; Larrauti, J. A.; Saura-Calixto, F. Free Radical Scavenging of Selected Red, Rose and White Wines. Journal of the Science of Food and Agriculture 1999, 79(10), 1301–1304.

