1. Introduction
The evolving therapeutic landscape for rheumatoid arthritis has seen three major breakthroughs over the last decades, each corresponding to a step in the comprehension of the disease [Citation1]. First, understanding of the immunological basis of the disease has led to the systematic and precocious use of immunosuppressants, called conventional disease-modifying-drugs (csDMARDs), like methotrexate (MTX), that are still the cornerstone of RA treatment. Second, deeper comprehension of disease pathophysiology led to the development of targeted biological treatments (bDMARDs), monoclonal antibodies or soluble receptors, that block pro-inflammatory cytokines like TNF or IL-6, cellular populations, like mature B cells, or cellular interactions that are critical to T-cell activation.
The development of orally available small molecules that inhibit intracellular signaling of cytokines and growth factors is the third major advance in the treatment of RA. These compounds are referred to as targeted synthetic DMARDs (tsDMARDs).
The jakinibs are a novel family of inhibitors of the Janus-associated kinase (JAK) signaling pathway. The JAK/STAT (signal transducer and activators of transcription) system is a highly evolutionary conserved system that signals downstream type I and type II cytokine receptors () [Citation2]. Tofacitinib, the first developed jakinib, was approved by the FDA in 2012 at the dose of 5 mg twice daily. Originally designed to be a JAK3-selective inhibitor, tofacitinib is considered a pan-JAK inhibitor that inhibits, in descending order of potency, JAK3, 1 and 2. Tofacitinib was developed in a wide phase 3 program, in which it was administered at 5 or 10 mg twice daily. In patients with established RA that were insufficient responders to csDMARDs [Citation3–Citation6] or bDMARds [Citation6,Citation7], tofactinib showed higher rates of clinical response vs. placebo both as monotherapy [Citation6] and as add-on therapy to MTX or other csDMARDs [Citation3–Citation5]. In the latter setting showed similar efficacy to adalimumab as add-on therapy in MTX insufficient responders (to note, the study was not designed to formally test the noninferiority of tofacitinib vs. adalimumab tofacitinib) [Citation3]. Tofacitinib is now approved for clinical use in over 40 countries worldwide, including USA, Japan, Russia, and Switzerland, while it failed to obtain marketing license in the European Union. The reasons for license refusal were unresolved safety concerns, mainly infections, which were dose-dependent, and the fact that the dose of 5 mg did not provide sufficient benefit in terms reduction of structural progression [Citation5]. Nevertheless, a subsequent trial in early csDMARDs naïve RA showed that even at the dose of 5 mg, tofacitinib monotherapy was superior to MTX in preventing radiological progression [Citation8]. In USA, tofacitinib was approved with a warning highlighting the risk of serious infections in particular tuberculosis and malignancy. Pooled analyses of phases 2, 3, and open-label extension studies confirmed the dose-dependency of the infectious risk but suggested that overall risk was comparable to that of RA patients on bDMARDs [Citation9]. Additionally, it was shown that the rates and types of malignancies observed in clinical trials remained stable over time with increasing tofacitinib exposure and were within the expected range of patients with moderate-to-severe RA [Citation10]. There was an increase in the rate of varicella–zoster virus (VZV) infections across the studies, consistent with inhibition of interferon IFN type I and II signaling (). Other frequent events like dysplidemia and liver enzyme elevation are reminiscent of those seen on anti-IL-6 treatments [Citation11] and are consistent with JAK1 inhibition. Increased rates of anemia and cytopenia, notably neutropenia, ascribed to inhibition of JAK2 signaling were also observed. To overcome potential limitations of generalized blockade of JAK signaling, other jakinibs with more restricted JAK specificities have been developed. There are currently five jakinibs between phases 2 and 3 of clinical development.
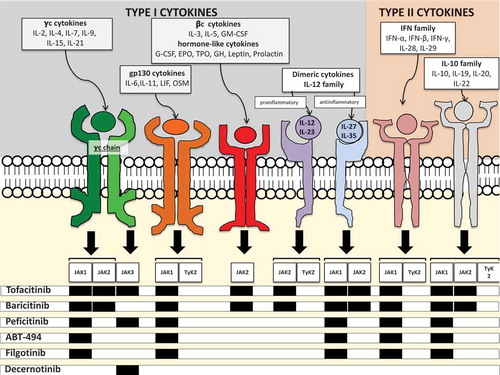
Figure 1. Selectivity of jakinibs for different JAK isoforms and cytokine signaling via JAK/STAT pathways. JAKs are tyrosine kinases that become capable to bind the intracellular portion of several type I and II cytokine receptors upon ligation of the receptor by the cytokine.
A plethora of cytokines with disparate functions signal through JAK/STAT: γc-cytokines are involved in lymphocyte development and homeostasis; the gp-130 cytokine and the related dimeric cytokine family involve both pro- (IL-6, IL-12, IL-23) and anti-inflammatory (IL-27, IL-35) cytokines, the βc- and the hormone-like cytokines family involves critical growth factors for hematopoiesis like EPO, GM-CSF and G-CSF, and other hormones and growth factors. Important type II cytokines that use JAK/STAT pathways are the interferons (both type I and II) and the IL-10 family. The latter involves the anti-inflammatory IL-10, IL-20 critical for osteoclast formation and 22 involved in epithelial barriers integrity.The interaction between the activated receptors and homo- or heterodimers of JAK leads to the phosphorylation of the receptors, which, in turns allows the ligation phosphorylation and activation of STATs that enter the nucleus and regulate gene transcription.
Each cytokine receptor can activate more than one isoform of JAK, except for the γc chain of the receptor of γc -cytokines, which can only activate JAK3. Further complexity is added by the fact that each JAK isoform can activate different isoforms of STATs for downstream signaling. Tofacitinib is considered a pan-JAK inhibitor, active on JAK1, 2 and 3. Baricitinib is selective for JAK 1 and 2, peficitinib for JAK 1 and 3. Filgotinib and ABT-494 are JAK-1 selective agents, while decernotinib is a selective JAK3 inhibitor.
In the figure the cytokine pathways that are supposed to be predominantly blocked based on jakinibs selectivity are shown. Nevertheless, current data do not allow to determine whether those different selectivities do result in real differences in terms of efficacy or safety. Likewise, as each JAK isoform is involved in a number of cytokine pathways, it is not clear whether blockade of a single JAK isoform interferes with all these pathways and, if so, with the same potency. Hence, the patterns of (potentially) blocked cytokines can only partially explain the efficacy and tolerance profile of jakinibs.
2. JAK1/2 inhibitors
Baricitinib is a potent JAK1/2 inhibitor with moderate activity on Tyk2 and negligible activity on JAK 3 at both enzymatic and cellular assays [Citation12]. Baricitinib is currently developed in a phase 3 program encompassing at least four clinical trials involving more than 2000 patients that aims to position baricitinib as a treatment choice in both early and long-standing RA. In the RA-BEGIN trial, 4 mg daily baricitinib monotherapy was superior to MTX at inducing clinical response, and remission in early csDMARDs naïve RA, with no added benefit in patients receiving concomitant MTX [Citation13]. In the 24-week RA-BUILD trial (NCT01721057), baricitinib, given as add-on therapy to MTX in csDMARDs nonresponders, ameliorated clinical symptoms and inhibited radiologic progression. In the RA-Beacon trial [Citation14], 2 or 4 mg daily baricitinib with background csDMARDs was superior to placebo in patients that were nonresponders or intolerant to anti-TNF inhibitors, other bDMARDs, or both. During the 24-week study duration, there were more adverse events (AE), including infections, with baricitinib vs. placebo, while serious AE did not differ. Two cardiovascular events and two nonmelanoma skin cancers were reported in the 4 mg baricitinb group. Interestingly, despite baricitinib selectivity for JAK2, no difference in the number of cases of anemia was seen vs. placebo. Transient small increases in liver enzymes and creatinine and increased levels of both low- and high-density lipoprotein cholesterol were seen in the baricitinib groups. The RA-BEAM trial showed that 4 mg/day baricitinib as add-on therapy in MTX insufficient responders induced higher rates of clinical response vs. adalimumab treatment, with comparable tolerance [Citation15]. The manufacturer recently announced that the 4 mg dose kept preventing structural damage progression at 1 year of treatment in the extension study of the four phase 3 clinical trials, RA-BEYOND (NCT0188507).
3. JAK1/3 inhibitors
Peficitinib (formerly ASP015K) showed 14 times higher selectivity for JAK1/3 over JAK2. Peficitinib at once daily doses of 100 and 150 mg QD was superior to placebo in a 12-week phase 2b study in 289 RA patients with long-standing disease and not on MTX, but that could receive background sulphasalazine or hydroxychloroquine [Citation16]. The ACR20 response rates for the 100 and the 150 mg peficitinib group were around 48% and 55%, respectively. Nausea was reported in 5% of peficitinib treated patients vs. 0% in the placebo group; diarrhea (3.8% vs. 2%) und upper respiratory tract infections (5.5 vs. 3.9%) were also more frequently reported. Conversely, no differences were seen in lymphocyte and neutrophil counts and in hemoglobin levels vs. placebo. These doses are currently tested in two phase 3 studies in RA patients with insufficient response to MTX or other csDMARDs (NCT02308163, NCT02305849). Another report from a Japanese phase IIb trial suggests that the safety profile of peficitinib resembles that of other jakinibs [Citation17].
4. JAK1 inhibitors
4.1. Filgotinib (GLPG0634)
Filgotinib (GLPG0634) is a highly selective inhibitor of JAK1 over JAK2, JAK3, and TYK2 in biochemical and cell assays. Moreover, 4-week treatment at a dose range of 30–300 mg/day in RA dose-dependently increased hemoglobin and did not impact reticulocyte and erythrocyte counts, indicating JAK1 over JAK2 selectivity at these doses and regimen. The absence of effect on NK and CD8+ T cells supports the absence of substantial JAK3 inhibition [Citation18]. Two 12-week phase 2b dose ranging studies conducted in MTX-insufficient responders showed a dose-dependent efficacy of filgotinb both with concomitant MTX (DARWIN1) [Citation19] and as monotherapy (DARWIN 2) [Citation20], respectively. The highest doses held ACR20 response rates between 65% and 80%. The onset of action was rapid with a detectable difference vs. placebo by week 1 of treatment for the 200 mg dose. AE were equally distributed between filgotinib and placebo. Filgotinib induced a dose-dependent decrease in mean neutrophil and platelet counts consistent with JAK1 inhibition. A mild increase in mean creatinine concentration was apparent.
4.2. Abt-494
Different doses of ABT-494, a JAK-1 selective jakinib, were superior to placebo in two large phase 2b studies in RA patients that were insufficient responders to MTX (Balance II, 300 patients) or anti-TNF (Balance I, 276 patients), respectively. Doses ranging from 3 to 24 mg in one or two daily administrations were tested. There was a dose-dependent efficacy, with the highest doses attaining ACR response rates over 70%. Overall rates of discontinuation and serious AE were <5% and <3%, respectively, across both studies. In the Balance I study, there were 5 nonserious VZV infections that were equally distributed between the ABT-494 and the placebo groups. One case of anemia and two cases of neutropenia were observed in the higher-dose ABT-494 groups [Citation21]. Five phase 3 trials are currently ongoing, to test the efficacy of 15 or 30 mg once daily ABT-494.
5. JAK3 inhibitors
Decernotinib (VX-509) selectively inhibits JAK3 over the other JAK family members at both enzyme and cellular assays [Citation22]. In a phase 2a trial, decernotinib monotherapy was superior to placebo in csDMARD-insufficient responders, with the highest drug doses attaining an ACR20 response rate of about 65% [Citation23]. In a dose-escalating phase 2b trial of decernotinib with concomitant MTX in MTX-insufficient responders, ACR20 response rates over 65% were observed for the once-daily 150 mg and the twice-daily 100 mg doses [Citation24]. Headache was the most frequently reported AE (8.7%), followed by hypercholesterolemia (5.2%), diarrhea (4.5%), nausea (3.5%), and increased hepatic enzyme levels (3.1%). The rate of infections, mainly airway infections, and 6 cases of VZV infection were reported, while lymphopenia was observed in all decernotinib groups, which is coherent with JAK 3 selectivity. No higher rates of anemia were observed in decernotinib groups, which pleads in favor of limited effect on JAK2. Conversely, liver enzyme elevation, neutropenia, and hypercholesterolemia may suggest a partial selectivity of decernotinib for JAK 1 in vivo.
6. Conclusion
Tofacitinib, an inhibitor of JAK’s isoforms 1, 2, and 3, opened the way to JAK inhibition in RA, with established efficacy on both clinical activity and structural damage. Five new jakinibs with more restricted JAK isoform selectivity are currently between phases 2 and 3 of clinical development. All these jakinibs are effective on signs and symptoms of RA, and baricitinib also proved efficacy on radiological progression. At present, clinical data do not allow to establish whether the different profiles of JAK selectivity impact differently on relevant outcomes like clinical efficacy or safety profile. Likewise, data on the effect of the different jakinibs on radiological progression is awaited.
7. Expert opinion
Even if data are still limited, all the five jakinibs that are between phases 2 and 3 of development seem to be effective in RA, with a favorable tolerance profile, regardless of their different JAK specificity. It is then reasonable to say that several new jakinibs will be marketed in a near future.
The incoming availability of multiple jakinibs holds promise to redefine therapeutic attitudes for RA. Tofacitinib set a high standard of therapeutic efficacy in different scenarios: early, long-standing, and refractory RA (). Nevertheless, the unavailability of tofacitinib in Europe has limited the clinical experience in everyday practice for a large part of the rheumatological community. Hence, the position of jakinibs in the therapeutic strategy for RA can be discussed based on the results of clinical trials and the recommendations of scientific societies ().
Figure 2. Simplified scheme of the three main scenarios of therapeutic intervention in RA: early cDMARDs naïve RA (a), methotrexate (or other cDMARDs) monotherapy therapeutic failure (b), cDMARDs combination or bDMARDs±cDMARDs therapeutic failure (c). The current or potential place of tofacitnib, and of newer jakinibs, based either on the FDA approval or on the recommendation of the scientific societies (yellow boxes) is shown. The clinical trials that support the positioning of the different jakinibs at the three different steps of therapeutic intervention are reported in blue boxes, with the corresponding reference in the text or clinical trial number. A) At present tofacitinib is not indicated in DMARDs naïve arthritis (dotted line). Several scientific societies recommend early intervention with a combination of cDMARDs or with bDMARDs in early, severe arthritis. The Oral-Start and the RA-Begin trials showed that tofacitinib and baricitinib monotherapies, respectively, were superior to methotrexate in controlling clinical activity in the setting of DMARDs naïve RA. This may support early therapeutic intervention with jakinibs in the future. B) The FDA approved tofacitinib in methotrexate insufficient responders both as sequential monotherapy and as add-on therapy to methotrexate or other cDMARDs. The 2015 ACR recommendations and the preliminary 2016 update of EULAR recommendations allow the use of tofacitinib in this setting, but with a suggestion to prioritize other approaches if possible. The Oral-Standard study showed that, in methotrexate insufficient responders, add-on therapy with tofacitinib was superior to placebo and numerically similar to adalimumab in efficacy. Both phase 2 and 3 studies support the use of other jakinibs as add-on or sequential monotherapy in this setting (blue box). C) In insufficient responders to cDMARDs combination, or to bDMARDs+/- cDMARDs, the use of tofacitinib is approved by the FDA and recommended both by the ACR and the EULAR [Citation25]. The clinical trials that support the use of the different jakinibs in this setting are reported in the blue box. ACR: American College of Rheumatology; bDMARDs biological disease-modifying drugs; cDMARDs: conventional disease-modifying drugs; EULAR European League Against Rheumatism; FDA: Food and Drug Administration.
![Figure 2. Simplified scheme of the three main scenarios of therapeutic intervention in RA: early cDMARDs naïve RA (a), methotrexate (or other cDMARDs) monotherapy therapeutic failure (b), cDMARDs combination or bDMARDs±cDMARDs therapeutic failure (c). The current or potential place of tofacitnib, and of newer jakinibs, based either on the FDA approval or on the recommendation of the scientific societies (yellow boxes) is shown. The clinical trials that support the positioning of the different jakinibs at the three different steps of therapeutic intervention are reported in blue boxes, with the corresponding reference in the text or clinical trial number. A) At present tofacitinib is not indicated in DMARDs naïve arthritis (dotted line). Several scientific societies recommend early intervention with a combination of cDMARDs or with bDMARDs in early, severe arthritis. The Oral-Start and the RA-Begin trials showed that tofacitinib and baricitinib monotherapies, respectively, were superior to methotrexate in controlling clinical activity in the setting of DMARDs naïve RA. This may support early therapeutic intervention with jakinibs in the future. B) The FDA approved tofacitinib in methotrexate insufficient responders both as sequential monotherapy and as add-on therapy to methotrexate or other cDMARDs. The 2015 ACR recommendations and the preliminary 2016 update of EULAR recommendations allow the use of tofacitinib in this setting, but with a suggestion to prioritize other approaches if possible. The Oral-Standard study showed that, in methotrexate insufficient responders, add-on therapy with tofacitinib was superior to placebo and numerically similar to adalimumab in efficacy. Both phase 2 and 3 studies support the use of other jakinibs as add-on or sequential monotherapy in this setting (blue box). C) In insufficient responders to cDMARDs combination, or to bDMARDs+/- cDMARDs, the use of tofacitinib is approved by the FDA and recommended both by the ACR and the EULAR [Citation25]. The clinical trials that support the use of the different jakinibs in this setting are reported in the blue box. ACR: American College of Rheumatology; bDMARDs biological disease-modifying drugs; cDMARDs: conventional disease-modifying drugs; EULAR European League Against Rheumatism; FDA: Food and Drug Administration.](/cms/asset/e0d8555c-1939-44dc-8326-7875fda1b6c0/ieid_a_1249565_f0002_oc.jpg)
Head-to-head comparison trials showed that both tofacitinib and baricitinib are superior to MTX in early RA, but it is unlikely that these drugs may replace MTX as first line of intervention. Conversely, with wider clinical practice, it is conceivable that early intervention with jakinibs may be reserved to early RA patients with severe disease, for whom several national scientific societies currently recommend precocious treatment with csDMARDs combination or bDMARDs.
According to current approved indications, tofacitinib (with or without concomitant csDMARDs) is to be reserved to RA that does not respond to csDMARDs or bDMARDs, and there are clinical trials that support this positioning for all the five jakinibs in development. Before clinical experience accumulates, practitioners will probably be reluctant to prescribe jakinibs in case of MTX-insufficient response, and will more frequently use jakinibs in case of failure of csDMARDs combination or of a first bDMARDs. Accordingly, in the latest ACR recommendations for the treatment of RA, there is a conditional suggestion to prioritize agents with longer postmarketing experience [Citation26].
Another element that emerges from clinical trials is that the rate and type of adverse events can hardly be forecast on the basis of the presumed JAK selectivity. This, together with the similar efficacy of these agents, argues against the importance of the profile of JAK selectivity for clinical purposes.
It is then supposable that all the jakinibs will require the same kind of clinical and laboratory vigilance. In particular, the risk of VZV will likely induce the scientific societies to recommend VZV vaccination early in the care of RA patients, as live vaccines are contraindicated when taking bDMARDs. The relevance of anemia and cytopenias also needs to be established. The latters were not associated with major adverse events in clinical trials, but this needs to be confirmed in clinical practice. The significance of other frequent events, like liver enzyme and creatinine elevation and changes in blood lipids, also need to be evaluated with long-term experience. The creation of patient registries is part of the research agenda to monitor outcomes and optimize practices with these new agents.
Declaration of interest
The authors have received unrestricted research grants from Pfizer Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Semerano L, Minichiello E, Bessis N, et al. Novel immunotherapeutic avenues for rheumatoid arthritis. Trends Mol Med. 2016;22:214–229.
- Schwartz DM, Bonelli M, Gadina M, et al. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12:25–36.
- Van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–519.
- Kremer J, Li Z-G, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253–261.
- Van Der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–570.
- Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507.
- Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–460.
- Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386.
- Cohen S, Radominski SC, Gomez-Reino JJ, et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2924–2937.
- Curtis JR, Lee EB, Kaplan IV, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016;75:831–841.
- Semerano L, Thiolat A, Minichiello E, et al. Targeting IL-6 for the treatment of rheumatoid arthritis: Phase II investigational drugs. Expert Opin Investig Drugs. 2014;23:979–999.
- Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184:5298–5307.
- Fleischmann R, Takeuchi T, Schlichting DE, et al. Baricitinib, methotrexate, or baricitinib plus methotrexate in patients with early rheumatoid arthritis who had received limited or no treatment with Disease-Modifying Anti-Rheumatic Drugs (DMARDs): phase 3 trial results [abstract]. Arthritis Rheumatol. 2015;67(Suppl 10):1045.
- Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243–1252.
- Taylor PC, Keystone EC, Van Der Heijde D, et al. Baricitinib versus placebo or adalimumab in patients with active Rheumatoid Arthritis (RA) and an inadequate response to background methotrexate therapy: results of a Phase 3 study [abstract]. Arthritis Rheumatol. 2015;67(Suppl 10):2L.
- Genovese MC, Greenwald M, Codding C, et al. A Phase 2b, randomized, double-blind, parallel-group, placebo-controlled, dose-finding, multi-center study to evaluate the safety and efficacy of ASP015K in moderate to severe rheumatoid arthritis subjects not on concomitant methotrexate [abstract]. Arthritis Rheumatol. 2014;66(Suppl 10):2826.
- Takeuchi T, Tanaka Y, Iwasaki M, et al. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75:1057–1064.
- Galien, R., Brys R, Van der Aa A, et al. Absence of effects of filgotinib on erythrocytes, CD8+ and NK cells in rheumatoid arthritis patients brings further evidence for the JAK1 selectivity of filgotinib [abstract]. Arthritis Rheumatol. 2015;67(Suppl10):2781.
- Westhovens R, Alten R, Pavlova D, et al. Filgotinib (GLPG0634), an oral JAK1 selective inhibitor is effective in combination with methotrexate in patients with active rheumatoid arthritis: results from a Phase 2B dose ranging study [abstract]. Arthritis Rheumatol. 2015;67(Suppl10):1048.
- Kavanaugh A, Ponce L, Cseuz R, et al. Filgotinib (GLPG0634), an Oral JAK1 selective inhibitor is effective as monotherapy in patients with active rheumatoid arthritis: results from a Phase 2B dose ranging study [abstract]. Arthritis Rheumatol. 2015;67(Suppl10):1049.
- Kremer JM, Keystone EC, Emery P, et al. Safety and Efficacy of ABT-494, a novel selective JAK1 inhibitor, in patients with active rheumatoid arthritis and inadequate response or intolerance to anti-TNF biologic therapy [abstract]. Arthritis Rheumatol. 2015;67:Suppl10:14L.
- Mahajan S, Hogan JK, Shlyakhter D, et al. VX-509 (decernotinib) is a potent and selective janus kinase 3 inhibitor that attenuates inflammation in animal models of autoimmune disease. J Pharmacol Exp Ther. 2015;353:405–414.
- Fleischmann RM, Damjanov NS, Kivitz AJ, et al. A randomized, double-blind, placebo-controlled, twelve-week, dose-ranging study of decernotinib, an oral selective JAK-3 inhibitor, as monotherapy in patients with active rheumatoid arthritis. Arthritis Rheumatol. 2015;67:334–343.
- Genovese MC, Van Vollenhoven RF, Pacheco-Tena C, et al. VX-509 (Decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol. 2016;68:46–55.
- Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509.
- Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26.
