ABSTRACT
The objective of this study was to determine the in vitro inhibition effects of seven commonly used pesticides including 2,4-d-acid dimethylamine, fenoxaprop-p-ethyl, glyphosate isopropylamine, haloxyfop-p-methyl, cypermethrin, λ-cyhalothrin, and dichlorvos on the peroxidase purified from turnip (Brassica rapa L.) and black radish (Raphanus sativus L.) using 4-amino benzohydrazide affinity column chromatography. The purification factors for the turnip and black radish peroxidases were found to be 263.29-fold (with a yield of 12.89%) and 36.20-fold (with a yield of 6.90%), respectively. Among these compounds, λ-cyhalothrin showed the strongest inhibitory effect against turnip peroxidase (Ki: 1.23 × 10−2 ± 0.21 × 10−2 mM) as noncompetitive inhibition. On the other hand, cypermethrin demonstrated the highest inhibition effect against black radish peroxidase (Ki: 2.14 × 10−2 ± 0.08 × 10−2 mM) as competitive inhibition.
Introduction
Horticultural plants including have been an indispensable part of human life for ages. Ever since ancient times, their fruits, seeds, and even roots and branches have been used to meet personal and social needs such as severing food, curing diseases, and beautifying the planet.[Citation1–Citation4] Plant peroxidases (PODs) are found in all plants and control plant growth and development. Also they are found in the cell wall and are responsible for diverse cellular processes in plant metabolism for construction, lignifications, senescence, organogenesis, phenol oxidation, cross-linking of cell wall proteins, and protection of tissue.[Citation5–Citation7] PODs (E.C.1.11.1.7) contain a heme protein, which are part of the oxidoreductases. They have antioxidant features that catalyzed the reactive oxygen species (ROS) using hydrogen peroxide (H2O2) generated during metabolism and are converted into harmless molecules.[Citation8,Citation9] PODs are widely present in prokaryotes, eukaryotes, and photosynthetic cells. As noted, PODs have a crucial function in plant metabolism.
To increase crop protection and to affect the growth of productivity, a range of chemicals has been increasingly used in agriculture.[Citation10–Citation12] Some of these are pesticides including carbamates, organophosphates, organochlorines, and bipyridyls. Pesticides are chemical substances which are used for many purposes in nature as a biological agent, commonly to control pests that are considered harmful, and on unwanted plants, and microorganisms.[Citation13] There are many researches related to PODs in many manufacturing processes. Some studies have shown that PODs may be used successfully to polymerize anilines and phenols in organic solvent matrices.[Citation14] However, lactoperoxidase is one of the most studied members of the POD family.[Citation15–Citation17]
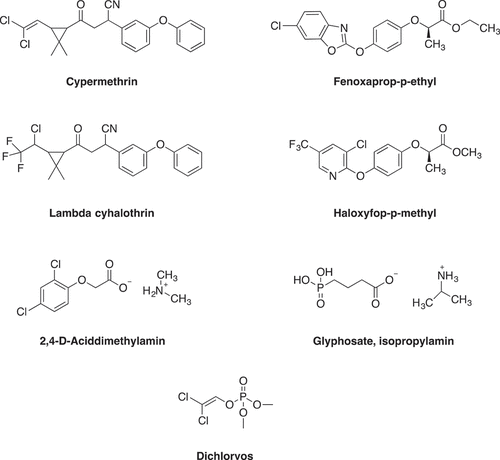
On the other hand, pesticides can become a risk factor for living organisms and other nontargeted creatures due to the negative effects such as metabolic disorders, and severe acute and chronic poisoning. Some pesticides are highly toxic and are still widely used for pest insect control.[Citation18] They cause serious concerns over food safety and environmental pollution. Pesticides can cause oxidative stress by the formation of free radicals and change in antioxidants, generate ROS, and alter enzyme systems in metabolism.[Citation19] 2,4-d-acid dimethylamine, fenoxaprop-p-ethyl, glyphosate isopropylamine, and haloxyfop-p-methyl are used as herbicides. Cypermethrin, λ-cyhalothrin, and dichlorvos are used as insecticides in agricultural applications.[Citation20] 2,4-d-acid dimethylamine is used as a selective herbicide that kills many terrestrial and aquatic broadleaf weeds. It is effective on the growth hormone auxin, which results in uncontrolled growth.[Citation16] Fenoxaprop p-ethyl is a widely used effective herbicide which is used to control grass weeds, crops, vegetables, feed, and forage crops.[Citation21] Glyphosate isopropylamine is commonly used as a post-emergence herbicide, which is used to kill weeds, broadleaf weeds, and grasses. It prevents the synthesis of aromatic amino acids in the plant-restricted shikimate pathway. However, this compound had a cardiotoxic effect in mammals.[Citation22] Haloxyfop-p-methyl is frequently used as a selective herbicide for control in plants such as sugar beets, canola, and various vegetables.[Citation23] Cypermethrin is a synthetic pyrethroid which demonstrates insecticidal properties. It is used during food storage, in public health, animal husbandry, and on cotton, cereals, vegetables, and fruits.[Citation24]
λ-Cyhalothrin is an insecticide belonging to a class of pyrethroid chemicals, which has an effect on the nervous system.[Citation25] Dichlorvos is known as an insecticide belonging to a group of organophosphates. It is usually preferred to control household pests, for public health, and protecting stored products from pests. It has negative effects against fruit and vegetable crops. It has effects on acetylcholinesterase, which damages the DNA of insects.[Citation26,Citation27]
In living organisms, all chemical reactions are catalyzed by enzymes, which are effected by many chemical substances including pesticides, drugs, and metal ions. Scientific researches have indicated that pesticides show toxic effects by inhibiting the enzymes at low concentration usage. So, many problems occur in the living metabolism.[Citation27] Pesticides play an important role in high agricultural productivity, but due to inevitable toxicity, often toward nontarget organisms, their uncritical release into the environment has serious consequences.[Citation28]
In the literature, there are many studies focused on the investigation of POD’s new inhibitors. However, there are no detailed studies about the effect of pesticides on PODs in organisms. For this reason, this is the first study investigating inhibition effects of some commonly used pesticides against POD purified from turnip (tPOD) (Brassica rapa L.) and black radish (rPOD) (Raphanus sativus L.) using 4-amino benzohydrazide affinity column chromatography.
Materials and methods
Chemicals and instruments
In affinity chromatography, 4-amino benzohydrazide was used as a ligand; L-Tyrosine was used as a spacer arm, and CNBr-activated-Sepharose-4B was used as a matrix. Glycerol, bovine serum albumin (BSA) (lyophilized powder), guaiacol, H2O2, potassium phosphate (monobasic), and all other chemical reagents used were obtained from Sigma Aldrich. 2,4-d-acid dimethylamine, fenoxaprop-p-ethyl, glyphosate-isopropylamine, haloxyfop-p-methyl, cypermethrin, λ-cyhalothrin, dichlorvos, and all chemicals were of analytical grade. An ultraturrax (Heidolph Silient Crusher M, Germany) and a centrifuge (HermleZ 323K, Germany) were used to prepare a homogenate, a pH-meter (SCHOTT CG840, Germany) was used for preparation of a buffer solution, a peristaltic pump (Ismatec) was used to ensure stable flow rate, and a UV-VIS spectrophotometer (Beckman Coulter Du 730, USA) was used for kinetic assay.
Preliminary processing of plant materials
Fresh turnip (B. rapa L.) and black radish (R. sativus L.) were supplied from a local market, Erzurum. They were washed, drained, separated into small pieces, and stored at −20°C until use, in this order.[Citation10]
Preparation procedure of the homogenate
The black radish and turnip samples (approximately 15 g) were shred with mixer then treated with liquid nitrogen to freeze, and then pestled to a grain size of sand. Buffer solution (KH2PO4, 0.3 M, pH 7.0) was added to the homogenate. Then the mixture was stirred for about 10 min and centrifuged at 10,000×g for 60 min at +4°C. Supernatant was stored at −20°C in small aliquots before usage.[Citation10]
POD activity assay
The POD activity was measured at 470 nm by using UV-VIS spectrophotometer.[Citation11] The POD activity assay was prepared as follows: 1 mL of guaiacol (45 mM), 1 mL of H2O2 (22.5 mM) was mixed in a tube, then an aliquot of POD enzyme (0.1 mL) was added to the mixture. The final volume of the mixture was adjusted to 3 mL by the addition of phosphate buffer (0.2 M). The absorbance was measured every 60 s. One unit of POD enzyme was defined as the amount of enzyme able to catalyze the oxidation of 1 µmol of guaiacol/min−1 at 25°C (molar absorption coefficient, 5000 M−1 cm−1).[Citation10]
Protein determination
The quantity of protein was determined according to the Bradford method (1976) as described previously.[Citation29–Citation31] BSA was used as standard protein and described in detail in our previous studies.[Citation32–Citation34]
Kinetic and inhibition studies
To identify the kinetic parameters, POD activity was determined. Also, for determination of inhibition parameters for each pesticide, IC50 and Ki values were measured. IC50 value was determined with constant guaiacol concentration in the presence of five different concentrations of pesticides. Likewise, three different concentrations of pesticides and five different concentrations (9.0, 10.5, 12.0, 13.5, and 15.0 mM) of substrate were used to determine the Ki constant. The Ki values and the inhibition types were calculated from Lineweaver–Burk plots (1/V – 1/[S]).[Citation35]
Results and discussion
PODs are found in a wide range of living organisms including plants, animals, vertebrates, fungi, and microorganisms. They have varying fields of application in biotechnology, food, and chemical industries. Some PODs such as horseradish peroxidase and chloroperoxidase have been used to prepare enantiomerically pure alkyl hydroperoxides and alcohols. In addition, lactoperoxidase conserves food quality and extends shelf life.[Citation9,Citation26,Citation36]
Plant PODs have several important physiological functions such as construction, lignifications of cell walls, senescence, organogenesis, phenol oxidation, cross-linking of cell wall proteins, and protection of tissue damage; they also prevent infection caused by pathogenic microorganisms.[Citation17] They catalyze the oxidation reaction of a wide variety of substrates including 2,2ʹ-azino-bis(3-etilbenztiazolin-6-sulfonic acid, p-phenylenediamine, pyrogallol, guaiacol, catechol, simple phenols, aromatic amines, ascorbates, epinephrine, and tetramethylbenzidine in the presence of H2O2.[Citation37]
With the development of new technology areas, there is attention on the investigation of PODs in order to find various POD sources and inhibitors. It was reported that PODs have been purified and characterized from a wide variety of plant sources including turnip (B. rapa) roots, black radish, papaya (Carica papaya) fruit, sweet potato tubers, sweet gourd (Cucurbita moschata), chard leaves (Beta vulgaris), olives (Olea europaea), black gram, leucaena (Leucaena leucocephala), Caribbean plant (Euphorbia cotinifolia), cauliflower (Brassica oleracea) buds, buckwheat, palm oil (Elaeis guineensis) leaf, cotton (Gossypium hirsutum), and white cabbage (Brassica oleracea).[Citation10,Citation26]
Some POD inhibitors can be used as the ligands for plant POD purification by the chromatographic methods. 4-Aminobenzohydrazide is a well-known POD inhibitor that inhibits the myeloperoxidase enzyme. Therefore, 4-aminobenzohydrazide and its derivatives can be used as inhibitors.[Citation37,Citation38] In the present study, POD was purified from black radish (R. sativus L.) 36.02-fold with a yield of 6.90% and from turnip (B. rapa L.) 263.29-fold with a yield of 12.89% using 4-aminobenzohydrazide affinity column as shown in .
Table 1. Purification steps of rPOD and tPOD with a sepharose-4B-tyrosine-4-aminobenzohydrazide affinity column chromatography.
It is well known that enzymes catalyze almost all chemical reactions in the metabolism of the living organisms. Many chemical substances including pesticides, fungicides, drugs, and metal ions influenced their activities even at low concentrations.[Citation39,Citation40] The POD inhibitors were identified as nonselective monoamine reuptake inhibitors including opipramol, lofepramine, dibenzepin, protriptyline, melitracen, butriptyline, dimetacrine, dosulepin, and quinapyramine. Also, they contain selective serotonin reuptake inhibitors including alaproclate and etoperidone; nonselective monoamine oxidase inhibitors including moclobemide, toloxatone, and isocarboxazid; and other antidepressants are viloxazine, minaprine, bifemelane, oxaflozane, and medifoxamine.[Citation41]
Exposure to pesticides is considered to be one of the most important occupational health risks among farmers in developing countries.[Citation42] The uses of pesticides for the protection of fruits and vegetables have shown positive effects by increasing agricultural production. It was reported that only 5% of pesticides reach the target plant organism.[Citation43] More serious effects were produced by direct inhalation of pesticide than by absorption or ingestion of toxins. The rest runs off into water or dissipates in the soil or air.[Citation44] Researchers are looking for either persistent or less toxic pesticides. While organophosphorus, carbamate, and paraquat aluminum phosphide are highly dangerous, pyrethroids, glyphosate, and neonicotinoids have very few side effects.[Citation45,Citation46]
In this section, we analyze the inhibition of POD activity by investigated pesticides. For this purpose, the inhibition kinetics of 2,4-d-acid dimethylamine, fenoxaprop-p-ethyl, glyphosate isopropylamine, haloxyfop p-methyl, cypermethrin, λ-cyhalothrin, and dichlorvos (Ki and IC50) values were determined on rPOD and tPOD ( and ). IC50 is the inhibitor concentration required for 50% inhibition of an enzyme and the Ki value is the constant. The IC50 value was determined by Activity (%)–[Pesticide] graphs for each pesticide.[Citation47–Citation48] Lineweaver–Burk curves were used to determine the Ki value and the inhibition type of the pesticide.[Citation34,Citation49–Citation51] Three different pesticide concentrations were tested at five different substrate concentrations for each pesticide.[Citation52–Citation54]
Table 2. IC50 value, Ki constant, and inhibition type of pesticides for rPOD
Table 3. IC50 values, Ki constants, and inhibition types of pesticides for peroxidase purified from turnip (Brassica rapa L.) (tPOD).
As seen in , the obtained IC50 values of pesticides against rPOD are as follows: λ-cyhalothrin (1.82 × 10−2 mM, r2: 0.9591) > cypermethrin (3.85 × 10−2 mM, r2: 0.9412) > fenoxaprop p-ethyl (7.70 × 10−2 mM, r2: 0.9482) > 2,4-d-acid dimethylamine (2.68 mM, r2: 0.9874) > glyphosate isopropylamine (7.70 mM, r2: 0.9151) > dichlorvos (16.0 mM, r2: 0.9882) > haloxyfop p-methyl (23.0 mM, r2: 0.9180). On the other hand, Ki values of these pesticides decreased in the following order: cypermethrin (2.14 × 10−2 ± 0.08 × 10−2 mM) > λ-cyhalothrin (3.80 × 10−2 ± 0.72 × 10−2 mM) > fenoxaprop p-ethyl (7.12 × 10−2 ± 1.83 × 10−2 mM) > 2,4-d-acid dimethylamine (5.33 ± 1.43 mM) > glyphosate isopropylamine (6.76 ± 1.59 mM) > dichlorvos (11.7 ± 4.4 mM) > haloxyfop p-methyl (220.0 ± 23.0 mM). Among them, cypermethrin, which induces the microglial activation and overexpression of pro-inflammatory proteins, had the most effective inhibition effects with Ki value of 3.85 × 10−2 mM. It was reported that the pesticides, which contain −Cl groups (electron-withdrawing groups) at different positions, had effective POD inhibition effects.[Citation44] Also, this pesticide showed inflammation could be critical in degeneration of the nigrostriatal dopaminergic neurons.[Citation55]
As shown in , the results of this study clearly demonstrate the kinetic properties (Ki and IC50 values) of pesticides on tPOD and their inhibition types. The obtained IC50 values decreased as follows: dichlorvos (1.48 × 10−2 mM, r2: 0.9364) > λ-cyhalothrin (3.01 × 10−2 mM, r2: 0.9320) > fenoxaprop p-ethyl (4.07 × 10−2 mM, r2: 0.9044) > cypermethrin (63.0 × 10−2 mM, r2: 0.9718) > glyphosate isopropylamine (0.147 mM, r2: 0.8990) > 2,4-d-acid dimethylamine (7.07 mM, r2: 0.9558) > haloxyfop p-methyl (17.30 mM, r2: 0.9475). On the other hand, Ki values were found to be 1.23 × 10−2 ± 0.21 × 10−2 mM for λ-cyhalothrin, 1.89 × 10−2 ± 0.26 × 10−2 mM for cypermethrin, 2.87 × 10−5 ± 0.41 × 10−2 mM for fenoxaprop p-ethyl, 4.76 × 10−5 ± 1.2 × 10−2 mM for dichlorvos, 0.137 ± 0.024 mM for haloxyfop p-methyl, 1.230 ± 0.024 mM for glyphosate isopropylamine, and 6.98 ± 2.97 mM for 2,4-d-acid dimethylamine. According to these Ki values of pesticides for tPOD, the most effective inhibition profile was demonstrated by λ-cyhalothrin, which is one of the most commonly used pyrethroid insecticides against soybean aphids, with Ki value of 1.23 × 10−2 ± 0.21 × 10−2 mM. λ-Cyhalothrin contains –Cl, –F, and –CN groups. It is well known that the compounds of these reactive groups effectively inhibit enzyme activities[Citation56-Citation58] Also, it was reported that this chemical was one of the top three insecticides used to control the outbreak of soybean aphids in the United States in 2005 ().[Citation59]
Among these compounds, λ-cyhalothrin showed the strongest inhibitory effect on both POD enzymes. λ-Cyhalothrin belongs to a group of pyrethroid insecticides that are known as synthetic chemicals. They show high toxicity against a wide range of insects and low toxicity against mammals that contact or consume the chemical. While the irresponsible usage of pyrethroids has increased, the damage for nontarget species has increased.[Citation60,Citation61] The obtained IC50 values were 3.01 × 10−5 M and 1.82 × 10−5 M for tPOD and rPOD, respectively, and the Ki values were 1.23 × 10−5 ± 0.21 × 10−5 M and 3.80 × 10−6 ± 0.72 × 10−6 M for tPOD and rPOD enzymes, respectively. Also, the inhibition effect of λ-cyhalothrin on tPOD was found to be a noncompetitive inhibition. In this type of inhibition, the inhibitor shows its effect by binding the enzyme at a site other than the active site.[Citation62] For the rPOD, λ-cyhalothrin demonstrated competitive inhibition in this type of inhibitor and the substrate was competitive between binding the enzyme active site.[Citation63–Citation64] The present study considered the molecular structure of pesticides (), all of which have a Cl atom. In addition, λ-cyhalothrin has –CN chromophore groups,[Citation25] which are part of a molecule responsible for its color. Of the pesticides that were used in our study, only glyphosate isopropylamine and dichlorvos have phosphate groups in their structures. –CN is a polar group and has a high dipole moment.[Citation65]
Figure 1. (A) Activity (%)–[λ-cyhalothrin] plot for the tPOD in five different λ-cyhalothrin concentrations; (B) Lineweaver–Burk graph of λ-cyhalothrin for the tPOD using three different λ-cyhalothrin concentrations for the determination of Ki and inhibition type; (C) activity (%)–[λ-cyhalothrin] plot for the rPOD in five different λ-cyhalothrin concentrations; (D) Lineweaver–Burk graph of λ-cyhalothrin for the rPOD using three different λ-cyhalothrin concentrations for the determination of Ki and inhibition type.
![Figure 1. (A) Activity (%)–[λ-cyhalothrin] plot for the tPOD in five different λ-cyhalothrin concentrations; (B) Lineweaver–Burk graph of λ-cyhalothrin for the tPOD using three different λ-cyhalothrin concentrations for the determination of Ki and inhibition type; (C) activity (%)–[λ-cyhalothrin] plot for the rPOD in five different λ-cyhalothrin concentrations; (D) Lineweaver–Burk graph of λ-cyhalothrin for the rPOD using three different λ-cyhalothrin concentrations for the determination of Ki and inhibition type.](/cms/asset/3acdea01-d746-4248-82a0-90a692a78f93/ljfp_a_1424197_f0001_oc.jpg)
Pesticides show their toxic effects by affecting the activation mechanism of the enzymes at low concentrations.[Citation24] In the activation mechanism of PODs, in the first step, the POD reacts with one equivalent of the peroxide to form a Fe (IV) containing compound I that is the porphyrin cation radical. In the second step, in the cation radical compound I, by taking a proton (H+) of the substrate and reduced (Substrate) – Fe (IV) form, the substrate becomes radical to lose a H+. The compound II takes a H+ from the substrate and returns the first reduced form. In this case many problems can occur in the plant metabolism.[Citation66]
Conclusion
The results clearly indicate that all used pesticides effectively inhibited rPOD and tPOD; however, among these commonly used pesticides, λ-cyhalothrin showed the strongest inhibitory effect against rPOD and tPOD. Although the use of pesticides has commercial benefits in the agriculture, it is necessary to adjust the dose of pesticides. Excessive amounts of the pesticides act on the metabolic processes in plants and have deleterious effects on the environment.
References
- Canan, I.; Gundogdu, M.; Seday, U.; Oluk, C. A.; Karasahin, Z.; Eroglu, E. C.; Yazici, E.; Unlu, M. Determination of Antioxidant, Total Phenolic, Total Carotenoid, Lycopene, Ascorbic Acid, and Sugar Contents of Citrus Species and Mandarin Hybrids. Turkish Journal of Agriculture and Forestry 2016, 40, 894–899. DOI: 10.3906/tar-1606-83.
- Erturk, Y.; Ercisli, S.; Haznedar, A.; Cakmakci, R. Effects of Plant Growth Promoting Rhizobacteria (PGPR) on Rooting and Root Growth of Kiwifruit (Actinidia Deliciosa) Stem Cuttings. Biological Research 2010, 43(1), 91–98. DOI: 10.4067/S0716-97602010000100011.
- Hricova, A.; Fejer, J.; Libiakova, G.; Szabova, M.; Gazo, J.; Gajdosova, A. Characterization of Phenotypic and Nutritional Properties of Valuable Amaranthus Cruentus L. Mutants. Turkish Journal of Agriculture and Forestry 2016, 40, 761–771. DOI: 10.3906/tar-1511-31.
- Yazici, K.; Sahin, A. Characterization of Pomegranate (Punica Granatum L.) Hybrids and Their Potential Use in Further Breeding. Turkish Journal of Agriculture and Forestry 2016, 40, 813–824. DOI: 10.3906/tar-1604-120.
- Gulcin, I.; Yıldırım, A. Purification and Characterization of Peroxidase from Brassica Oleracea Var. Asian Journal of Chemistry 2005, 17, 2175–2183.
- Koksal, E.; Aggül, A. G.; Bursal, E.; Gulcin, I. Purification and Characterization of Peroxidase from Sweet Gourd (Cucurbita Moschata Lam. Poiret). International journal of food properties 2012, 15, 1110–1119. DOI: 10.1080/10942912.2010.513216.
- Sisecioglu, M.; Gulcin, I.; Cankaya, M.; Atasever, A.; Sehitoglu, M. H.; Kaya, H. B.; Ozdemir, H. Purification and Characterization of Peroxidase from Turkish Black Radish (Raphanus Sativus L.). al of Medicinal Plants Research 2010, 4, 1187–1196.
- Davies, K. J.;. Oxidative Stress: The Paradox of Aerobic Life. Biochemical Society Symposia 1995, 61, 1–31. DOI: 10.1042/bss0610001.
- Hussain, S.; Slikker, W.; Ali, S. F. Age Related Changes in Antioxidant Enzymes, Superoxide Dismutase, Catalase, Glutathione Peroxidase and Glutathione in Different Region of Mouse Brain. International Journal of Developmental Neuroscience 1995, 13, 811–817. DOI: 10.1016/0736-5748(95)00071-2.
- Kalin, R.; Atasever, A.; Ozdemir, H. Single-Step Purification of Peroxidase by 4-Aminobenzohydrazide from Turkish Blackradish and Turnip Roots. Food Chemistry 2014, 150, 335–340. DOI: 10.1016/j.foodchem.2013.10.125.
- Koksal, E.; Gülcin, I. Purification and Characterization of Peroxidase from Cauliflower (Brassica Oleracea L.). Protein and Peptide Letters 2008, 15, 320–326. DOI: 10.2174/092986608784246506.
- Kwak, S. S.; Kim, S. K.; Park, I. H.; Liu, J. R. Enhancement of Peroxidase Activity by Stress-Related Chemicals in Sweet Potato. Phytochemistry 1996, 43, 565–568. DOI: 10.1016/0031-9422(96)00315-9.
- Mehrpour, O.; Karrari, P.; Zamani, N.; Tsatsakis, A. M.; Abdollahi, M. Occupational Exposure to Pesticides and Consequences on Male Semen and Fertility. Toxicology letters 2014, 230, 146–156. DOI: 10.1016/j.toxlet.2014.01.029.
- Tuhela, L.; Sims, G. K.; Tuovinen, O. Polymerization of Substituted Anilines, Phenols, and Heterocyclic Compounds by Peroxidase in Organic Solvents. The Ohio State University: Columbus, Ohio, 1989; pp 58.
- Gulcin, I.; Scozzafava, A.; Supuran, C. T.; Akıncıoğlu, H.; Koksal, Z.; Turkan, F.; Alwasel, S. The Effect of Caffeic Acid Phenethyl Ester (CAPE) Metabolic Enzymes Including Acetylcholinesterase, Butyrylcholinesterase, Glutathione S-Transferase, Lactoperoxidase and Carbonic Anhydrase Isoenzymes I, II, IX and XII. Journal of enzyme inhibition and medicinal chemistry 2016a, 31, 1095–1101. DOI: 10.3109/14756366.2015.1094470.
- Koksal, Z.; Kalin, R.; Gulcin, I.; Ozdemir, H.; Atasever, A. The Impact of Some Avermectins on Lactoperoxidase from Bovine Milk. International journal of food properties 2016, 19, 1207–1216. DOI: 10.1080/10942912.2015.1076457.
- Sisecioglu, M.; Gulcin, I.; Cankaya, M.; Ozdemir, H. The Inhibitory Effects of L-Adrenaline on Lactoperoxidase Enzyme (LPO) Purified from Buffalo Milk. International journal of food properties 2012, 15, 1182–1189. DOI: 10.1080/10942912.2010.511924.
- Deng, S.; Chen, Y.; Wang, D.; Shi, T.; Wua, X.; Ma, X.; Lia, X.; Hua, R.; Tang, X.; Li, Q. X. Rapid Dehalogenation of Pesticides and Organics at the Interface of Reduced Graphene Oxide-Silver Nanocomposite. Journal of hazardous materials 2016, 308, 192–198. DOI: 10.1016/j.jhazmat.2016.01.004.
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and Oxidative Stress. Medical Science Monitor 2004, 10, 141–147.
- Gianessi, P. L.;. The Increasing Importance of Herbicides in Worldwide Crop Production. Pest management science 2013, 69, 1099–1105. DOI: 10.1002/ps.3598.
- Pornprom, T.; Mahatamnuchoke, P.; Usui, K. The Role of Altered acetyl-CoA Carboxylase in Conferring Resistance to Fenoxaprop-P-Ethyl in Chinese Sprangletop (Leptochloa Chinensis (L.). Nees. Pest management science 2006, 62, 1109–1115. DOI: 10.1002/ps.1287.
- Eddleston, M.;. Pesticides. Medicine 2016, 44, 193–196. DOI: 10.1016/j.mpmed.2015.12.005.
- Thomas, A. U.;. Pesticide Synthesis Handbook, 1st ed. Park Ridge (TN): Noyes Publications, 1996; pp 771.
- Ekinci, D.; Beydemir, S. Risk Assessment of Pesticides and Fungicides for Acid-Base Regulation and Salt Transport in Rainbow Trout Tissues. Pesticide biochemistry and physiology 2010, 97, 66–70. DOI: 10.1016/j.pestbp.2009.12.006.
- Dogan, S.;. The in Vitro Effects of Some Pesticides on Carbonic Anhydrase Activity of Oncorhynchus Mykiss and Cyprinus Carpio Fish. Journal of hazardous materials 2006, 132, 171–176. DOI: 10.1016/j.jhazmat.2005.10.006.
- Erdem, H. U.; Kalın, R.; Ozdemir, N.; Ozdemir, H. Purification and Biochemical Characterization of Peroxidase Isolated from White Cabbage (Brassica Oleracea Var. Capitata F. Alba). International Journal of Food Properties 2015, 18, 2099–2109. DOI: 10.1080/10942912.2014.963868.
- Demirdag, R.; Yerlikaya, E.; Aksakal, E.; Küfrevioglu, O. I.; Ekinci, D. Influence of Pesticides on the pH Regulatory Enzyme, Carbonic Anhydrase, from European Seabass Liver and Bovine Erythrocytes. Environmental toxicology and pharmacology 2012, 34, 218–222. DOI: 10.1016/j.etap.2012.04.007.
- Lazarevic-Pašti, T.; Momic, T.; Radojevic, M. M.; Vasic, V. Influence of Organophosphorus Pesticides on Peroxidase and Chlorination Activity of Human Myeloperoxidase. Pesticide biochemistry and physiology 2013, 107, 55–60. DOI: 10.1016/j.pestbp.2013.05.004.
- Aksu, K.; Ozgeris, B.; Taslimi, P.; Naderi, A.; Gulcin, I.; Goksu, S. Antioxidant Activity, Acetylcholinesterase and Carbonic Anhydrase Inhibitory Properties of Novel Ureas Derived from Phenethylamines. Archiv der pharmazie 2016, 349, 944–954. DOI: 10.1002/ardp.201600183.
- Kocyigit, U. M.; Aslan, O. M.; Gulcin, I.; Temel, Y.; Ceylan, M. Synthesis and Carbonic Anhydrase Inhibition of Novel 2-(4-(Aryl)Thiazole-2-Yl)-3a,4,7,7a-Tetrahydro-1h-4,7-Methanoisoindole-1,3(2h)-Dione Derivatives. Archiv der Pharmazie 2016, 349, 955–963. DOI: 10.1002/ardp.201600092.
- Topal, F.; Gulcin, I.; Dastan, A.; Guney, M. Novel Eugenol Derivatives: Potent Acetylcholinesterase and Carbonic Anhydrase Inhibitors. Bioorganic & medicinal chemistry 2017, 94, 845–851. DOI: 10.1016/j.ijbiomac.2016.10.096.
- Aksu, K.; Nar, M.; Tanç, M.; Vullo, D.; Gülçin, İ.; Göksu, S.; Tümer, F.; Supuran, C. T. The Synthesis of Sulfamide Analogues of Dopamine Related Compounds and Their Carbonic Anhydrase Inhibitory Properties. International journal of biological macromolecules 2013, 21, 2925–2931. DOI: 10.1016/j.bmc.2013.03.077.
- Boztas, M.; Cetinkaya, Y.; Topal, M.; Gülçin, İ.; Menzek, A.; Şahin, E.; Tanc, M.; Supuran, C. T. Synthesis and Carbonic Anhydrase Isoenzymes I, II, IX, and XII Inhibitory Effects of Dimethoxy-Bromophenol Derivatives Incorporating Cyclopropane Moieties. Journal of medicinal chemistry 2015, 58, 640–650. DOI: 10.1021/jm501573b.
- Kucukoglu, K.; Oral, F.; Aydin, T.; Yamali, C.; Algul, O.; Sakagami, H.; Gulcin, I.; Supuran, C. T.; Gul, H. I. Synthesis, Cytotoxicity and Carbonic Anhydrase Inhibitory Activities of New Pyrazolines. Journal of enzyme inhibition and medicinal chemistry 2016, 31, 20–24. DOI: 10.1080/14756366.2016.1217852.
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. Journal of the American chemical society 1934, 56, 658–666. DOI: 10.1021/ja01318a036.
- Dayan, S.; Somturk, B.; Kayaci, N.; Ozdemir, N.; Ozpozan, N. K.; Ozdemir, H. Inhibition of Peroxidase Activity by N-(3-Aminophenyl)-Arylsulfonamides Hydrochloride. Chemistry Journal 2015, 1, 51–57.
- Marquez, O.; Waliszewski, K. N.; Oliart, R. M.; Pardio, V. T. Purification and Characterization of Cell Wall-Bound Peroxidase from Vanilla Bean. Lebensmittel-Wissenschaft & Technologie 2008, 41, 1372–1379. DOI: 10.1016/j.lwt.2007.08.017.
- Kettle, A. J.; Gedye, C. A.; Wınterbourn, C. C. Mechanism of Inactivation of Myeloperoxidase by 4 Amino Benzoic Acid Hydrazide. Biochemical Journal 1997, 321, 503–508. DOI: 10.1042/bj3210503.
- Ekinci, D.; Beydemir, S.; Kufrevioglu, O. I. In Vitro Inhibitory Effects of Some Heavy Metals on Human Erythrocyte Carbonic Anhydrases. Journal of enzyme inhibition and medicinal chemistry 2007, 22, 745–750. DOI: 10.1080/14756360601176048.
- Kucuk, M.; Gulcin, I. Purification and Characterization of Carbonic Anhydrase Enzyme from Black Sea Trout (Salmo Trutta Labrax Coruhensis) Kidney and Inhibition Effects of Some Metal Ions on the Enzyme Activity. Environmental toxicology and pharmacology 2016, 44, 134–139. DOI: 10.1016/j.etap.2016.04.011.
- Kumar, R.; Bhatla, K. L. Purification, Crystallization and Preliminary X-Ray Crystallographic Analysis of Lactoperoxidase from Buffalo Milk. Acta Crystallographica Section D: Biological Crystallography 1995, 51, 1094. DOI: 10.1107/S0907444995004422.
- Thundiyil, J. G.; Stober, J.; Besbelli, N.; Pronczuk, J. Acute Pesticide Poisoning: A Proposed Classification Tool. Bulletin of the World Health Organization 2008, 86, 205–209. DOI: 10.2471/BLT.07.041814.
- Rea, W. J.;. Pesticides. Journal of Nutritional & Environmental Medicine 1996, 6, 55–124. DOI: 10.3109/13590849608999136.
- Davila-Vazquez, G.; Tinoco, R.; Pickard, M. A.; Vazquez-Duhalt, R. Transformation of Halogenated Pesticides by Versatile Peroxidase from Bjerkandera Adusta. Enzyme and Microbial Technology 2005, 36, 223–231. DOI: 10.1016/j.enzmictec.2004.07.015.
- Ceyhun, S. B.; Sentürk, M.; Erdogan, O.; Küfrevioglu, O. I. In Vitro and in Vivo Effects of Some Pesticides on Carbonic Anhydrase Enzyme from Rainbow Trout (Oncorhynchus Mykiss) Gills. Pesticide biochemistry and physiology 2010, 97, 177–181. DOI: 10.1016/j.pestbp.2010.01.003.
- Ekinci, D.; Beydemir, S.; Alım, Z. Some Drugs Inhibit in Vitro Hydratase and Esterase Activities of Human Carbonic anhydrase-I and II. Pharmacological Reports 2007a, 59, 580–587.
- Aydin, B.; Gülcin, I.; Alwasel, S. H. Purification and Characterization of Polyphenol Oxidase from Hemşin Apple (Malus Communis L.). International journal of food properties 2015, 18, 2735–2745. DOI: 10.1080/10942912.2015.1012725.
- Ozbey, F.; Taslimi, P.; Gulcin, I.; Maras, A.; Goksu, S.; Supuran, C. T. Synthesis, Acetylcholinesterase, Butyrilcholinesterase, Carbonic Anhydrase Inhibitory and Metal Chelating Properties of Some Novel Diaryl Ether. Journal of enzyme inhibition and medicinal chemistry 2016, 31, 79–85. DOI: 10.1080/14756366.2016.1189422.
- Genc, H.; Kalin, R.; Koksal, Z.; Sadeghian, N.; Kocyigit, U. M.; Zengin, M.; Gülçin, İ.; Özdemir, H. Discovery of Potent Carbonic Anhydrase and Acetylcholinesterase Inhibitors: 2-Aminoindan β-lactam Derivatives. International journal of molecular sciences 2016, 17, 1736. DOI: 10.3390/ijms17101736.
- Gulcin, I.; Scozzafava, A.; Supuran, C. T.; Koksal, Z.; Turkan, F.; Çetinkaya, S.; Bingöl, Z.; Huyut, Z.; Alwasel, S. H. Rosmarinic Acid Inhibits Some Metabolic Enzymes Including Glutathione S-Transferase, Lactoperoxidase, Acetylcholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Isoenzymes. Journal of enzyme inhibition and medicinal chemistry 2016, 31, 1698–1702. DOI: 10.3109/14756366.2015.1135914.
- Koçak, R.; Akin, E. T.; Kalin, P.; Talaz, O.; Saraçoğlu, N.; Daştan, A.; Gülçin, İ.; Durdagi, S. Synthesis of Some Novel Norbornene-Fused Pyridazines as Potent Inhibitors of Carbonic Anhydrase and Acetylcholinesterase. Journal of heterocyclic chemistry 2016, 53, 2049–2056. DOI: 10.1002/jhet.2558.
- Ozmen Ozgun, D.; Yamali, C.; Gül, H. I.; Taslimi, P.; Gülçin, İ.; Yanik, T.; Supuran, C. T. Inhibitory Effects of Isatin Mannich Bases on Carbonic Anhydrases, Acetylcholinesterase and Butyrylcholinesterase. Journal of enzyme inhibition and medicinal chemistry 2016, 31(6), 1498–1501. DOI: 10.3109/14756366.2016.1149479.
- Sujayev, A.; Garibov, E.; Taslimi, P.; Gülçin, İ.; Gojayeva, S.; Farzaliyev, V.; Alwasel, S. H.; Supuran, C. T. Synthesis of Some Tetrahydropyrimidine-5-Carboxylates, Determination of Their Metal Chelating Effects and Inhibition Profiles against Acetylcholinesterase, Butyrylcholinesterase and Carbonic Anhydrase. Journal of enzyme inhibition and medicinal chemistry 2016, 31, 1531–1539. DOI: 10.3109/14756366.2016.1156104.
- Yılmaz, S.; Akbaba, Y.; Ozgeris, B.; Polat Köse, L.; Göksu, S.; Gülçin, İ.; Alwasel, S. H.; Supuran, C. T. Synthesis and Inhibitory Properties of Some Carbamates on Carbonic Anhydrase and Acetylcholine Esterase. Journal of enzyme inhibition and medicinal chemistry 2016, 31(6), 1484–1491. DOI: 10.3109/14756366.2016.1149477.
- Singh, A. K.; Tiwari, M. N.; Dixit, A.; Upadhyay, G.; Patel, D. K.; Singh, D.; Prakash, O.; Singh, M. P. Nigrostriatal Proteomics of Cypermethrin-Induced Dopaminergic Neurodegeneration: Microglial Activation-Dependent and -Independent Regulations. Toxicological Sciences 2011, 122, 526–538. DOI: 10.1093/toxsci/kfr115.
- Bi, R.; Pan, Y.; Shang, Q.; Peng, T.; Yang, S.; Wang, S.; Xin, X.; Liu, Y.; Xi, J. Comparative Proteomic Analysis in Aphis Glycines Mutsumura under Lambda-Cyhalothrin Insecticide Stress. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 2016, 19, 90–96.
- Yıldırım, A.; Atmaca, U.; Keskin, A.; Topal, M.; Çelik, M.; Gülçin, İ.; Supuran, C. T. N-Acylsulfonamides Strongly Inhibit Human Carbonic Anhydrase Isoenzymes I and II. Bioorganic & medicinal chemistry 2015, 23, 2598–2605. DOI: 10.1016/j.bmc.2014.12.054.
- Güney, M.; Coşkun, A.; Topal, F.; Daştan, A.; Gülçin, İ.; Supuran, C. T. Oxidation of Cyanobenzocycloheptatrienes: Synthesis, Photooxygenation Reaction and Carbonic Anhydrase Isoenzymes Inhibition Properties of Some New Benzotropone Derivatives. Bioorganic & medicinal chemistry 2014, 22, 3537–3543. DOI: 10.1016/j.bmc.2014.04.007.
- Inter-Organization Programme for the Sound Management of Chemicals, & World Health Organization, WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009, World Health Organization, 2010. Environmental Health Criteria Cyhalothrin International Programme on Chemical Safety 1990, 99, 106.
- Pesticide Tolerances, Lambda-cyhalothrin. Federal Register 1998, 63, 7291–7299.
- Atasever, A.; Ozdemir, H.; Gülcin, I.; Küfrevioglu, O. I. One-Step Purification of Lactoperoxidase from Bovine Milk by Affinity Chromatography. Food chemistry 2013, 136, 864–870. DOI: 10.1016/j.foodchem.2012.08.072.
- Camadan, Y.; Ozdemir, H.; Gulcin, I. Purification and Characterization of Dihydropyrimidine Dehydrogenase Enzyme from Sheep Liver and Determination of the Effects of Some Anaesthetic and Antidepressant Drugs on the Enzyme Activity. Journal of enzyme inhibition and medicinal chemistry 2016, 31, 1335–1341. DOI: 10.3109/14756366.2015.1132710.
- Ozgeris, B.; Göksu, S.; Köse, L. P.; Gülçin, I.; Salmas, R. E.; Durdagi, S.; Tümer, F.; Supuran, C. T. Acetylcholinesterase and Carbonic Anhydrase Inhibitory Properties of Novel Urea and Sulfamide Derivatives Incorporating Dopaminergic 2-Aminotetralin Scaffolds. Bioorganic & medicinal chemistry 2016, 24, 2318–2329. DOI: 10.1016/j.bmc.2016.04.002.
- Artunc, T.; Cetinkaya, Y.; GöCer, H.; GüLçIn, İ.; Menzek, A.; Sahin, E.; Supuran, C. T. Synthesis of 4-[2-(3,4-Dimethoxybenzyl)Cyclopentyl]-1,2-Dimethoxybenzene Derivatives and Evaluations of Their Carbonic Anhydrase Isoenzymes Inhibitory Effects. Chemical biology & drug design 2016, 87, 594–607. DOI: 10.1111/cbdd.12695.
- Karakida, K. K.; Fukuyama, T.; Kuchitsu, K. Molecular Structures of Hydrogen Cyanide and Acetonitrile as Studied by Gas Electron Diffraction. Bulletin of the Chemical Society of Japan 1974, 47, 299–304. DOI: 10.1246/bcsj.47.299.
- Veitch, N. C.;. Horseradish Peroxidase: A Modern View of A Classic Enzyme. Phytochemistry 2004, 65, 249–259. DOI: 10.1016/j.phytochem.2003.10.022.