?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Fruit leathers, which are also known as fruit rolls, are dehydrated fruit-based products. Despite the popularity of Lapsi fruit leather, its production is limited to home-scale industries, which lack uniformity in quality. Moreover, scientific research to address this issue is very limited. Therefore, in this study, Lapsi fruit leather was prepared and its nutritional, phytochemicals, and sensorial properties were studied. The fruit pulp and sugar were mixed at the proportion of 50:50, 80:20, 57.5:42.5, 72.5:27.5, and 65:35 to prepare leather and labeled as samples A, B, C, D, and E, respectively. The drying behavior was studied at 55°C in cabinet dryer. Among these samples, B had higher amount of crude fat (0.88 g/100 g sample), crude protein (3.87 g/100 g sample), crude fiber (10.95 g/100 g sample), and total ash (1.09 g/100 g sample); however, carbohydrate and energy value was significantly higher in sample A with the value of 83.21 g and 284 kcal per 100 g leather, respectively. Similarly, sample B was rich in phytochemicals containing 67 mg vitamin C/, total polyphenol of 88.33 mg GAE/g dry extract, total flavonoid of 37.57 mg QE/g dry extract and DPPH inhibition activity of 43.17%. Sample A was dried in 8.83 h, which had moisture diffusivity of 8.723 × 10−7 m2/sec, and sample B was dried in 7.33 h, which had moisture diffusivity of 10.0 × 10−7 m2/sec. Though there was significant effect (p < .05) on the taste, flavor, texture, and overall acceptability except for color of the product, sample C was preferred by the panelists. Thus, nutritionally rich and underutilized lapsi fruit can be processed into fruit leather with high consumer acceptance.
Introduction
Fruit leathers are dehydrated fruit-based products, a practical method of incorporating fruit solids, since they do not require refrigeration to avoid microbial growth[Citation1,Citation2] Also known as fruit rolls, taffies, kome, or pestil in some countries, fruit leathers are produced by various methods and using different ingredients.[Citation3,Citation4] Besides diversifying the consumption options for fruits, preparation of fruit leathers is a feasible alternative for preserving as well as adding value of various fruits.[Citation5,Citation6] These fruit snacks were originally developed at a home-scale as an alternative preservation method but considering their nutritional benefits, they have recently emerged at an industrial scale.[Citation7,Citation8] Since it has very low moisture content and is lightweight, it is convenient for normal storage as well as transportation, thereby making it an effective way to preserve nutritious fruits.[Citation1,Citation9,Citation10]
Choerospondias axillaris (Lapsi) is a popular deciduous fruit, native to Nepal,[Citation11] and is recognized as having a great potentiality as a cash generating commodity. According to the National Center for Fruit Development (NCFD), 8651 metric tons of lapsi fruit was produced in Nepal in the year 2019.[Citation12] In Nepal, it is consumed fresh or pickled, and processed for preparing a variety of sweet and sour food products like fruit leathers (locally called as Mada), candies, jam, chutney, and powdered tea additives.[Citation11,Citation13] Similarly, reports have shown the production of juices and pastilles in China from lapsi fruit.[Citation14] Lapsi fruit is an abundant source of several essential macro- and micro-nutrients. It has been found to be very rich in essential amino acids like arginine, glutamic acid, glutamine; vitamin C and minerals like potassium, calcium, and magnesium.[Citation15] It is also gaining popularity for its antibacterial, antimicrobial, and antioxidant properties.[Citation11,Citation16] C. axillaris contains many biologically active substances like phenolic compounds, flavonoids, sterols, organic acids, and polysaccharides, which are considered to be the effective constituents behind its antioxidant activity and medicinal usage.[Citation14,Citation16] It has been reported to possess several properties for the treatment of myocardial ischemia, calming nerves, ameliorating blood circulation, and improving microcirculation in Mongolia.[Citation16,Citation17] The Newari community of Nepal has been practicing a tradition of serving a thin lapsi gravy, called paun kwa, after meal to assist digestion.[Citation18]
Despite of all the health benefits and the variety of products that can be prepared out of the fruit pulp, lapsi is confined within home-scale processors of Nepal. Almost all lapsi fruit available in Nepalese market to-date originated from wild grown trees. Although it possesses a huge potential to be sold in international markets, majority of the produce is consumed within Nepal only.[Citation15] Consumer awareness about the product and lack of uniformity in product quality are the major hindrances to lapsi commercialization. Nepalese farmers have been producing lapsi fruit leather for decades as an effective method of preservation, and the leather is used in lean season as a flavoring agent and also for the production of several candies. C. axillaris fruits having the promising benefits, they have potential in the areas of food and health industries, and possess great opportunity to flourish in the international market.[Citation19] The studies are being conducted to utilize it in product development due to their potential benefits like some studies done in amala fruit leather Citation20. However, not much research has been done on lapsi fruit leather. So this study aimed to establish the nutritional significance of lapsi fruit leather and familiarize the product.
Materials and methods
Collection of raw materials
Lapsi was bought from Dhankuta district (26.9835° N, 87.3215° E) and were stored under refrigeration until further analysis. Sugar was bought from the local market of Itahari (26.6646° N, 87.2718° E).
Preparation of Lapsi fruit leather
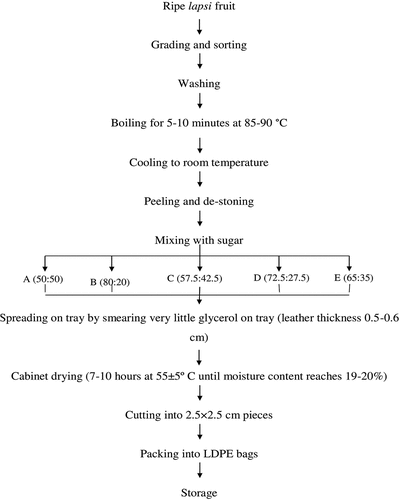
Lapsi fruit leather was prepared as described by Gujral & Brar[Citation21] with modifications (). Fresh ripe lapsi of uniform size and maturity were washed and then boiled for 8–10 min. It was drained off immediately and cooled with tap water. Pulping was then done to remove seeds and thus obtained pulp was mixed in different proportions with sugar (). The glycerol was used as a releasing agent in the tray and pulp mixture was spread for drying. Drying process was carried in a cabinet dryer for 7–9 h at 55°C to obtain moisture content of 19–20%. Thus, obtained fruit leather was cut to uniform square shapes (approx. 2.5 cm × 2.5 cm) using a sharp knife. Low-Density Poly-ethylene (LDPE) plastic was used for packing final product. They were labeled and stored in a cool and dry place for further analysis.
Table 1. Sample formulations with different proportions of lapsi fruit pulp and sugar
Table 2. Proximate composition of lapsi fruit pulp
Drying behavior of fruit leather and estimation of moisture diffusivity
Fruit leathers were dried at 55°C in cabinet dryer (model: CD-5 by model: CD-5, Shini Plastics Technologies India) continuously till the moisture content of product reached 20%. Changes in weight were noted in regular bases (every 30 min) which give moisture on wet basis (Mwb). Fick’s Second law of diffusion was used to interpret the experimental drying data for the determination of moisture diffusivity.[Citation22] Assuming uni-dimensional moisture movement without volume change, constant diffusivity, uniform initial moisture distribution, and negligible resistance can be transformed in the form of the following equation[Citation23,Citation24]:
by comparing the above equation 1to
The thickness (rs) of the sample was 0.005 m in the tray used for drying.
Proximate and chemical analysis
Moisture, crude fat, crude protein, total ash, and crude fiber content of samples were determined as per described by AOAC (1990),[Citation25] while carbohydrate content was estimated by difference. Energy value of the fruit was expressed as Kcal/100 g and was calculated by multiplying the values of crude protein, lipids, and carbohydrates by recommended factors (4, 9, and 4, respectively).[Citation26] Ascorbic acid was determined by using 2, 6-Dichlorophenol-Indophenol visual titration method.[Citation27] Total soluble solids (TSS) of the samples were determined by using a hand refractometer, while pH was determined using a digital pH meter. Acidity was determined by titrimetric method,[Citation27] and reducing sugar content was determined by using Fehling’s solution as described in FSSAI (2015).[Citation28]
Extract preparation
In order to perform phytochemical analysis, lapsi pulp extract was prepared by using 80% ethanol.[Citation29] To 10 g of pulp, 100 mL of 80% ethanol was added and kept at room temperature for 12 h. It was then filtered through Whatmann no. 41 filter paper, and the filtrate was stored in screw capped bottles at 2–4°C until further analysis. Concentration of the extract obtained was determined by evaporating 10 mL of the extract to dryness at 80°C temperature and then measuring the weight of the residue.
Total phenolic content (TPC)
TPC was determined using the spectrophotometric method given by Waterhouse[Citation30] with slight modifications. To 0.5 mL of ethanolic pulp extract, 2.5 mL of 10% Folin-Ciocalteu’s reagent (dissolved in water) and 2.5 mL of 7.5% Na2CO3 aqueous solution were added. The mixture was then incubated in an incubator (accuracy ± 1°C, MYQ engineering Pvt. Ltd) at 45°C for 45 min. Absorbance of the samples was determined using UV-Vis spectrophotometer (LT-2203, wavelength range 190–1100 nm, Labtronics, India) at 765 nm. Similarly, absorbance for standard gallic acid solutions was determined to construct a calibration curve. Based on the observed absorbance, total phenolic content of samples was expressed as mg Gallic acid equivalent per g dry extract.
Total flavonoid content (TFC)
TFC of lapsi pulp was determined using a modified aluminum chloride assay method.[Citation31] 1 ml of pulp extract was mixed with 4 mL distilled water and 0.3 mL 5% sodium nitrate. After 5 min, 0.6 mL 10% aluminum chloride was added to the mixture and again the mixture was allowed to stand for 5 min. Finally, 2 mL of 1 M sodium hydroxide was added and volume was made up to 10 mL using distilled water. After 15 min, the mixture was mixed properly and absorbance was measured by using a UV Vis spectrophotometer at 510 nm wavelength. Similarly, absorbance values were determined for standard quercetin solutions (20, 40, 60, 80, and 100 μg/mL) and calibration curve was drawn. The values were expressed as mg quercetin equivalents.
DPPH radical scavenging activity
Spectrophotometric method was used to determine the DPPH free radical scavenging activity of lapsi pulp extract.[Citation32] Briefly, 1 mL of the extract was mixed with 2 mL of 0.1 mM DPPH solution (4 mg DPPH of 100 mL methanol). The mixture was stoppered and incubated for 30 min in dark. Then the absorbance was read at 517 nm wavelength using a UV Vis spectrophotometer. Finally, percentage scavenging activity was determined using the following equation:
% scavenging activity = (Ac − As) × l00/ Ac
where Ac and As refer to absorbance of control and test samples, respectively.
Sensory evaluation
Sensory analysis of prepared lapsi leather samples was performed by using 9-point hedonic rating system as defined by Ranganna (1987). Twenty-five healthy panelists (20–35 years old) were trained for 4 days (2 h each day) to familiarize panel members with sensory attributes. The panelists were then provided with uniform quantity of prepared samples from stainless steel plate and evaluation of appearance, texture, taste, flavor, and overall acceptability was done.
Statistical analysis
Each analysis was performed in triplicates, and the values were subjected to one-way analysis of variance (ANOVA) using Genstat Release 12.1 (Copyright 2009, VSN International Ltd.). Means were separated using Tukey’s HSD post hoc test at 5% level of significance.
Results and discussion
Yield and weight distribution
Each kg of lapsi fruit contained 450 g pulp, 200 g peel, and 350 g seed, while the yield of dried leather was found to be 57.51%. Similar weight distributions were reported by Paudel et al.[Citation15] and Shrestha et al.[Citation33]
Nutritional, chemical, and organoleptic evaluation of fresh lapsi pulp
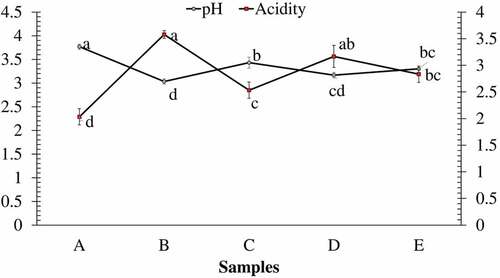
Lapsi pulp obtained after removing of peels and stone was grayish white in color, sour in taste, and slippery to touch. The proximate and chemical constituents of the pulp are shown in respectively. Moisture content of the pulp was found higher than reported by Rai et al.,[Citation34] Paudel et al.,[Citation15] and Chen et al.,[Citation35] while slightly lower values have been mentioned by DFTQC.[Citation36] TSS was found to be 10.5%, which is higher than the findings of Paudel et al.[Citation15] Crude protein content was found to be greater than reported by Chen et al.,[Citation35] DFTQC,[Citation36] Bajracharya,[Citation37] and Rai et al.[Citation34] whereas less than the findings of Bhutia et al..[Citation38] Lapsi fruit is reported to be rich in several amino acids like arginine, glutamic acid, glycine, and lysine.[Citation15] Crude fat content was found to be similar to that of Rai et al.[Citation34] but greater than recorded by DFTQC[Citation36]and Chen et al.[Citation35] Crude fiber was found to be significantly greater than the values reported by Chen et al.[Citation35] and DFTQC.[Citation36] Regarding total ash content, findings of this study were significantly higher than the values reported in DFTQC[Citation36] and Bajracharya.[Citation37] The values for titratable acidity were comparable with the findings of Paudel et al.[Citation15] but significantly greater than the values reported by Chen et al.[Citation35] The pH value of the pulp was 2.9 ± 0.1 which indicates acidic nature of the pulp, due to which it has a strong sour taste. Total carbohydrate content was found to be significantly lower than the values reported by DFTQC[Citation36] and Paudel et al.[Citation15] The pectin content in lapsi fruit pulp was found to be 1.7 ± 0.26 g/100 g pulp. Pectin is used in fruit leather production to thicken the pulp, modify the flexible texture, and ensure shape retention of the leather.[Citation21] So, presence of pectin in lapsi fruit pulp might contribute to the quality of fruit leather produced. The variation in chemical parameters is a result of genetic diversity of the trees, elevation, light availability, and water availability.[Citation19,Citation39,Citation40]
Table 3. Chemical, phytochemical analysis, and anti-oxidant activity of lapsi fruit pulp
Phytochemical and DPPH inhibition of fresh lapsi pulp
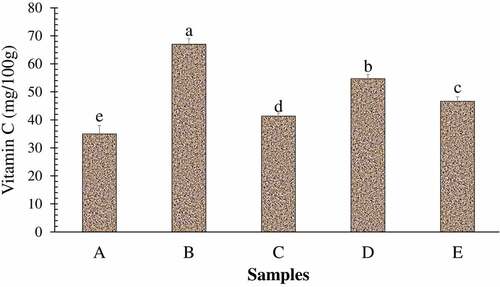
Lapsi is a rich source of Vitamin C.[Citation11] The Vitamin C content in this study was found to be 130 ± 2.64 mg/100 g, which is higher than the value reported by DFTQC.[Citation36] The average recommended intake of Vitamin C for an adult man is 110 mg per day.[Citation41] A large portion of this recommended level can be fulfilled by the consumption of a small quantity of lapsi pulp products.
Similarly, lapsi fruit is said to possess medicinal value due to the presence of several flavonoid and phenolic compounds,[Citation16] because of which it has been used to treat heart diseases for centuries in Mongolia. The TPC of lapsi fruit pulp was found to be 118 ± 1.3 mg GAE/g dry extract (). A wide variation in TPC of lapsi fruit has been reported.[Citation14,Citation16,Citation42] Likewise, TFC of the fruit pulp was found to be 63 ± 1.7 mg QE/g dry extract. Much smaller findings have been reported by other workers.[Citation14,Citation16] Concentration of phenolic compounds in plants can be affected by genetic backgrounds, agronomic practices, as well as several environmental factors, such as soil composition, temperature, rainfall, and ultraviolet radiation.[Citation43–45] The concentration of phenols and flavonoids also depends on the polarity of the solvents used for their extraction.[Citation46]
Similarly, lapsi fruit pulp also exhibited antioxidant activity and was found to show DPPH inhibition of 53%. Slightly higher percent inhibition was reported by 80 μg/ml ethanolic extract of Choerospondias axillaris.[Citation47] Natural antioxidants present in plants are responsible for preventing harmful consequences of oxidative stress.[Citation48] Presence of polyphenols, flavonoids, and phenolic compounds in plants are mainly responsible for their antioxidant activity as they act as reducing agents, hydrogen donors, and are capable of scavenging free radicals.[Citation49,Citation50]
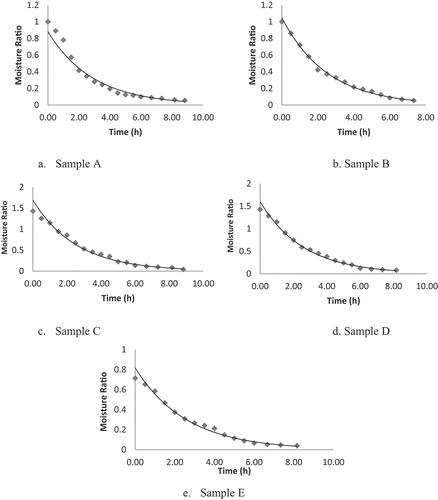
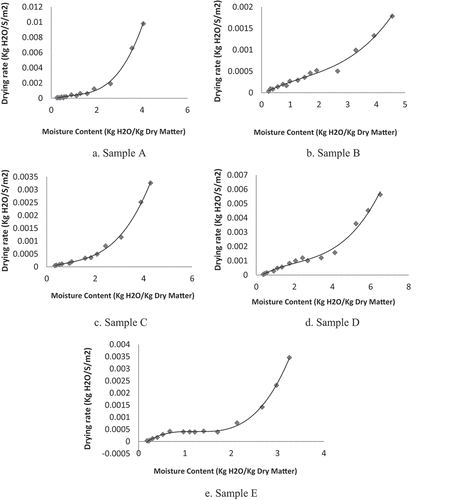
Drying behavior of fruit leather
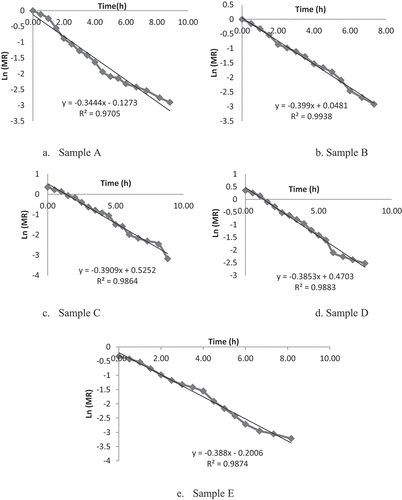
The initial moisture content of lapsi fruit pulp was found to be 433.7 (db). The moisture content (db) of all the samples of leather decreased with increase in drying time (). Drying rates of all samples are shown in . It was clear that drying of fruit leather falls in falling rate period. That means, critical moisture content was not found on the drying rate curve. The drying rate was decreased, and less moisture was available at the surface to evaporate. Time taken to reach the final moisture of 20% (wb) was 8.83 h and 7.33 h for sample A and sample B, respectively. Interval of 30 min was used to record the weight after drying. This showed that the drying time of sample C was 8.17 h. Similarly, Sample D and sample E were dried to final moisture of 20% (wb) at 8.17 h. Sugar might have increased the drying time due to the increase of total solids and binding of water molecules.[Citation22] Gowda et al.[Citation51] also concluded that addition of sugar increased drying time, yield, moisture, and sugar content in the mango fruit bar. The graphical representation of ln (MR) and time for drying of fruit leather samples is used in determining the moisture diffusivity as shown in . The experimental effective moisture diffusivity for the drying of lapsi fruit leather was calculated from the law of diffusion (EquationEq. 1)(1)
(1) . Its value represents the moisture movement and a key parameter in drying process. Sample A, which took longer time to dry, has low moisture diffusivity, i.e., 8.723 × 10−7 m2/sec while sample B, which took shorter time of 7.33 h, has effective moisture diffusivity of 10.1 × 10−7 m2/sec. It was found to be 9.9 × 10−7, 9.76 × 10−7 , and 9.83 × 10−7 m2/sec for sample C, sample D, and sample E, respectively.
Nutritional, phytochemical, and DPPH inhibition of lapsi fruit leather
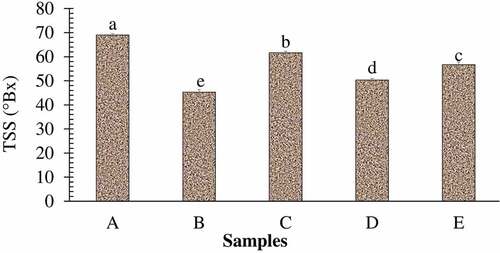
The proximate composition of lapsi fruit leathers prepared by varying the composition of pulp and sugar is shown in . On increasing the proportion of pulp in the leather, proximate constituents were found to increase significantly. Sample A contained the least crude fat, protein, fiber, and ash content, while sample B was found to have the highest level of proximate constituents among the samples. Fruit leathers are generally considered a healthy snack as they are naturally high in fiber and carbohydrates, whilst low in fats.[Citation41] All of the lapsi fruit leathers can be considered healthy owing to their lower fat content as well as higher fiber content. On the contrary, sample A had the highest energy value among the leather samples, which is obviously due to higher proportion of sugar in that sample. Increasing the proportion of sugar in lapsi fruit leather significantly increased the TSS content of the product (). It is obviously due to high TSS in sugar in comparison to very low TSS of the fruit pulp. Similarly, increasing the proportion of fruit pulp increased the acidity of lapsi leather significantly (P < .05), with a simultaneous decrease in pH (). Although no work has been done yet to identify different acids present in lapsi fruit pulp, the acidity is obviously contributed by different organic acids present in lapsi fruit pulp. The vitamin C content (), TPC and TFC of lapsi fruit leather samples increased significantly on increasing the proportion of lapsi pulp in leather (). From the obtained values of fresh lapsi pulp, the proportionate values of vitamin C, TPC, and TFC should have been higher. For instance, sample A must have contained 65 mg/100 g Vitamin C, 54 mg GAE/100 g TPC, and 31.5 mg QE/100 g TFC owing to the proportion of lapsi fruit pulp used. But the actual values were found to be significantly lower. This indicates a drastic reduction of phytochemicals during processing to produce lapsi fruit leather, although the topic seeks further research for more clarity. Studies have shown that ascorbic acid is highly unstable and is easily oxidized to dehydroascorbic acid.[Citation52] Similarly, degradation of heat labile polyphenols during high temperature drying might have lowered the TPC and TFC.[Citation53] Similarly, the fruit leather samples containing higher proportion of lapsi pulp demonstrated greater DPPH inhibition activity. It might be due to higher TPC in those samples. A strong relationship between total phenolic content and antioxidant activity in fruits has been well reported.[Citation54–56]
Table 4. Nutritional analysis of different samples of lapsi fruit leather
Table 5. Phytochemical analysis and anti-oxidation activity of different samples of lapsi fruit leather
Figure 5. TSS (°BX) of the different samples of lapsi fruit leather.
Sensory evaluation of lapsi fruit leather
Lapsi fruit leather samples were subjected to sensory evaluation based on a 9-point Hedonic scale (). Altering the proportion of fruit pulp and sugar did not affect the color of fruit leathers significantly (P > .05). Although there was no significant difference in color, the panelists preferred lighter color of lapsi fruit leather containing lower proportion of fruit pulp. Sugar might have acted as a diluent to make the product color lighter. Similar findings have been reported for mango fruit leather where increasing sucrose proportion increased lightness and thus improved the color of the leather.[Citation57] Samples B and D, had a higher proportion of lapsi pulp, exhibited brownish color which was not preferred by the panelists. Thermal treatment of fruits causes degradation of color pigments and formation of brown pigments by enzymatic as well as non-enzymatic browning reactions.[Citation58]
Figure 8. Sensory analysis of the different samples of lapsi fruit leather.
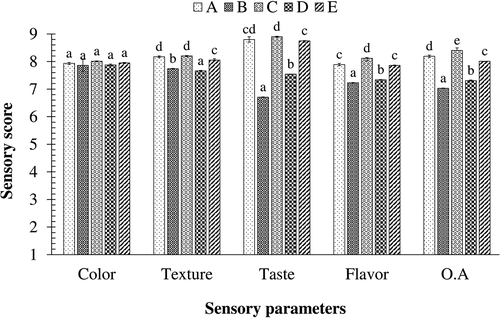
Similarly, it was observed that increasing the proportion of lapsi fruit pulp in the fruit leathers resulted in a significant decrease (P < .05) in scores of texture and the panelists reported a softer texture in samples containing higher proportion of sugar, thereby making the sample less chewy. Similar findings have been reported by Gujral & Khanna[Citation57] for mango leather, where increasing the proportion of sucrose decreased the extensibility of leather, thus making the product softer and brittle. However, in a study by Mardhatilah et al.,[Citation59] sensory panelists preferred the texture of dragon fruit leather containing higher proportion of fruit pulp. Pectin is present in lapsi pulp. Pectin has a significant impact on the texture of fruit leathers and it increases the hardness, cohesiveness, springiness, and chewiness of the products.[Citation60] Higher pectin content in samples B and D might be the reason for hard texture, which was not preferred by the panelists. Taste and flavor were significantly (P < .05) affected by the proportion of lapsi fruit pulp and sugar. Sample C had significantly higher scores than other samples. This might be due to a perfect balance between acidity and sweetness in the sample. The balance between sweetness and acidity is a basic perception through which a person judges the quality of many fruits and fruit products.[Citation61] Thus, sugar–acid balance is an important quality criterion for consumer acceptance.[Citation61] Panelists reported very sour and astringent taste for samples B and D, which might be due to higher acidity and presence of phenolics in those samples. Similarly, sugars, acids, phenolics, and hundreds of volatile compounds present in the pulp contribute to the flavor of the fruit in conjunction.[Citation62] Majority of the flavor must have been contributed by the acids and phenolics present. Regarding overall acceptability, the fraction of pulp and sugar used had a significant impact on the overall acceptability scores. Samples A, C, and E (with lower pulp content) received higher sensory scores than samples B and D with higher pulp content. Similar findings have been reported for soursop fruit leather[Citation63] and mango fruit leather.[Citation57]
Conclusion
Lapsi is an underutilized wild fruit in Nepal that is enriched with vital nutrients and thus bears the potential to uplift the economic status of farmers. Drying of lapsi pulp to produce lapsi leather is a simple and effective technique for preservation. As practiced in Nepal, lapsi leather can be mixed with sugar during leather making to make it more appealing to consumers. The nutritional quality, phytochemical contents, and antioxidant activity of the product increased with the level of lapsi pulp in the formulation. Thus, for maximum nutritional benefits, the formulation with the highest level of pulp (80% pulp, 20% sugar) is recommended. However, lapsi leather with 57.5% pulp and 42.5% sugar was more appealing to consumers in terms of sensory quality. These findings could be helpful for small- and medium-scale industries and could aid in providing nutritional delicacy to the consumers.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Diamante, L. M.; Bai, X.; Busch, J. Fruit Leathers: Method of Preparation and Effect of Different Conditions on Qualities. Int. J. Food Sci. 2014, 2014. DOI: 10.1155/2014/139890.
- Demarchi, S. M.; Quintero Ruiz, N. A.; Concellón, A.; Giner, S. A. Effect of Temperature on Hot-air Drying Rate and on Retention of Antioxidant Capacity in Apple Leathers. Food Bioprod. Process. 2013, 91(4), 310–318. DOI: 10.1016/j.fbp.2012.11.008.
- Oktay, Y. Physicochemical and Sensory Properties of Mulberry Products: Gümüşhane Pestil and Köme. Turk. J. Agric. For. 2013, 37(6), 762–771. DOI: 10.3906/TAR-1301-41.
- da Silva Simão, R.; de Moraes, J. O.; Carciofi, B. A. M.; Laurindo, J. B. Recent Advances in the Production of Fruit Leathers. Food Eng. Rev. 2020, 12(1), 68–82. DOI: 10.1007/S12393-019-09200-4.
- Concha-Meyer, A. A.; D’Ignoti, V.; Saez, B.; Diaz, R. I.; Torres, C. A. Effect of Storage on the Physico-chemical and Antioxidant Properties of Strawberry and Kiwi Leathers. J. Food Sci. 2016, 81, C569–C577. DOI: 10.1111/1750-3841.13214.
- Sai Srinivas, M.; Jain, S. K.; Jain, N. K.; Lakhawat, S. S.; Kumar, A.; Jain, H. K. A Review on the Preparation Method of Fruit Leathers. Int. J. Curr. Microbiol. App. Sci. 2020, 9(5), 773–778. DOI: 10.20546/ijcmas.2020.905.085.
- Quintero Ruiz, N. A.; Demarchi, S. M.; Giner, S. A. Research on Dehydrated Fruit Leathers: A Review. 11th International Congress on Engineering and Food - “Food Process Engineering in a Changing World, 2011: 6. doi:10.1155/2014/139890.
- Torres, C. A.; Romero, L. A.; Diaz, R. I. Quality and Sensory Attributes of Apple and Quince Leathers Made without Preservatives and with Enhanced Antioxidant Activity. LWT - Food Sci. Technol. 2015, 62(2), 996–1003. DOI: 10.1016/j.lwt.2015.01.056.
- Maskan, A.; Kaya, S.; Maskan, M. Hot Air and Sun Drying of Grape Leather (Pestil). J. Food Eng. 2002, 54(1), 81–88. DOI: 10.1016/S0260-8774(01)00188-1.
- Moyls, A. L. Drying of Apple Purees. J. Food Sci. 1981, 46(3), 939–942. DOI: 10.1111/j.1365-2621.1981.tb15387.x.
- Poudel, K. Domesticating Lapsi, Choerospondias Axillaris Roxb. (B.l.Burtt & A.W. Hill) for Fruit Production in the Middle Mountain Agroforestry Systems in Nepal. Himal. J. Sci. 2003, 1(1), 55–58. DOI: 10.3126/hjs.v1i1.188.
- NCFD. National Fruit Development Programme: Annual Progress and Statistical Report (2075/76). National Centre for Fruit Development, Ministry of Agriculture and Livestock Development, Kirtipur, Kathmandu. 2021. https://ncfd.gov.np/sites/default/files/2020-08/finalbook2077.pdf.
- Seber, C. An Analysis of Choerospondias Axillaris “Lapsi” regarding Production, Product Development and Its Effect on Rural Lapsi Farmers in Nepal. 2016; (December 2016). http://repository.library.fresnostate.edu/handle/10211.3/185203.
- Li, Q.; Chen, J.; Li, T.; Liu, C.; Liu, W.; Liu, J. Comparison of Bioactivities and Phenolic Composition of Choerospondias Axillaris Peels and Fleshes. J. Sci. Food Agric. 2016, 96(7), 2462–2471. DOI: 10.1002/jsfa.7366.
- Paudel, K. C.; Eder, R.; Paar, E.; Pieber, K. Chemical Composition of Lapsi (Choerospondias Axillaris) Fruit from Nepal. Mitteilungen Klosterneubg Rebe und Wein, Obs und Fruchteverwertung. 2002;52(1/2), 45–53. https://eurekamag.com/research/003/676/0036.
- Wang, H.; Gao, X. D.; Zhou, G. C.; Cai, L.; Yao, W. B. In Vitro and in Vivo Antioxidant Activity of Aqueous Extract from Choerospondias Axillaris Fruit. Food Chem. 2008, 106(3), 888–895. DOI: 10.1016/j.foodchem.2007.05.068.
- Labh, S. N.; Shakya, S. R. Medicinal Importance of Choerospondias Axillaris (Roxb.) Burtt & Hill Fruits in Nepal. 2016, 3(2), 463–469. DOI:10.1002/acn3.316.
- Sijapati, A. The Love of Lapsi. Nepali Times. https://www.nepalitimes.com/banner/the-love-of-lapsi/. Published 2020. (accessed Jun 27, 2021).
- Mann, S.; Chakraborty, D., and Biswas, S. An alternative perspective of an underutilized fruit tree Choerospondias axillaris in health promotion and disease prevention: A review. Food Biosci. 2021, 101609. DOI: 10.1016/j.fbio.2022.101609.
- Guragain, N., & KC, Y. Preparation and Quality Evalution of Amala (Phyllanthus emblica L.) Fruit Leather. NUTA Journal, 2020; 7(1–2):61–67. DOI:10.3126/nutaj.v7i1-2.39933
- Gujral, H. S.; Brar, S. S. Effect of Hydrocolloids on the Dehydration Kinetics, Color, and Texture of Mango Leather. Int. J. Food Prop. 2003, 6(2), 269–279. DOI: 10.1081/JFP-120017846.
- Gujral, H. S.; Oberoi, D. P. S.; Singh, R.; Gera, M. Moisture Diffusivity during Drying of Pineapple and Mango Leather as Affected by Sucrose, Pectin, and Maltodextrin. Int. J. Food Prop. 2012, 16(2), 359–368. DOI: 10.1080/10942912.2011.552016.
- Crank, J. The Mathematics of Diffusion., 2nd ed.; London: Oxford University Press, 1975. https://vdocuments.mx/crank-j-the-mathematics-of-diffusion-elsevier1975.html. (accessed Jan 14, 2022).
- Karathanos, V. T.; Villalobos, G.; Saravacos, G. D. Comparison of Two Methods of Estimation of the Effective Moisture Diffusivity from Drying Data. J. Food Sci. 1990, 55(1), 218–223. DOI: 10.1111/J.1365-2621.1990.TB06056.X.
- AOAC. Official Methods of Analysis., 15th ed.; Association of Official Analytical Chemists: Rockville, Md, USA, 1990.
- Valdez-Solana, M. A.; Mejía-García, V. Y.; Téllez-Valencia, A.; García-Arenas, G.; Salas-Pacheco, J.; Alba-Romero, J. J., and Sierra-Campos, E. 2015. Nutritional Content and Elemental and Phytochemical Analyses of Moringa Oleifera Grown in Mexico. J. Chem. doi:10.1155/2015/860381.
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products., 2nd ed.; Tata McGraw-Hill Publishing Company Limited, New Delhi: New Delhi, 1987.
- FSSAI. Manual of Methods of Analysis of Foods: Beverages (Coffee, Tea, Cocoa, Chicory), Sugar and Sugar Products and Confectionery Products. 2021. https://old.fssai.gov.in/Portals/0/Pdf/Draft_Manuals/BEVERAGES_AND_CONFECTIONARY.pdf.
- KC, Y.; Rai, R.; Katuwal, N.; Shiwakoti, L. D.; Pant, B. R.; Bajgai, T. R.; Dura, S.; Chaudhary, D. K.; Raghavan, V.; Upadhyaya, J. Phytochemicals, Nutritional, Antioxidant Activity, and Sensory Analyses of Moringa Oleifera Lam. Collected from Mid-hill Region of Nepal. Nat. Prod. Res. 2020. DOI: 10.1080/14786419.2020.1781113.
- Waterhouse, A. L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; USA:John Wiley & Sons, Inc, 2003, Vol. 6, pp I1.1.1–I1.1.8. DOI:10.1002/0471142913.fai0101s06.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64(4), 555–559. DOI: 10.1016/S0308-8146(98)00102-2.
- Casagrande, R.; Georgetti, S. R.; Verri, W. A.; Borin, M. F.; Lopez, R. F. V.; Fonseca, M. J. V. In Vitro Evaluation of Quercetin Cutaneous Absorption from Topical Formulations and Its Functional Stability by Antioxidant Activity. Int. J. Pharm. 2007, 328(2), 183–190. DOI: 10.1016/j.ijpharm.2006.08.006.
- Shrestha, K.; Shrestha, B. P.; Bhattarai, U. K. Preparation of Lapsi (Choerospondias Axillaries Roxb.) Pulp Stock Using IMF Technology and Study for Storage Stability and Food Safety Aspects. J. Food Sci. Technol. Nepal. 2006, 2, 98–101. https://biblio.ugent.be/publication/906511/file/930973.
- Rai, A. K.; Sharma, R. M.; Tamang, J. P. Food Value of Common Edible Wild Plants of Sikkim. J. Hill Res. 2005, 18(2), 99–103.
- Chen, J.; Deng, X. B.; Bai, Z. L.; Yang, Q.; Chen, G. Q.; Liu, Y.; Liu, Z. Q. Fruit Characteristics and Muntiacus Muntijak Vaginalis (Muntjac) Visits to Individual Plants of Choerospondias Axillaris. Biotropica. 2001, 33(4), 718–722. DOI: 10.1111/j.1744-7429.2001.tb00231.x.
- DFTQC. Food Composition Table for Nepal 2012. Ministry of Agricultural Development, Department of Food Technology and Quality Control, Nepal, 2021. http://www.fao.org/fileadmin/templates/food_composition/documents/regional/Nepal_Food_Composition_table_2012.pdf.
- Bajracharya, D. Nutritive Values of Nepalese Edible Wild Fruits. Z. Lebensm. Unters. Forsch. 1980, 171(5), 363–366. DOI: 10.1007/BF01087135.
- Bhutia, K.; Suresh, C.; Amar, R.; Subba, P. Nutritional Composition of Some Minor Fruits of the Sikkim Himalayas. Proceedings of the International Symposium on Minor Fruits and Medicinal Plants for Health and Ecological Security (ISMF & MP), West Bengal, India, 2011 ( 22nd ed., Vol. 19, pp. 344–346.
- Brodie, J. F.; Helmy, O. E.; Brockelman, W. Y., and Maron, J. L. Functional differences within a guild of tropical mammalian frugivores. Ecology. 2009;90(3):688-698. https://scholarworks.umt.edu/biosci_pubs/322.
- Paudel, K. C.; Pieber, K.; Klumpp, R.; de C. Machado, M. L. Collection and Evaluation of Germplasm of Lapsi (Choerospondias Axillaris (Roxb.) B.L. Burtt and A.W. Hill), an Indigenous Fruit Tree of Nepal. Plant Genet Resour. Newsl FAO Biodivers. 2002;130:36–46. https://www.bioversityinternational.org/fileadmin/PGR/article-issue_130-art_58-lang_en.html (accessed Jun 14, 2021).
- Bechthold, A. New Reference Values for Vitamin C Intake. Ann. Nutr. Metab. 2015, 67(1), 13–20. DOI: 10.1159/000434757.
- Chalise, J. P.; Acharya, K.; Gurung, N.; Bhusal, R. P.; Gurung, R.; Skalko-basnet, N.; Basnet, P. Antioxidant Activity and Polyphenol Content in Edible Wild Fruits from Nepal. Int. J. Food Sci. Nutr. 2010, 61(4), 425–432. DOI: 10.3109/09637481003591590.
- Kouki, M.; Manetas, Y. Resource Availability Affects Differentially the Levels of Gallotannins and Condensed Tannins in Ceratonia Siliqua. Biochem. Syst. Ecol. 2002, 30(7), 631–639. DOI: 10.1016/S0305-1978(01)00142-9.
- Doshi, P.; Adsule, P.; Banerjee, K. Phenolic Composition and Antioxidant Activity in Grapevine Parts and Berries (Vitis Vinifera L.) Cv. Kishmish Chornyi (Shared Seedless) during Maturation. Int. J. Food Sci. Technol. 2006, 41(SUPPL. 1), 1–9. DOI: 10.1111/j.1365-2621.2006.01214.x.
- Haytowitz, D. B.; Bhagwat, S.; Holden, J. M. Selection and Peer-review under Responsibility of National Nutrient Databank Conference Steering Committee 36 Th National Nutrient Databank Conference Sources of Variability in the Flavonoid Content of Foods-review under Responsibility of the National Nutrient Databank Conference Editorial Board. Procedia Food Sci. 2013, 2, 46–51. DOI: 10.1016/j.profoo.2013.04.008.
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant Potential, Total Phenolic and Total Flavonoid Contents of Rhododendron Anthopogonoides and Its Protective Effect on Hypoxia-induced Injury in PC12 Cells. BMC Complement. Altern. Med. 2015, 15(1), 1–12. DOI: 10.1186/s12906-015-0820-3.
- Narayan Labh, S.; Ratna Shakya, S.; Labh Kayasta, B.; Shyam Narayan Labh, C. Extract of Medicinal Lapsi Choerospondias Axillaris (Roxb.) Exhibit Antioxidant Activities during in Vitro Studies. J. Pharmacogn. Phytochem. 2015, 4(3), 194–197.
- Phuyal, N.; Jha, P. K.; Raturi, P. P.; Rajbhandary, S. Total Phenolic, Flavonoid Contents, and Antioxidant Activities of Fruit, Seed, and Bark Extracts of Zanthoxylum Armatum DC. Sci. World. J. 2020, 2020. DOI: 10.1155/2020/8780704.
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105(3), 940–949. DOI: 10.1016/j.foodchem.2007.04.038.
- Mansouri, A.; Embarek, G.; Kokkalou, E.; Kefalas, P. Phenolic Profile and Antioxidant Activity of the Algerian Ripe Date Palm Fruit (Phoenix Dactylifera). Food Chem. 2005, 89(3), 411–420. DOI: 10.1016/j.foodchem.2004.02.051.
- Gowda, D.; Dan, A.; Ramanjaneya, K. H. Studies on Mango Fruit Bar Preparation. Indian Food Cracker. 1995;49(2), 17–24. https://www.researchgate.net/publication/280564176_Studies_on_mango_fruit_bar_preparation (accessed Jan 14, 2022).
- Achinewhu, S. C.; Hart, A. D. Effect of Processing and Storage on the Ascorbic Acid (Vitamin C) Content of Some Pineapple Varieties Grown in the Rivers State of Nigeria. Plant Foods Hum. Nutr. 1994, 46(4), 335–337. DOI: 10.1007/BF01088433.
- Wani, S. M.; Riyaz, U.; Wani, T. A.; Ahmad, M.; Gani, A.; Masoodi, F. A.; Dar, B. N.; Nazir, A.; Mir, S. A. Influence of Processing on Physicochemical and Antioxidant Properties of Apricot (Prunus Armeniaca L. Variety Narmo). Cogent Food Agric. 2016, 2(1). DOI: 10.1080/23311932.2016.1176287.
- Dorman, H. J. D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant Properties and Composition of Aqueous Extracts from Mentha Species, Hybrids, Varieties, and Cultivars. J. Agric. Food Chem. 2003, 51(16), 4563–4569. DOI: 10.1021/jf034108k.
- Duraipandiyan, V.; Ignacimuthu, S.; Gabriel Paulraj, M. Antifeedant and Larvicidal Activities of Rhein Isolated from the Flowers of Cassia Fistula L. Saudi J. Biol. Sci. 2011, 18(2), 129–133. DOI: 10.1016/j.sjbs.2010.12.009.
- Kumbhare, M. R.; Guleha, V.; Sivakumar, T. Estimation of Total Phenolic Content, Cytotoxicity and In-vitro Antioxidant Activity of Stem Bark of Moringa Oleifera. Asian Pacific J. Trop. Dis. 2012, 2(2), 144–150. DOI: 10.1016/S2222-1808(12)60033-4.
- Gujral, H. S.; Khanna, G. Effect of Skim Milk Powder, Soy Protein Concentrate and Sucrose on the Dehydration Behaviour, Texture, Color and Acceptability of Mango Leather. J. Food Eng. 2002, 55(4), 343–348. DOI: 10.1016/S0260-8774(02)00112-7.
- Bejar, A. K.; Kechaou, N.; Mihoubi, N. B. Effect of Microwave Treatment on Physical and Functional Properties of Orange (Citrus Sinensis) Peel and Leaves. J. Food Process. Technol. 2011, 2(2). DOI: 10.4172/2157-7110.1000109.
- Mardhatilah, D.; Partha, I. B. B.; Hartati, H. Influence of Types of Fatty Materials and Addition of Sugar Concentration on Fruit Leather Quality from Dragon Fruit Albedo (Hylocereus Polyrhizus). IOP Conf. Ser. Earth Environ. Sci. 2018, 209, 012030. DOI: 10.1088/1755-1315/209/1/012030.
- Huang, X.; Hsieh, F. H. Physical Properties, Sensory Attributes, and Consumer Preference of Pear Fruit Leather. J. Food Sci. 2005, 70(3). DOI: 10.1111/j.1365-2621.2005.tb07133.x.
- Jayasena, V.; Cameron, I. °brix/acid Ratio as a Predictor of Consumer Acceptability of Crimson Seedless Table Grapes. J. Food Qual. 2008, 31(6), 736–750. DOI: 10.1111/j.1745-4557.2008.00231.x.
- Gonçalves, B.; Oliveira, I.; Bacelar, E.; Morais, M. C.; Aires, A.; Cosme, F.; Ventura-Cardoso, J.; Anjos, R., and Pinto, T. Factors that influence biosynthesis of volatile flavor compounds in apple fruits. HortScience. 2000;35(6):1026-1033. DOI: 10.5772/intechopen.76231.
- Ávila de Hernández, R. M.; Mujica de Soto, M. V.; Caraballo, E. A. H.; Machado, A. J. G.; González de Rangel, M. T.; Pérez de Camacaro, M. Physicochemical Properties, Sensory Attributes and Consumer Preference of Soursop Leather. Rev. Fac. Nac. Agron Medellin. 2020, 73(2), 9189–9199. DOI: 10.15446/rfnam.v73n2.83402.