ABSTRACT
In this study, various fruit compounds including phenolic acid, flavonoid, anthocyanin, tannin, moisture content, carotenoid, soluble, and reducing sugar date varieties were investigated. The results showed that there were differences among varieties of date palm in terms of synthesis of chemical compounds. The maximum and minimum SOD, APX, and CAT activity were detected in the Klote and Khasab variety, respectively. The highest amount of phenolic compounds, flavonoids, and anthocyanins were found in a variety of Klote and the lowest amount were observed in the Khenizi variety. The analysis of tannin’s content among different date varieties showed that the highest amount of tannin in the Khenizi variety and the lowest amount in the variety of Klote were observed. There is a positive correlation between phenolic compounds and antioxidant capacity in different date palm varieties. The comparison of antioxidant’s capacity of different varieties showed that Klote had the highest amount, while Khenizi had the lowest amount of antioxidant. Also, the highest amount of soluble sugar in the Klote and the lowest amount in Khasab were observed. The amount of reducing sugar in the variety of Klote and Gorbani were higher than other varieties and lower level have been seen in Khenizi. The amount of carotenoids was the highest in the Klote variety and the lowest in the Khenazi and Halili varieties. The highest amount of moisture was in the Mazafati and the lowest amount viewed in the Khenizi.
Introduction
Date palm (Phoenix dactylifera) is the second most important horticultural product in Iran after pistachio. More than 400 varieties, among 3000 named varieties worldwide, are found in Iran, thus assembling a main worldwide date palm gene source. Researches indicate that although Iran is one of the top four countries in the world in terms of date production, about 51% of the world’s dates are produced in Iran.[Citation1] Date fruits are a main source of important phytochemicals, including carotenoids, phenolics, and flavonoids. Also, date fruits contain fat, protein, dietary fiber, and vitamins.[Citation2] Oxidative stress is one of the most common denominator in the pathogenesis of chronic diseases[Citation3] and dietary antioxidants have positive role in control of degenerative disorders such as cardiovascular, neurological diseases and cancer[Citation4] and gastric ulcer.[Citation5] Plants have various protective mechanisms to eliminate ROS through both enzymatic and non-enzymatic defense mechanisms.[Citation6] Phenolic compounds are active antioxidants playing an important role in the neutralization of free radicals and decomposition of peroxides.[Citation7] Antioxidants have attracted more and more attention as potential agents for preventing and treating oxidative stress-related diseases. Plants have antioxidant systems that are capable of elimination of active oxygen species and finally can reduce the effects of oxidative stress.[Citation9] These antioxidant enzymes including catalase, peroxidase, superoxide dismutase, and ascorbate peroxidase (APX) eliminate ROS to protect plant cells against stress conditions.[Citation10] Natural antioxidants are expected to be an alternative to the synthetic ones because of their potential health benefits.[Citation11] Although CAT is widely distributed in the cell, high concentrations are found in both peroxisomes and mitochondria SOD and CAT are known as primary antioxidant enzymes and each one performs reduction of particular ROS. Superoxide radicals are reduced by SOD so that H2O2 and O2 are formed.[Citation12] The sugar levels are known to change during the development of the fruit and the process of maturation and ripening. Vinson and Freeman (1911) reported that the total sugars and sucrose increase with ripening and noted the rapid accumulation of total sugars and the inversion of sucrose at the later stages of development.[Citation13] Depending upon the type of sugars, dates can be classified into two types: dates containing sucrose and dates containing reducing sugars.[Citation14] The main objectives of this study were to determine the polyphenolic content (total phenolics, total flavonoid, tannin, and anthocyanin), antioxidant capacity, reducing sugar, and total soluble in seven varieties of Phoenix dactylifera palm fruits.
Materials and methods
Plant materials
The test was performed in a randomized complete block design with three replications. Three trees were selected from each variety. The fruits were randomly harvested from different clusters. Seven date palm varieties named Khenizi, Khasab, Shahani, Mazafati, Qorbani, Kloteand, and Halili were collected at ripening stage from a commercial orchard in south of Kerman, Iran, and transported to the laboratory immediately. Then, only the ripe fruits with the best quality, and free from fungal and pest damage were used for this study. After the harvesting of the ripe fruits, the chemical properties including ascorbate peroxidase, superoxide dismutase, catalase, phenolic acid, flavonoid, anthocyanin, tannin, carotenoids, moisture content, soluble, and reducing sugar date varieties were analyzed.
Preparing of enzymes extract
Samples extractions were prepared for the analyses by homogenizing 1 g of fruit in 4 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 2 mM Na–EDTA and 1% (w/v) polyvinyl–polypirrolidone (PVP). The experiment was performed on ice cold and then the homogenate was centrifuged at 10,000 × g for 10 min. The supernatants were collected and stored at −20°C until using. The total protein content of samples was determined according to Bradford protein assay using bovine serum albumin (BSA) as a standard. The absorbance is recorded at 595 nm.[Citation15]
Determination of APX activity
Peroxidase Ascorbate peroxidase was assayed according to Nakona and Asada (1981).[Citation16] Reaction mixture in a total volume of 1 ml contained 50 mM K-phosphate buffer (pH 7.0), 0.2 mM ascorbic acid, 0.2 mM EDTA, 20 mM H2O2 and enzyme. H2O2was the last component to be added and the decrease in absorbance was recorded at 290 nm (extinction coefficient of 2.8 mM−1 cm−1) using a UV–vis spectrophotometer. Correction was made for the low, non-enzymic oxidation of ascorbic acid by H2O2. The specific activity of enzymes expressed as unit mg protein−1.
Catalase
Peroxidase Catalase and peroxidase activity was analyzed according to Dhindsa et al. (1981), [Citation17] with a slight modification. The reaction mixture consisted of 2 ml sodium phosphate buffer (50 mM, pH 7.0), 0.5 ml H2O2 (40 mM) and 0.5 ml enzyme extract. The decomposition of H2O2 was measured by the decline in absorbance (A) at 240 nm. CAT specific activity was expressed as U kg−1of FW, where U = − A at 240 nm s−1.
Superoxide dismutase
Superoxide dismutase (SOD) was assayed in accordance with the method of Beauchamp and Fridovich (1971).[Citation18] The reaction mixture was prepared by mixing 50 µl crude enzyme extract with the SOD assay solution (50 mM potassium phosphate buffer [pH 7.8], 75 mM nitrobluetetrazolium [NBT], 13 mM L-methionine, 0.1 mM EDTA, and 2 µM riboflavin). The test tube was shaken and placed in a light box for 15 min. One unit of enzyme activity was determined as the amount of enzyme needed for inhibition of 50% NBT reduction by monitoring absorbance at 560 nm.
Total phenolic
Total phenolic contents were determined using the procedures described by Zieslin and Ben- .[Citation19]1 g of plant tissue in 2 ml of 80% methanol was combined. Then 0.5 mL of methanolic extract was mixed with 0.5 mL Folin-Ciocalteu reagent by annual shaking for 15–20 sec. After 3 min, 1 ml saturated sodium carbonate and 1 ml of distilled water were added. The reaction mixture was incubated in the dark at room temperature for 2 h and its absorbance was measured at 725 nm against deionized water using spectrophotometer.
Antioxidant capacity by DPPH
Analysis was carried out following the methods of Brand-Williams et al.[Citation20] The stock solution (24 mg DPPH/100 mL methanol) was diluted with methanol to obtain an absorbance of 1.1 at 515 nm using spectrophotometer. Total antioxidant activity in diluted extracts was determined by using the following formula.
Total antioxidant activity = Control absorption – Sample absorption x 100
Control absorption
Total tannins
The total condensed tannins were determined using the method modified by Heimler et al. (2006), [Citation21] 400 mL of the date fruit extract was mixed with 3 mL of methanolic solution of vanillin (4%) and 1.5 mL of concentrated hydrochloric acid. The mixture was incubated at room temperature for 15 min and the absorbance was determined at 500 nm.
Total flavonoids
Total flavonoids content (TFC) of the date extracts were measured according to the colorimetric assay of Kim et al.[Citation22] 0.5 g of fruit tissue mixed with2 ml of 80% methanol was homogenized and centrifuged for 10 min.1 ml of the methanolic extract was added to 300 μl sodium nitrite solution (5%) followed by 300 μl aluminum chloride (10%). Test tubes were incubated at room temperature for 5 min, and then 2 ml sodium hydroxide was added. Immediately, the volume of reaction mixture was made to 10 ml with distilled water and the mixture was thoroughly vortexed. The absorbance of the mixture was determined at 510 nm.
Anthocyanin
The total Anthocyanin content was measured using a modified pH differential method.[Citation23] 2 g of plant tissue was well homogenized with 5 ml of acidic methanol (99.5 ethyl alcohol and 99.1 hydrochloric acid). The absorbance of the methanolic extract of anthocyanins was measured at 510 and 700 nm in different pH buffers (pH 1.0 and 4.5, respectively). Absorbance readings were converted to total mg of cyanidin-3- glucoside per 100 g fresh weight using the molar extinction coefficient of 26,900.
Reducing sugar and soluble sugars
The content of reducing sugar was determined by the dinitrosalicylic acid (DNS) method adapted from Miller.[Citation24] 1 g of plant tissue was homogenized in 2 ml of distilled water. 1 ml of DNS solution was added to 1 ml of sample. The mixture was placed in boiling water for 10 min, cooled and then 9 ml of distilled water was added. After homogenizing the reaction mixture, the absorbance at 540 nm was measured (GENESYS 20 Spectrophotometer, Thermo Fisher Scientific, California, USA). A standard curve for reducing sugar was prepared using serial concentrations of glucose (0–700 mg/g). The results were expressed as reducing sugar concentration (mg) per sample weight (g). Total soluble solids (TSS) were measured using a hand-held refractometer (American Optical Co., Keene, N.H.).
Carotenoid
For carotenoids extraction, 2 g of plant tissue was placed in a falcon tube, and after adding 18 ml of hexane, acetone, and ethanol (at the ratio of 1:1:2), it was shaken for 20 min. The mixture was centrifuged at 5000 rpm for 15 min. The absorbance of the hexane layer was read at 450 nm and the concentration of carotenes was calculated by comparison against a calibration curve prepared with the 0–24 µg/mL standard solutions of β-carotene.[Citation25]
Moisture content
From each group, ten samples were selected. Seeds were removed and then the samples were weighed then oven-dried at 105°C for 24 h until a constant mass.
Statistical analysis
The experimental design was a completely randomized factorial with three replications. Data were analyzed by analysis of variance (ANOVA) and the means were compared (p ≤ .05) by Duncan’s multiple range test (DMRT). All analyses were performed using a version of the software SAS (SAS Institute, Cary, NC, USA).
Results and discussions
Enzyme activities
The enzymes activities are presented in . Enzyme activity varied among species, however, maximum SOD, APX, and CAT activities were detected in the Klote fruits and the minimum activities of SOD, APX, and CAT have shown in Khasab variety. No significant difference (p < .05) were observed between the Shahani and Mazafati fruits, whereas significant differences in SOD, APX, and CAT activity had been seen in the other varieties of date palm ().
Table 1. Ascorbate peroxidase (APX), superoxide dismutase (SOD), and catalase (CAT) activities in seven date palm varieties at ripening stage.
In normal conditions in cells, the antioxidant defense system is efficient and adequate against reactive oxygen species.[Citation26] Plants have developed several mechanisms whereby the endogenous content of antioxidant enzymes provides protection against the harmful effects of oxidative stress generated by abiotic/biotic sources.[Citation27] The enzymes CAT, SOD, and APX, as well as non-enzymatic antioxidant compounds such as phenols, ascorbate, and glutathione, are considered to be components of the synergistic antioxidant defensive system. Superoxide Dismutase is an important antioxidant enzyme, responsible for the catalysis of the dismutation of superoxide in oxygen and hydrogen. Catalase is an intracellular enzyme, responsible for the decomposition of hydrogen.[Citation28]
Phenolic compounds
Total polyphenols, flavonoids, and anthocyanins content of examined date palm varieties showed significant differences as shown in . The results showed that the highest amount of phenolic compounds were found in varieties of Klote (98.31 mg/g) and the lowest amount was observed in the Khenizi variety (82.47 mg/g). Wide variation was observed in the total phenolics contents among the date varieties. Also, the trend of changes flavonoids and anthocyanins between varieties of date palm are similar to phenolic compounds (). So that the highest and lowest levels of flavonoids and anthocyanins are found in Klote (0.45 mg/g, 0.35 mg/g) and Khenizi (0.28 mg/g, 0.27 mg/g) varieties, respectively (). Analysis of tannins content among different date varieties showed that the highest amount of tannins in the Khenizi variety and the lowest amount was observed in the variety of Klote ().
Table 2. Total phenolics, flavonoids, anthocyanins, and tannins content in seven date palm varieties at ripening stage.
Phenolic compounds are receiving much attention because of their antioxidant properties. Five flavonoids (catechin, epicatechin, rutin, myricetin, and quercetin) and 10 phenolic acids (gallic, vanillic, chlorogenic, caffeic, syringic, p-coumaric, ferulic, sinapic, salicylic, ellagic, and trans-cinnamic) were identified and quantified in date palm fruits.[Citation29] Wu et al. (2004) reported that dates contain relatively higher amounts of total phenolics as compared to other fresh or dried fruits in a comparative study of total phenolics in different fruits.[Citation30]
The research showed that the total phenol contents ranged from 199.43 to 576.48 mg/100 g FW in six Tunisian cultivars and 226 to 955 mg/100 g FW in ten analyzed Algerian date varieties, respectively.[Citation31] These variations in the total phenolic contents may be due to variety, growing conditions, maturity, seasons, geographic origin, fertilizer, soil type, and storage conditions.[Citation32] The examined date fruit cultivars are richer in phenolic content than the raisins (194 mg/100 g DW), apricots (333 mg/100 g DW), and figs (256 mg/100 g DW) but poorer than plums (551 mg/100 g DW).[Citation13] Tannins are bitter plant polyphenolic compounds that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids. The significant reduction of astringency after turning Khalal into Rutab can be attributed to the deposition of hydrolysable tannins in developing fruits.[Citation33] Our results are lower than those reported by Benmeddour et al. (2013) who found condensed tannins content varied between 82.81 and 525.06 mg/100 g DW.[Citation31] Flavonoids have antioxidant properties that prevent the synthesis of active oxygen species.[Citation34] Phenolic compounds in plants have antioxidant activity and may protect cells against the oxidative damage caused by free radicals.[Citation35] Studies have shown that the total flavonoid content varied from 43.28 to 84.95 mg/100 g and from 1.62 to 81.79 mg/100 g DW in the Moroccan date cultivars and the Iranian date cultivars, respectively.[Citation36,Citation37] The results showed that flavonoid content compared to other fruits such as raisins (30.9 mg/100 g DW), apricots (31.9 mg/100 g DW), prune (46.6 mg/100 g DW), and figs (79.9 mg/100 g DW).[Citation38]
Antioxidant capacity
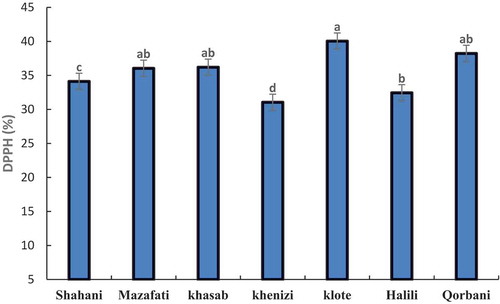
The results of the antioxidant assays showed that the antioxidant activities of date varieties analyzed by DPPH method. The antioxidant capacity of different date varieties varied considerably (P < .05) and ranged from 31.05% to 40.05%. Comparison of antioxidant capacity among different date varieties showed that the highest amount was founded in the Klote and the lowest level was observed in the variety of Khenizi. Other varieties of dates, the amount of antioxidant capacity are similar ().
Figure 1. Comparison of antioxidant capacity in seven date palm varieties at ripening stage. Means with the same letter are not significantly different from each other (p ≤.05).
Studies have shown that antioxidant activity of date fruits from three palm varieties in Oman. Antioxidant activity was expressed as 9.97 mmol of Trolox per gram and total phenol values was reported as 167–343 mg/100 g of the dry plant, respectively.[Citation39]
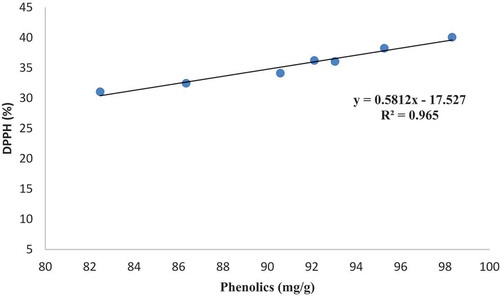
Correlation between phenolic compounds and antioxidants
shows that there is a positive correlation between phenolic compounds and antioxidant capacity in different date varieties. The same trend of changes for antioxidant capacity and total phenolics strongly suggested the main role of phenolic compounds in fruit antioxidant capacity. Phenolic compounds are widely distributed in plants. Not only do they contribute to color and flavor and are responsible for astringent and bitter tastes in fruits and vegetables, they also have antioxidant properties.[Citation40] Studies on various date fruit varieties demonstrated a linear relationship between antioxidant activity and phenolic content.[Citation41]
Soluble and reducing sugar
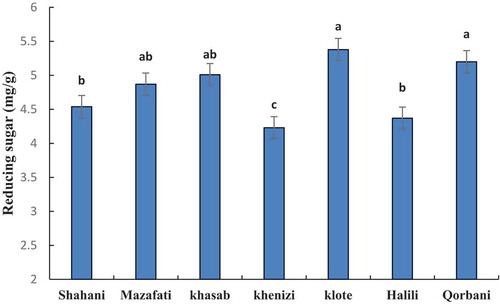
The results in show that the total sugar content varies between varieties of date palm. The highest amount of total sugar in the Klote and the lowest amount in Khasab were observed. However, the amount of sugar in Shahani, Mazafati, and Qorbani were the same (). The amount of reducing sugar in the variety of Klote and Qorbani were higher than other varieties and lower level was observed in Khenizi ().
Figure 3. Comparison of soluble sugar content in in seven date palm varieties at ripening stage. Means with the same letter are not significantly different from each other (p ≤.05).
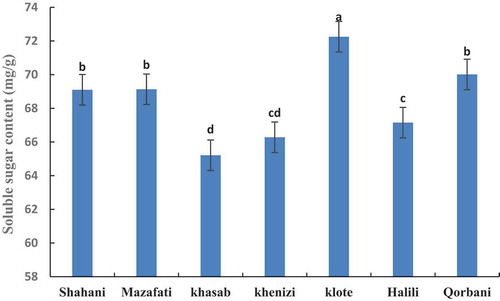
Figure 4. Comparison of reducing sugar content in seven date palm varieties at ripening stage. Means with the same letter are not significantly different from each other (p ≤.05).
Total soluble is the main factors affecting fruit taste. The major sugars in analyzed date fruit are glucose, fructose, and sucrose. The reducing sugars (glucose and fructose) were predominant in varieties of date which contains a large amount of sucrose. Studies had shown that sugar content of Zahedi and Halavi in the Tamar stage, the amount of glucose from 46.3 to 52.4 mg\g and the amount of fructose in the samples was from 40.8 to 57.7 mg\g and the sucrose was from 5 to 6.5 mg/g.[Citation42] The present results confirm the previous finding that the total sugar content varied from 54.79 to 75.56 g/100 g DW in 14 different Moroccan date varieties and between 56.1 and 62.2 g/100 g FW in three Omani date varieties, respectively.[Citation37,Citation43]
Carotenoid content
The highest amount of carotenoids was observed in the Klote variety and the lowest level of showed in the Khenizi and Halili varieties. The amount of carotenoids in the varieties of Khasab, Mazafati, and Qorbani are the same ().
Figure 5. Comparison of carotenoid content in seven date palm varieties at ripening stage. Means with the same letter are not significantly different from each other (p ≤.05).
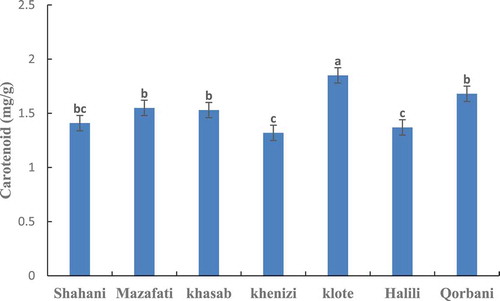
Pigment content including chlorophylls, carotenoids, and anthocyanins are responsible for green, yellow, and red colors in Khalal date fruit, respectively.[Citation43] The concentration and composition of some date fruit carotenoids have been studied by different authors who determined their major role in fruit yellow color at Khalal stage.[Citation44] The amount of carotenoids in Fard, Khasab, and Khalas dates were100.39, 1.31, and 3.03 mg in 100 g, respectively.[Citation39]
Moisture content
Moisture level in different varieties dates showed a significant difference. So that the highest amount of moisture in the Mazafti (30%) and the lowest amount has seen in the variety Khenizi (20%) ().
Figure 6. Comparison of moisture content in in seven date palm varieties at ripening stage. Means with the same letter are not significantly different from each other (p ≤.05).
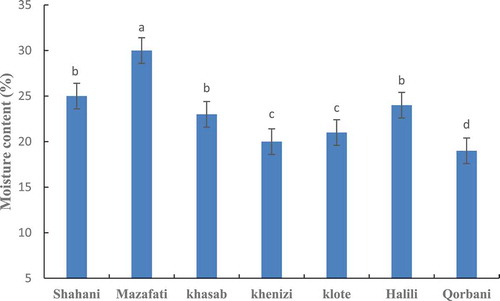
The percentage of moisture in the Tamar stage varies depending on the variety of dates, which basis on the amount of moisture, dates are classified into dry, soft, and semi-dry varieties. In a study of ten varieties in the country of Tunisia, the moisture content of date varieties was reported 12% to 47%. The results obtained in this study were in line with the results of other researchers.
Conclusion
The results showed that there are differences among varieties of date palm in terms of synthesis of chemical compounds measured in this study. Date palm fruits considered in this study could serve as a good source of natural antioxidant. Date fruits are valuable in certain nutrients and provide a rich pool of energy, due to their high carbohydrate content. However, the amount of these compounds varies in different species. The essential role of these compounds in human health has been confirmed.
Acknowledgments
The authors declare that there is no conflict of interest.
References
- Al-Farsi, M.; Alasalvar, C.; Al-Abid, M.; Al-Shoaily, K.; Al-Amry, M.; Al-Rawahy, F. Compositional and Functional Characteristics of Dates, Syrups, and Their By-products. Food Chem. 2007, 104, 943–947.
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-dried Date (Phoenix Dactylifera L.) Varieties Grown in Oman. J Agric Food Chem. 2005, 53, 7592–7599.
- Al-Shahib, W.; Marshall, R. J. The Fruit of the Date Palm: Its Possible Use as the Best Food for the Future? Inte J Food Sci Nut. 2003, 54, 247–259.
- Allaith, A. A. A.;. Antioxidant Activity of Bahraini Date Palm (Phoenix Dactylifera L.) Fruit of Various Varieties. Inte J Food Sci and Technol. 2008, 43, 1033–1040.
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaiee, A. Pesticides and Oxidative Stress: A Review. Med Scimonit. 2004, 10, 144–147.
- Al-Qarawi, A. A.; Abdel-Rahman, H.; Ali, B. H.; Mousa, H. M.; El-Mougy, S. A. The Ameliorative Effect of Dates (Phoenix Dactylifera L.) On Ethanol-induced Gastric Ulcer in Rats. J. Ethnopharmacol. 2005, 98, 313–317.
- Benmeddour, Z.; Mehinagic, E.; Le Meurlay, D.; Louaileche, H. Phenolic Composition and Antioxidant Capacities of Ten Algerian Date (Phoenix Dactylifera L.) Cultivars: A Comparative Study. J. Funct. Foods. 2013, 5(1), 346–354.
- Besbes, S.; Drira, L.; Blecker, C.; Deroanne, C.; Attia, H. Adding Value to Hard Date (Phoenix Dactylifera L.): Compositional, Functional and Sensory Characteristics of Date Jam. Food Chem. 2009, 112(2), 406–411.
- Blokhina, O.; Virolainen, E.; Fagerstedt, K. V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Annal Bot. 2003, 91, 179–194.
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287.
- Biglari, F.; AlKarkhi, A. F.; Easa, A. M. Antioxidant Activity and Phenolic Content of Various Date Palm (Phoenix Dactylifera) Fruits from Iran. Food Chem. 2008, 107(4), 1636–1641.
- Barry, G. H.; Wyk, A. A. Low-temperature Cold Shock May Induce Rind Colour Development of ‘Nules Clementine’ Mandarin (Citrus Reticulata Blanco) Fruit Postharvest. Biol Technol. 2006, 40(1), 82–88.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci Technol. 1995, 28, 25–30.
- Beak, K. H.; Skinner, D. Z. Alteration of Antioxidant Enzyme Gene Expression during Cold Acclimation of Near-isogenic Wheat Lines. Plant Sci. 2003, 165,1221-1227.
- Bradford, M. M.;. A rapid and Sensitive Method for Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-dye Binding. Anal. Biochem. 1976, 72, 248–254.
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic Acids and Flavonoids Profiles of Extracts from Edible Wild Fruits and Their Antioxidant Properties. Inte J Food Prop. 2017, 20(12), 3124–3134.
- Ding, C. K.; Chachin, K.; Ueda, Y.; Imahori, Y.; Wang, C. Y. Metabolism of Phenolic Compounds during Loquat Fruit Development. J Sci Food Agric. 2001, 49, 2883–2888.
- Dhindsa, R. A.; Plumb-Dhindsa, P.; Thorpe, P. A. Leaf Senescence: Correlated with Increased Membrane Permeability and Lipid Peroxidation, and Decreases Levels of Superoxide Dismutase and Catalase. J Exp Bot. 1981, 32, 93–101.
- Foyer, C.; Halliwell, B. The Presence of Glutathione and Glutathione Reductase in Chloroplasts: A Proposed Role in Ascorbic Acid Metabolism. Plant. 1976, 133, 21–25.
- Fu, L.; Xu, B. T.; Xu, X. R.; Qin, X. S.; Gan, R. Y.; Li, H. B. Antioxidant Capacities and Total Phenolic Contents of 56 Wild Fruits from South China. Mole. 2010, 15, 8602–8617.
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, Y.; Jiang, Y. Antioxidant Activities of Peel, Pulp and Seed Fractions of Common Fruits as Determined by FRAP Assay. Nut Res. 2003, 23, 1719–1726.
- Hasnaoui, A.; ElHoumaizi, M. A.; Asehraou, A.; Sindic, M.; Deroanne, C.; Hakkou, A. Chemical Composition and Microbial Quality of Dates Grown in Figuig Oasis of Morocco. Int. J. Agric. Biol. 2010, 12(2), 311–314.
- Halliwell, B.; Gutteridge, J. M. Iron Toxicity and Oxygen Radicals. In Free Radicals in Biology and Medicine, 2nd ed.; Clarendon Press: Oxford, 1998.
- Hajian, S.; HamidiEsfahani, Z. Date Palm Status and Perspective in Iran. In Date Palm Genetic Resources and Utilization. (Illustrated Edn.); Al-Khayri, J. M., Jain, S. M., Johnson, D. V., Eds.; CRC Press: Asia and Europe, 2015; Vol. 2, pp 16.
- Heimler, D.; Vignolini, P.; Dini, M. G.; Vincieri, F. F.; Romani, A. Antiradical Activity and Polyphenol Composition of Local Brassicaceae Edible Varieties. Food Chem. 2006, 99(3), 464–469.
- Jithesh, M. N.; Prashanth, S. R.; Sivaprakash, K. R.; Parida, A. K. Antioxidative Response Mechanisms in Halophytes: Their Role in Stress Defence. J Gene. 2006, 85, 237–254.
- Jaakola, L.; Hohtola, A. Effect of Latitude on Flavonoid Biosyn-thesis in Plants. Plant Cell Environ. 2010, 33, 1239–1247.
- Kim, H. B.; Kim, A. J.; Kim, S. Y. The Study on the Functional Materials and Effects of Mulberry Leaf. Food Sci Ind. 2003, 36, 2–14.
- Kirakosyan, A.; Seymour, E.; Warber, K. S.; Bolling, S.; Chang, S. C. Antioxidant Capacity of Polyphenolic Extracts from Leaves of Crataeguslaevigata and Crataegusmonogyna (Hawthorn) Subjected to Drought and Cold Stress. J Agric Food Chem. 2003, 51, 3973–3976.
- Lee, J.; Durst, M. R.; Wrolstad, R. E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J AOAC Int. 2005, 88(5), 1269–1278.
- Mohamed, A. A.; Al-Qurashi, A. D.; Mohamed, S. A. Antioxidant Capacity, Antioxidant Compounds and Antioxidant Enzyme Activities in Five Date Varieties during Development and Ripening. SciHortic. 2011, 129, 688–693.
- Mousavi, A.; Shavandi, M. Quality and Quantity Sugar Analyses in Four Date Palm by HPLC for Feasibility Study of Its Waste in Fermentation Industry. 18th National Iranian Congress of Food Sci and Technol. 2008.
- Mrabet, A.; Ferchichi, A.; Chaira, N.; Mohamed, B. S.; Baaziz, M.; Penny, T. M. Physico-chemical Characteristics and Total Quality of Date Palm Varieties Grown in the Southern of Tunisia. Pak J BiolSci. 2008, 11, 1003–1008.
- Muthusamy, V. R.; Kannan, S.; Sadhaasivam, K.; Gounder, S. S.; Davidson, C. J.; Boeheme, C.; Hoidal, J. R.; Wang, L.; Rajasekaran, N. S. Acute Exercise Stress Activates Nrf2/ARE Signaling and Promotes Antioxidant Mechanisms in the Myocardium. Free Radi Biol Med. 2012, 52, 366–376.
- Maghsoudlou, Y.; Motamedzadegan, A.; Esmaeelzadeh, R.; Hamzeh, S. Evaluation of Chemical Composition and Energy Content of Three Common Varieties of Iranian Dates. J Agricnat Resour Sci. 2005, 2, 56–61.
- Mortazavi, S. M. H.; Physicochemical Changes during Fruit Growth and Ripening and Effect of Some Packing Conditions on the Postharvest Quality and Shelf Life of Date Palm Fruit (Phoenix Dactylifera L.), Cv. Barhee, Ph.D. thesis, TarbiatModaresUnivrsity. 2007, 192 p.
- Miller, G. L.;. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428.
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate Peroxidase in Spinach Chloroplasts. Plant Cell Physio. 1981, 22, 867–880.
- Orabi, K. Y.; Al-Qasoumi, S. I.; El-Olemy, M. M.; Mossa, J. S.; Muhammad, I. Dihydroagarofuran Alkaloid and Triterpenes from Maytenusheterophylla and Maytenusarbutifolia. Phytochem. 2001, 58, 475–480.
- Ouchemoukh, S.; Hachoud, S.; Boudraham, H.; Mokrani, A.; Louaileche, H. Antioxidant Activities of Some Dried Fruits Consumed in Algeria. LWT-Food Sci. Technol. 2012, 49(2), 329–332.
- Sriyook, S.; Siriatiwat, S.; Siriphanich, J. Durian Fruit Dehiscence-Water Status and Ethylene. HortSci. 1994, 29, 1195–1198.
- Sawaya, W. N.; Khalil, J. K.; Safi, W. M.; Al-Shalhat, A. Physical and Chemical Characterization of Three Saudi Date Varieties at Various Stages of Development. Can Inst Food SciTechnol J. 1983, 16, 87–92.
- Vinson, A. E.; Freeman, G. F. Chemistry and Ripening of the Date. Vol. 66, College of Agriculture; University of Arizona: Arizona, 1911; pp 403–435.
- Wu, W.; Beecher, G. R.; Holden, J. M.; Haytowitz, D. B.; Gebhardt, S. E.; Prior, R. L. Lipophilic and Hydrophilic Antioxidant Capacities of Common Foods in the United States. J Agric Food Chem. 2004, 52, 4026–4037.
- Zieslin, N.; Ben-Zaken, R. Peroxidase Activity and Presence of Phenolic Substances in Peduncles of Rose Flower. Plant Physio Biochem. 1993, 31, 333–339.