ABSTRACT
Background: This Phase I study (VOLTAIRE®-PK) aimed to evaluate three-way pharmacokinetic similarity (bioequivalence), safety, and immunogenicity of BI 695501 (a Humira® [adalimumab] biosimilar candidate) compared with US- and EU-approved Humira in healthy male subjects.
Methods: Subjects (N = 327) were randomized 1:1:1 to receive one 40-mg subcutaneous dose of BI 695501, US- or EU-approved Humira; safety was assessed for 70 days. Bioequivalence was evaluated using the average bioequivalence method to test if the 90% confidence intervals (CIs) of the geometric means (BI 695501 vs US- and EU-approved Humira) for the primary end points were within prespecified acceptance ranges (80–125%). Immunogenicity was assessed using a sensitive bridging method.
Results: Bioequivalence between BI 695501 and US- and EU-approved Humira was demonstrated with the 90% CIs of the ratios of all primary end points: Cmax, AUC0–inf, pred and AUC0–tz being within the prespecified acceptance ranges of 80–125%. Concentration vs time profiles were similar as were the time course and frequency of immunogenic responses. All study drugs showed similar safety and tolerability results.
Conclusions: Three-way bioequivalence of BI 695501 to US- and EU-approved Humira was demonstrated; safety and immunogenicity results of the three study drugs were also similar.
Clinical trial registration: 2013-003722-84 (EudraCT) and NCT02045979.
1. Introduction
Patients with immune-mediated diseases, such as rheumatoid arthritis (RA) and psoriasis, often have significant comorbidities that impact their quality of life [Citation1,Citation2] and increase their risk of death [Citation3,Citation4]. The direct and indirect costs of treating and managing these patients can place a large financial burden on health-care systems, patients, and society; thus, financial factors impact the availability of treatments [Citation5].
Biologics are a treatment option available to patients with immune-mediated diseases; one such example is Humira® (AbbVie Ltd; adalimumab), which is approved in the United States and Europe for the treatment of diseases such as RA, psoriasis, and Crohn’s disease [Citation6,Citation7]. Adalimumab is a tumor necrosis factor (TNF) antagonist that modulates biological responses induced or regulated by TNF, including a reduction in inflammation [Citation7].
A biosimilar is a biological product that is highly similar to, and has no clinically meaningful differences from, the approved reference product [Citation8]. Given that the cost of managing immune-mediated diseases with biologics is rising, biosimilars have the potential to offer significant cost savings to both health-care systems and patients [Citation9].
Complex antibody biologics, including biosimilars, are large glycoproteins that undergo an intricate manufacturing process using living cells [Citation10]. The focus of biosimilar development and the regulatory approval process is the extensive physicochemical and functional characterization to ensure biosimilarity in terms of structure, mechanism of action, and pharmacology to the reference product [8–10]. Additionally, demonstration of pharmacokinetic (PK) bioequivalence is key to establishing biosimilarity [Citation8–Citation10].
Biologics can induce immunogenicity, an antibody-mediated immune response, and can instigate a hypersensitivity reaction. Immunogenicity can additionally impact PK, clinical efficacy and alter the safety profile of the agent. Evaluation of immunogenicity is therefore an important consideration in biosimilar development [Citation11]. Importantly, immunogenicity results reported from different studies can vary because of the differences in the performance/sensitivity of the assays used [Citation12]. As such, a comparison of immunogenicity to historical data would be misleading; immunogenicity data should be directly compared to the reference data in the same study, using the same method.
In the United States and Europe, the regulatory approval process for biosimilars requires that a thorough physicochemical and functional characterization of the proposed biosimilar be performed, and that PK and clinical efficacy and safety be determined in clinical studies. Typically, a Phase I PK study is performed to compare the single-dose PK profiles in healthy subjects to ensure that the drug disposition of the proposed biosimilar is equivalent to the reference product [Citation8–Citation10]. This Phase I study also offers the opportunity to evaluate the immunogenicity profile of the proposed biosimilar in a sensitive population, without the effect of other confounding factors (such as prior exposure to biologics, concomitant medications, and immune status of patients).
BI 695501 is an Humira biosimilar candidate being developed by Boehringer Ingelheim and is under investigation for use in immune-mediated diseases, including RA. The Phase I study reported here (VOLTAIRE®-PK) aimed to evaluate three-way PK bioequivalence, safety, and immunogenicity of a single dose of BI 695501 compared with US- and EU-approved Humira.
2. Subjects and methods
2.1. Study design
This Phase I clinical study was a randomized, double-blind, single-dose, parallel arm, active comparator study and was conducted to assess three-way PK bioequivalence, safety, and immunogenicity of BI 695501 compared with US- and EU-approved Humira in healthy subjects. Subjects were enrolled at three sites in New Zealand and Belgium, stratified by site and randomized 1:1:1 to one of three treatment groups (; EudraCT: 2013-003722-84; NCT02045979). Subjects received one subcutaneous dose (40 mg/0.8 mL) of BI 695501, US- or EU-approved Humira on Day 1 of the study.
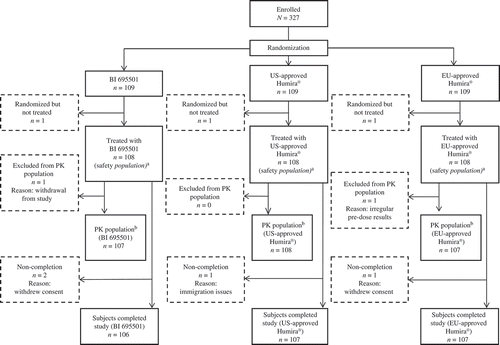
Figure 1. Patient flow.
PK: pharmacokinetic. aThe safety population consisted of subjects who received a single dose of study drug. bThe pharmacokinetic population consisted of all subjects who received a single dose of study drug, had at least one evaluable PK end point and were without protocol deviations or violations likely to impact the PK of the study drugs.
A single administration of the approved 40-mg dose was deemed to have an acceptable risk/benefit ratio when given to healthy subjects. The trial objective was to establish a three-way clinical PK equivalence bridge so that a single global Phase III study could be conducted using only one of the reference products, whilst satisfying approval requirements for BI 695501 across countries globally, including the US and EU regions. Both US- and EU-approved Humira were used in this Phase I clinical study to comply with US Food and Drug Administration (FDA) and European Medicines Agency (EMA) guidelines stating that comparisons must be made to the locally approved reference product [Citation8,Citation9].
Prior to the start of the study, the Independent Ethics Committees (IECs; located in Belgium and New Zealand) reviewed and approved the study. The IECs met the requirements of the International Conference on Harmonization (ICH) Harmonized Tripartite Guideline for Good Clinical Practice (GCP), the definition in 21 Code of Federal Regulations 312.3 and of the participating countries. The study was performed in compliance with the protocol, the principles of the Declaration of Helsinki, ICH GCP, and the applicable regulatory requirements. The standard operating procedures from the three clinical research organizations involved in the study were followed and were consistent with Boehringer Ingelheim standards, GCP, and local legislation requirements.
2.2. Study population
Healthy adult males (≥18–≤55 years) who passed a complete medical assessment, had a body mass index (BMI) of ≥18.5–≤29.9 kg/m [Citation2], and provided informed consent were eligible to enroll in this study. Key exclusion criteria included previous exposure to a biologic, exposure to restricted drugs within a defined time frame, specific lifestyles (including smoking and alcohol abuse), and an inability/unwillingness to comply with protocol requirements, including extensive blood draws for PK.
2.3. Study objectives and end points
The primary objective of this study was to evaluate three-way bioequivalence between BI 695501, US- and EU-approved Humira. The secondary objectives were to evaluate safety and tolerability and other PK parameters of BI 695501. Immunogenicity was also assessed. The primary end points were area under the concentration–time curve (AUC) from time zero to predicted infinity (AUC0–inf, pred), AUC from time zero to the last measurable concentration (AUC0–tz), and the maximum observed drug concentration in plasma (Cmax).
2.4. PK methodology
All PK analyses were conducted on the PK population set, those subjects who received a single dose of study medication, had at least one evaluable primary PK end point, and were without protocol deviations thought to significantly affect PK assessments.
2.4.1. Blood sampling and bioanalytical assays
PK sampling was performed on Days 1–9, 14, 21, 28, 35, 44, 56, and 71 (end of study). Plasma concentration was measured using a validated, indirect enzyme-linked immunosorbent assay (ELISA) method; a 96-well microtiter plate (Greiner, catalogue no. 655061) was coated with drug-binding ligand, TNFα (R&D Systems, catalogue no. 210-TA-10), and the free drug was detected with a horseradish peroxidase-conjugated mouse–anti-human immunoglobulin G Fcγ-specific antibody (Jackson Immunoresearch, catalogue no. 209-035-098). Tetramethylbenzidine was added to the wells to generate a chromophore and the development of color was stopped by the addition of a stopping solution. The absorbance at 450 nm was measured using a 190 microplate reader (Molecular Devices, Sunnyvale, California, USA) and BI 695501, US- and EU-approved Humira concentrations were calculated using a four-parameter standard calibration curve. The lower limit of quantification of the ELISA was 25 ng/mL; values lower than this were not reported. The upper limit of quantification of the assay was greater than that of the highest Cmax data point recorded. All samples were tested using the same technique, to ensure consistency in terms of methods used.
2.4.2. PK evaluations
Primary end points were AUC0–inf, pred, AUC0–tz, and Cmax. Secondary end points were truncated AUCs (time zero to 168, 312, 480, 648, and 1032 h postdose) and AUC from time zero to observed infinity (AUC0–inf, obs) based on the observed concentration of the analyte in plasma at the time of the last concentration measurement. Other PK parameters assessed were terminal half-life of the analyte in plasma (T1/2), time from administration to maximum observed concentration of the analyte in plasma (Tmax), terminal rate constant in plasma (λz), apparent volume of distribution during the terminal phase following an extravascular dose (Vz/F), and apparent clearance of the analyte in the plasma after extravascular administration (CL/F). Drug concentration measurements of BI 695501 were compared with both US- and EU-approved Humira. PK parameters were derived using non-compartmental methods with Phoenix WinNonlin Version 6.3 (Certara, St Louis, Missouri, USA). Graphics were prepared using SAS Version 9.2 (SAS, Cary, North Carolina, USA). Formal bioequivalence testing was performed, and the primary analysis used a statistical model to assess bioequivalence. In addition, all PK parameters were summarized using descriptive statistics.
2.5. Immunogenicity assays
Immunogenicity evaluations were conducted on the safety population. The proportion of antidrug antibody (ADA) positivity, titer, and neutralizing capacity of confirmed ADA-positive samples was reported separately for all three treatment groups. Blood samples for ADA and neutralizing antibody (nAb) analysis were taken prior to study start (predose) and during the study up to Day 71. The positivity of ADA status and titers was determined using a single bridging electrochemiluminescence assay on the MSD platform (Meso Scale Diagnostics LLC, Rockville, Maryland, USA), applying BI 695501 labeled with sulfo-tag and biotin as detection reagents. To improve drug tolerance in the assay, an acid dissociation step was introduced to detach existing conjugates of ADAs with circulating drug. ADAs (against BI 695501, US- and EU- approved Humira) were detected using a three-tiered approach, applying a screening, a confirmation, and a titer assay. A statistical cut point was set to deliver a 5% false-positive rate for the screening assay and a 0.1% false-positive rate for the confirmatory assay. The confirmatory assay comprised the addition of free drug (BI 695501, US- and EU-approved Humira) to reduce the assay signal by drug interference, and samples with more than 34.9% reduction of the signal were considered confirmed positive (confirmatory cut point).
Assay sensitivity was 50 ng/mL, and the drug tolerance was at least 30 µg/mL (free drug) at the low positive control level. During the whole study, free drug levels did not exceed this concentration and, as such, the assay was deemed able to detect ADA responses at any time point during the study.
The existence of nAb against BI 695501, US- and EU-approved Humira was determined using a cell-based, antibody-dependent cell-mediated cytotoxicity (ADCC) method, with a sensitivity of 1.5 µg/mL. The assay comprised membrane TNFα-expressing target cells and human peripheral blood mononuclear cells as effector cells. Cell killing was induced by the addition of 0.047 µg/mL BI 695501; the presence of nAb was confirmed by a drop in ADCC activity. A cut point where a signal reduction was deemed to be a positive nAb result was statistically set to result in a 5% false-positive rate. Assay methodologies were fully validated in accordance with ICH guidelines.
2.6. Safety evaluations
Safety evaluations were conducted on the safety population (subjects who received a single dose of study medication), and assessments were conducted continuously throughout the study. Adverse events (AEs) were classified by System Organ Class and Preferred Term using the Medical Dictionary for Regulatory Activities (MedDRA) Version 17.0. AEs were assessed for severity and relationship to the study drug. AEs of special interest (AESIs) were hepatic injuries, anaphylactic reactions, serious infections, and hypersensitivity reactions. All incidences of AEs during the study were collected, documented, and reported as per the protocol instructions. Any AEs occurring within 70 days of study drug administration were considered to be on-treatment; AEs after that period were not recorded.
2.7. Statistical analysis
The analysis strategy used in this study is in line with that recommended by the FDA, EMA, and the ICH [13–15].
The sample size was calculated so that the power to conclude three-way PK bioequivalence (with an acceptance range of 80–125%, one-sided significance level 5%, and taking into account all three comparisons) was approximately 90% assuming no treatment differences, and approximately 80% for a 5% treatment difference (ratio scale). The assumed variability was based on in-house as well as literature data on the approved versions of Humira.
The ratios of the geometric means (BI 695501 vs. US- and EU-approved Humira) and their two-sided 90% confidence intervals (CIs) were provided for all primary and secondary PK end points; the analysis was conducted using an analysis of covariance (ANCOVA) model on the logarithmic scale. Dependent variables were the logarithm of AUC0–inf, AUC0–tz, and Cmax; independent variables were treatment, study site, age at baseline, and body weight at baseline. Treatment and study site were categorical variables; age and body weight at baseline were continuous variables.
Bioequivalence was evaluated using the average bioequivalence method (using a statistical model) to test if the ratios of the geometric means (BI 695501 vs. US- and EU-approved Humira) for the primary end points were within the prespecified acceptance range of 80–125%. All three comparisons (i.e. BI 695501 vs. EU-approved Humira, BI 695501 vs. US-approved Humira, and US- vs. EU-approved Humira) needed to show statistical significance for bioequivalence to be declared; therefore, no adjustment of the type 1 error rate was required. All PK parameters were analyzed descriptively, with geometric coefficients of variation given to measure dispersion. Graphics and statistical analyses were generated using SAS Version 9.2 (SAS, Cary, North Carolina, USA).
3. Results
3.1. Subject demographics and baseline characteristics
Overall 460 subjects were screened, and 327 were randomized to the three treatment groups (n = 109 per treatment group, ). Subject demographics and baseline characteristics were comparable between the three groups; the mean age was 31 years (standard deviation [SD] 11), and the mean BMI was 24.4 kg/m2 (SD 2.6 kg/m2).
3.2. PK
The median time at which the maximum plasma concentrations (Tmax) were detected was 132, 109, and 120 h (median values) for BI 695501, US- and EU-approved Humira, respectively. Unadjusted geometric mean Cmax levels were 3.9, 3.9, and 4.1 µg/mL for BI 695501, US- and EU-approved Humira, respectively. Unadjusted geometric mean AUC0–inf, pred levels were 2630, 2470, and 2650 µg*h/mL for BI 695501, US- and EU-approved Humira, respectively. The 90% CIs for the adjusted geometric mean ratios of BI 695501 to reference product analyses, and between EU- and US-approved Humira, for the primary PK end points were all within the prespecified acceptance range of 80–125%. The mean plasma concentration–time profiles for BI 695501, US- and EU-approved Humira were comparable over the entire profiling period (). Results of the statistical comparison of primary PK end points corroborated the similarity of the mean plasma concentration–time curves (). An overview of the geometric mean and mean PK parameters obtained for the individual treatment groups is provided in .
Table 1. Summary of PK parameters in healthy subjects after a single dose of BI 695501, US- or EU-approved adalimumab.
Figure 2. Arithmetic mean plasma concentration–time profiles in healthy subjects after a single dose of study drug for (a) BI 695501, US- and EU-approved Humira, (b) Forest plot presenting point estimate and 90% confidence intervals for primary PK parameters for BI 695, 501, US- and EU-approved Humira (bioequivalence was declared if the 90% confidence intervals were within prespecified acceptance ranges of 80–125%).
AUC0–inf, pred: AUC of the analyte in plasma over the time interval from zero extrapolated to infinity; AUC0–tz: AUC of the analyte in plasma over the time interval from time zero to the last measurable concentration; Cmax: maximum observed drug concentration in plasma; PK: pharmacokinetic.
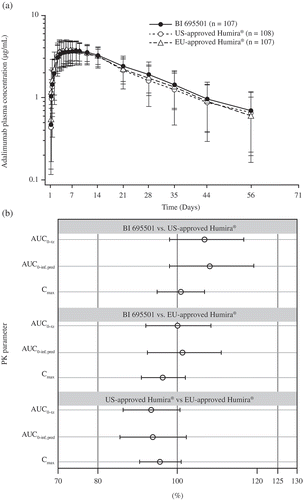
Point estimates of the adjusted geometric mean ratio and the 90% CIs for the ratios of the primary PK end points for the comparison of BI 695501 and US-approved Humira were 108.6% (98.5–119.8%) for AUC0–inf, pred, 107.3% (98.5–117.0%) for AUC0–tz, and 100.9% (95.2–106.9%) for Cmax. The point estimates of the adjusted geometric mean ratio and the 90% CIs for the ratios of the primary PK end points for the comparison of BI 695501 and EU-approved Humira were 101.3% (92.5–111.0%) for AUC0–inf, pred, 99.9% (92.2–108.4%) for AUC0–tz, and 96.4% (91.1–102.0%) for Cmax. The point estimates of the adjusted geometric mean ratio and the 90% CIs for the ratios of the primary PK end points for the comparison of US- and EU-approved Humira were 94.0% (86.0–102.8%) for AUC0–inf, pred, 93.7% (86.8–101.1%) for AUC0–tz, and 95.9% (90.8–101.3%) for Cmax ().
Three-way bioequivalence of BI 695501 to US- and EU-approved Humira was declared for all comparisons. The statistical comparison of secondary and further PK parameters showed that the 90% CIs of the adjusted geometric mean ratios were all within 80–125% limits and as such supported similarity between BI 695501, US- and EU-approved Humira.
3.3. Immunogenicity
Overall, 3.4% of subjects (n = 11) had ADAs at baseline. The frequency of predose-positive samples was equally distributed across the treatment groups with 3.7% (n = 4), 2.8% (n = 3), and 3.7% (n = 4) for BI 695501, US- and EU-approved Humira, respectively. Median (range) titer values for the predose subjects were: 5 (1–512), 2 (1–128), and 5 (1–32) for the BI 695501, US- and EU-approved Humira groups, respectively. A rapid development of ADAs was observed with all three drugs, and 4 weeks after dosing, 46.7% (n = 50), 56.1% (n = 60), and 37.4% (n = 40) of subjects were confirmed as ADA-positive in the BI 695501, US- and EU-approved Humira groups, respectively. The frequency of confirmed ADA-positive subjects further increased until the end of the study (Day 71) with 92.5% (n = 99), 88.0% (n = 95), and 84.3% (n = 91) of the subjects in the BI 695501, US- and EU-approved Humira groups, respectively ().
Figure 3. Development of ADAs in healthy subjects after a single dose of study drug at Days 28 and 71 for BI 695501, US- and EU-approved Humira. (a) Frequency of ADA-positive responses, (b) median ADA titer and (c) end-of-study titers for healthy subjects with ADA-positive responses. Median values are depicted by a line within the 25% and 75% percentile boxes; arithmetic mean = diamond shape; individual outliers = individual points; minimum and maximum values or 1.5× interquartile range = vertical lines out of the box plot. ADAs: antidrug antibodies.
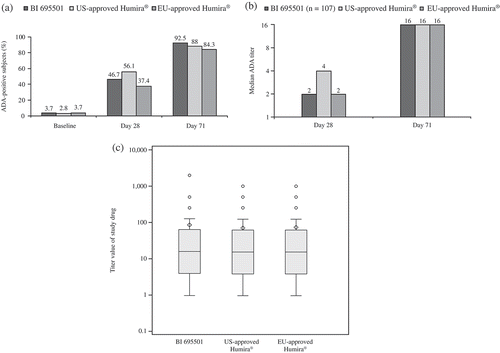
Despite the rapid development of ADA response, median titer values remained low until the end of the study. At Day 28, median (range) titer values were 2 (1–512), 4 (1–512), and 2 (1–256) for the BI 695501 group, US- and EU-approved Humira groups, respectively. At the end of the study (Day 71), median titer values remained at a relatively low level (16) for all groups with ranges of 1–2048 (BI 695501), 1–1024 (US-approved Humira), and 1–1024 (EU-approved Humira; ).
Confirmed ADA-positive samples were further characterized for their neutralizing capacity using an ADCC assay. At predose, 2.8% (n = 3), 0.9% (n = 1), and 1.9% (n = 2) of subjects in the BI 695501, US- and EU-approved Humira groups, respectively, were nAb-positive. After 4 weeks, 11.2% (n = 12), 17.8% (n = 19), and 8.4% (n = 9) of the subjects were nAb-positive in the BI 695501, US- and EU-approved Humira groups, respectively. By the end of the study, the percentage of nAb-positive subjects in the treatment groups had risen to 59.8% (n = 64), 63.9% (n = 69), and 58.3% (n = 63) for BI 695501, US- and EU-approved Humira, respectively ().
Figure 4. Neutralizing antibody development in healthy subjects after a single dose of study drug at baseline, Day 28 and Day 71 for BI 695501, US- and EU-approved Humira.
nAb: neutralizing antibody.
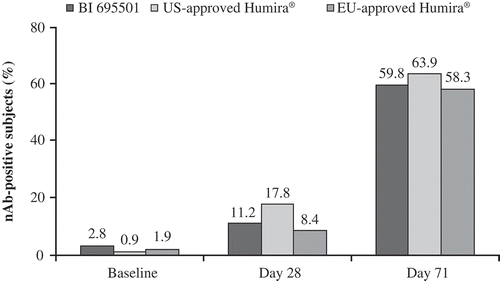
The development of nAb positivity was comparable across all three treatment groups. Taken together, these data suggest similar immunogenicity results between BI 695501, US- and EU-approved Humira.
Titer values ≤16 appeared to have a minor effect on the PK, while a decrease in exposure (AUC0–inf, pred) was observed for titer values >16. This effect was consistent across all treatment groups ().
Figure 5. Impact of antidrug antibody titer (high [>16] vs. low [≤16]) on the exposure (AUC0–inf, pred) to the study drugs in healthy subjects after a single dose of BI 695501, US- or EU-approved Humira. Median values are depicted by a line within the 25% and 75% percentile boxes; arithmetic mean = diamond shape; individual outliers = individual points; minimum and maximum values or 1.5× interquartile range = vertical lines out of the box plot.
AUC0–inf, pred: AUC of the analyte in plasma over the time interval from zero extrapolated to infinity.
![Figure 5. Impact of antidrug antibody titer (high [>16] vs. low [≤16]) on the exposure (AUC0–inf, pred) to the study drugs in healthy subjects after a single dose of BI 695501, US- or EU-approved Humira. Median values are depicted by a line within the 25% and 75% percentile boxes; arithmetic mean = diamond shape; individual outliers = individual points; minimum and maximum values or 1.5× interquartile range = vertical lines out of the box plot.AUC0–inf, pred: AUC of the analyte in plasma over the time interval from zero extrapolated to infinity.](/cms/asset/b5b2d476-600d-4e0a-ac43-ffef8aef403d/ieid_a_1255724_f0005_b.gif)
The time course of ADA development and ADA titers was similar for all three treatment groups. In addition, a similar impact of the ADAs on the PK profile of BI 695501, US- and EU-approved Humira was observed.
One subject within the US-approved Humira group developed a delayed hypersensitivity (erythema and itch at the injection site that became a generalized urticaria over the next 24 h) 11 days after administration of study drug, potentially linked with an immunogenicity response. This subject became ADA-positive at the next sampling time point (Day 14) and had a relatively high end-of-study ADA titer (128); a positive nAb response was observed at Day 28 only for this subject.
3.4. Safety
Safety and tolerability results were comparable between BI 695501, US- and EU-approved Humira. Overall, 72% (n = 232) of subjects reported at least one AE (). The most commonly reported AE was headache (78 in total; n = 25 in each of the BI 695501 and US-approved groups and n = 28 in the EU-approved group). There were no deaths during the study period.
Table 2. Summary of adverse events occurring in healthy subjects with one or more AEs, in any treatment group, after a single dose of BI 695501, US- or EU-approved Humira.
In total, 24.1% (n = 78) of subjects reported at least one drug-related AE, 19.4% (n = 21) in the BI 695501 group, 26.9% (n = 29) in the US-approved Humira group, and 25.9% (n = 28) in the EU-approved Humira group. The most commonly reported drug-related AE was headache (N = 27 in total; n = 6 in the BI 695501 group, n = 10 in the US-approved group, and n = 11 in the EU approved group, ).
Overall, 2.8% (n = 3) of subjects in each of the BI 695501 and US-approved Humira groups, and 1.9% (n = 2) in the EU-approved Humira group reported serious AEs (). Of these, two serious AEs (abdominal pain in the BI 695501 group and appendicitis in the US-approved Humira group) were considered related to the study drug. One subject reported two serious AEs (hand and ankle fracture), which were not considered related to the study drug.
Five subjects reported one AESI each (), injection site hypersensitivity (1.9% [n = 2] in the BI 695501 group and 0.9% [n = 1] in the EU-approved Humira group), hypersensitivity (0.9% [n = 1] in the US-approved Humira group), and urticaria (0.9% [n = 1] in the US-approved Humira group).
4. Discussion
BI 695501 is an Humira biosimilar candidate being developed for the treatment of a variety of inflammatory diseases. Current guidelines for biosimilarity require evaluation of the PK bioequivalence of the proposed biosimilar to the reference product. The primary objective of this study was to demonstrate the three-way PK bioequivalence between BI 695501 and US- and EU-approved Humira.
The 90% CIs for the geometric mean ratios of all primary PK parameters (for comparisons of BI 695501 to US- and EU- approved Humira, and for US- to EU-approved Humira) were within the prespecified acceptance ranges (80–125%), demonstrating three-way PK bioequivalence. Additionally, the statistical comparison of secondary and other PK parameters supported similarity between BI 695501, US- and EU-approved Humira.
Data obtained in this study indicate comparable immunogenicity results of BI 695501, US- and EU-approved Humira. Published data show variations in the incidence of ADA-positive subjects with approved Humira, highlighting the difference in data associated with individual assays and drug tolerance [Citation16–Citation24]. Although it is not appropriate to indirectly compare immunogenicity results across studies, it is evident that the results presented in this study are somewhat different to those reported in the literature. The bridging electrochemiluminescence method used in this study to detect ADAs was sensitive and highly drug tolerant and was able to detect very low ADA levels under conditions where high circulating drug levels would normally impair the sensitivity of other methods. Of note, the methodology used in this study was able to detect early immunogenicity events and discriminate between ADA levels that did or did not have an impact on PK parameters. The time course of ADA development and ADA titers is important an consideration when evaluating immunogenicity for biologics. In this study, these were similar for all three treatment groups. In addition, a similar impact of the ADAs on the PK profiles of BI 695501, US- and EU-approved Humira was observed. It can therefore be concluded that BI 695501 shows similar immunogenicity results to that of US- and EU-approved Humira in this study.
Likewise, the development of nAb was similar across all three treatment groups and the ADCC method used in this study appeared to be adequate to detect nAb responses over the course of the study. Originally published immunogenicity data relating to approved Humira results show that the rates of nAb development ranged from 3% to 9% in all approved indications over a 6–12-month period [Citation6,Citation7]. The rates of nAb development presented in this study ranged from 58.3% to 63.9% and were quite different from the published data. These results are, however, similar to the higher incidences recently observed in studies of other biosimilars, using state-of-the-art methods [Citation25]. As with the ADA results, differences could be attributed to study format, the type of assay used, and the fact that no concomitant immunosuppressive drugs (e.g. methotrexate) were allowed in the study. In this study, analyses of BI 695501 compared with US- and EU-approved Humira conducted using the same assay were similar. While assessment of immunogenicity incidence and understanding its impact on drug exposure and safety are important, comparison of ADA incidence rates generated in different studies or with previously published data is not considered appropriate, as assay methodologies for immunogenicity evaluations are generally not consistent between studies [Citation12].
Safety and tolerability results were similar between BI 695501, US- and EU-approved Humira.
5. Conclusions
The three-way PK bioequivalence between BI 695501, US- and EU-approved Humira was demonstrated. Safety, tolerability, and immunogenicity results across the three study treatments were similar.
Declaration of interest
C. Wynne is an employee of, and holds shares in Christchurch Clinical Studies Trust that received payment for carrying out the study. L. Gheyle is an employee of SGS, Clinical Pharmacology Unit, Antwerp, Belgium that received payment for carrying out the study. R. Ellis-Pegler is an employee of Auckland Clinical Studies Limited that received payment for carrying out the study. Mario Altendorfer, Ivo Sonderegger, Susanne Buschke, Benjamin Lang, Deepak Assudani, Sandeep Athalye and Niklas Czeloth are employees of Boehringer Ingelheim. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
Christopher Wynne, Lien Gheyle and Rod Ellis-Pegler were investigators in the study and provided a critical review of the manuscript. Mario Altendorfer, Ivo Sonderegger, Susanne Buschke, Benjamin Lang, Deepak Assudani, Sandeep Athalye and Niklas Czeloth were responsible for the interpretation of the study data and provided a critical review of the manuscript. The authors would like to thank the subjects who participated in the study, and the staff of the clinical research units who carried out the study. The authors would also like to thank Niraj Chhaya who was responsible for safety monitoring in the study. Medical writing support was provided by Melissa Purves of SciMentum (Manchester, UK).
The data in this manuscript have been presented previously at American Association of Pharmaceutical Scientists Annual Meeting and Exposition, Orlando, FL, USA, 25–29 October 2015 (poster number W4286) and American College of Rheumatology/Association of Rheumatology Health Professionals (ACR/ARHP) Annual Meeting, San Francisco, CA, USA, 6–11 November 2015 (poster number 2727).
Additional information
Funding
References
- Kuek A, Hazleman BL, Östör AJK. Immune‐mediated inflammatory diseases (IMIDs) and biologic therapy: A medical revolution. Postgrad Med J. 2007;83:251–260.
- Møller AH, Erntoft S, Vinding GR, et al. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167–177.
- Listing J, Kekow J, Manger B, et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFalpha inhibitors and rituximab. Ann Rheum Dis. 2015;74:415–421.
- Duffin KC. Identifying and managing complications and comorbidities in patients with psoriasis. Semin Cutan Med Surg. 2015;34:S30–S33.
- Jacobs P, Bissonnette R, Guenther LC. Socioeconomic burden of immune-mediated inflammatory diseases - focussing on work productivity and disability. J Rheumatol Suppl. 2011;88:55–61.
- Humira®. Prescribing information. Available from: http://www.rxabbvie.com/pdf/humira.pdf [ cited 2016 Aug].
- Humira®. Summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000481/WC500050870.pdf [cited 2016 Aug].
- Information for industry (biosimilars). U.S. Food and Drug Administration (FDA). Available from: http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm241720.htm.
- Guideline on similar biological medicinal products containing monoclonal antibodies - non-clinical and clinical issues. European Medicines Agency (EMA). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128686.pdf.
- McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91:405–417.
- Shankar G, Arkin S, Cocea L, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides—harmonised terminology and tactical recommendations. Aaps J. 2014;16:658–673.
- Carrascosa JM. Immunogenicity in biologic therapy: implications for dermatology. Actas Dermosifiliogr. 2013;104:471–479.
- Statistical principles for clinical trials. International Conference on Harmonisation (ICH). Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf.
- Guideline on the investigation of bioequivalence. European Medicines Agency (EMA). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf.
- Statistical approaches to establishing bioequivalence. U.S. Food and Drug Administration (FDA). Available from: http://www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdf.
- Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137:1628–1640.
- Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: A cohort study. Ann Rheum Dis. 2010;69:817–821.
- Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115.
- Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239.
- Bender NK, Heilig CE, Dröll B, et al. Immunogenicity, efficacy and adverse events of adalimumab in RA patients. Rheumatol Int. 2007;27:269–274.
- Van De Putte LB, Atkins C, Malaise M, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–516.
- Bartelds GM, Krieckaert CL, Nurmohamed MT, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305:1460–1468.
- De Vries MK, Brouwer E, Van Der Horst-Bruinsma IE, et al. Decreased clinical response to adalimumab in ankylosing spondylitis is associated with antibody formation. Ann Rheum Dis. 2009;68:1787–1788.
- Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66:921–926.
- BLA 761024. ABP 501, a proposed biosimilar to Humira (adalimumab). U.S. Food and Drug Administration (FDA). Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM510293.pdf.
