Abstract
Metabolic syndrome (MetS) is a diagnostic category, based on a cluster of risk factors (hyperglycemia/diabetes, abdominal obesity, hypertriglyceridaemia, low HDL cholesterol and hypertension), which identifies subjects at high risk for forthcoming type 2 diabetes mellitus and cardiovascular (CV) diseases. Recently, a close association between MetS, erectile dysfunction (ED) and male hypogonadism has been reported. In patients with MetS, hypogonadism can exacerbate sexual dysfunction and arteriogenic ED because of its typical symptoms, such as decreased sexual desire and mood disturbances. On the other hand, hypogonadism per se has been associated with an increased risk of CV and overall mortality. Obesity and in particular central obesity is nowadays considered the most important determinant of MetS-induced hypogonadism whereas hypertension and diabetes play a major role in ED associated with MetS. This review analyses the current literature regarding the relationship between ED, MetS and hypogonadism emphasising the epidemiological and psychopathological aspects and stressing the concept that ED subjects are ‘lucky’, because ED offers a unique chance to undergo medical examination and therefore to improve not only their sexual but, most importantly, their overall health.
Introduction
Metabolic syndrome (MetS), originally known as syndrome X, is a constellation of medical disorders that increase the overall risk of developing cardiovascular (CV) and metabolic diseases. Features of this syndrome include: insulin resistance (hyperinsulinemia, impaired glucose tolerance and type 2 diabetes mellitus), dyslipidemia (hypertriglyceridemia and low serum high-density lipoprotein cholesterol) and hypertension Citation[1]. The term ‘metabolic syndrome’ dates back to at least the late 1950s. More than 20 years later, Phillips Citation[2] developed the concept that risk factors for myocardial infarction concur to form a ‘constellation of abnormalities’ associated not only with heart disease, but also with aging, obesity and other clinical states, and originally suggested that sex hormones must be an underlying linking factor. It was an East German Professor, Markolf Hanefeld, who published in Deutsches Gesundheitswesen 1981 (in German), the first definition of the MetS, representing the common prevalence of obesity, hyper- and dyslipoproteinemia, maturity onset diabetes (type 2), gout and hypertension associated with increased incidence of atherosclerotic vascular disease, fatty liver and gallstones that develops on the basis of genetic susceptibility, combined with over-nutrition and physical inactivity (see Ref. Citation[3] for review).
The concept of MetS was later on introduced as a nosographic entity by Reaven in 1988 Citation[4] to identify subjects with an higher risk of metabolic and CV diseases, but its utility in clinical practice is still a matter of active debate Citation[1],Citation[5],Citation[6]. Although several definitions for MetS have been proposed Citation[7], criteria defined by the National Cholesterol Education Program-Third Adult Treatment Panel (NCEP-ATPIII) Citation[8] are the most widely used in clinical practice and research (). Recently, the International Diabetes Federation (IDF) Citation[9] proposed a revision of those criteria, lowering the thresholds for pathologic fasting glucose (from ≥110 to ≥100 mg/dL; 6.1 to 5.5 mmol/L) and abdominal adiposity (from >102 to ≥94 cm waist circumference). More importantly, abdominal adiposity is considered an essential, rather than an optional, component of MetS (). Obesity, and in particular visceral (android) adiposity, is in fact considered Citation[10-13] the key feature of MetS Citation[7],Citation[14],Citation[15]. A worldwide epidemic increase in obesity has taken place in the last decades and will steadily increase in the near future, due to substantial lifestyle changes Citation[16]. The National Center for Chronic Prevention and Health Promotion (http://www.cdc.gov/nccdphp/dnpa/obesity/trend/index.htm), on the basis of Health and Nutrition Examination Surveys (NHANES) Citation[17-19], estimates that, among US adults aged 20–74 years, the prevalence of obesity increased from 15.0% (in the 1976–1980 survey) to 32.9% (in the 2003–2004 survey). According to a recent study Citation[20] in Italy, overall, 38.4% of men were overweight and 7.4% were obese, with the highest proportions of overweight and obese subjects in the 45–64 year age group (51.4% overweight, 10.0% obese).
Table I. Comparisons of definition of metabolic syndrome: National Cholesterol Education Program-Third Adult Treatment Panel (NCEP-ATPIII) and International Diabetes Federation (IDF)
Longitudinal studies clearly demonstrated a direct association between obesity and the subsequent development of ED. On the other hand, a large body of evidence has substantiated the association between obesity and male hypogonadism, and a close association between MetS, erectile dysfunction (ED) and male hypogonadism has been reported. Therefore, these points will be discussed more extensively.
MetS and ED
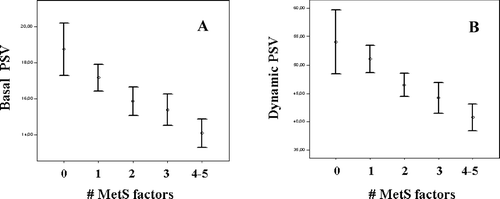
Although the relationship between CV and metabolic risk factors and impaired sexual health has been known for at least two decades, only recently it has been recognised that subjects with MetS have an increasing occurrence of sexual dysfunction, and in particular ED Citation[21],Citation[22]. The prevalence of ED in subjects with MetS was twice than in those without, and this MetS-associated increased risk in ED was coupled to a significant reduction in the endothelial function score Citation[22]. Prospective data from Massachusetts Male Aging Study in a population-based cohort observed at three time points during approximately 15 years showed that ED was predictive of MetS with an unadjusted relative risk of 1.35 Citation[23]. In subjects with sexual dysfunction, the prevalence of MetS is an age-dependent phenomenon that, at mid-life, could affect almost half of the patient population (). We found that, among patients with sexual dysfunction, those with MetS are characterised by the worst erectile function, essentially due to impairment in penile blood flow Citation[24]. Accordingly, they report a prevalent difficulty in obtaining vs. maintaining erection, a gradual onset of the disorder, and a decreased number of nocturnal erections, all of which were demonstrated to be associated more with an organic than a psychogenic origin of ED Citation[24]. shows, in a larger series of men with sexual dysfunction (n = 1007), the negative relationship between the number of MetS factors and penile blood flow, as measured at penile Doppler ultrasound. Correlations reported in for peak systolic velocity in the flaccid state (basal PSV) or after PGE1 administration (10 μg, dynamic PSV) were confirmed at multivariate regression analysis after adjustment for age (β = 0.155 and β = 0.147, respectively, both p < 0.0001). According to our studies in subjects with ED, the main MetS determinants of impaired penile blood flow were hypertension and insulin resistance (i.e. glycemia and triglyceride levels) Citation[24].
Figure 1. Prevalence of metabolic syndrome (MetS) in a consecutive series of 1536 patients afferent to our unit for sexual disorders. p < 0.0001 for trend.
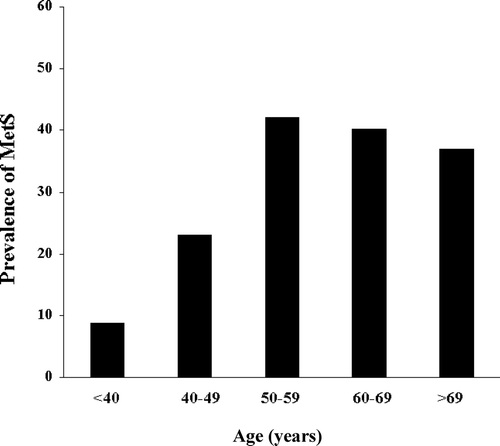
Figure 2. Peak systolic velocity (PSV) at penile Doppler ultrasound measured in the basal (A) and dynamic (B, after PGE1 stimulation) conditions as function of the number of metabolic syndrome (MetS) factors (n = 1007; both p < 0.0001 for trend).
In western countries, energy-dense diet and sedentary lifestyle determined the aforementioned dramatic increase in the incidence of obesity and MetS, which can lead to sexual disturbances and, in particular, to ED. Sexual disturbances can, in turn, affect psychological status, inducing dysfunctional behaviours, and therefore completing this vicious cycle Citation[24]. Although MetS is not characterised by any specific symptom, we previously found that patients with MetS and ED have a greater somatised anxiety than those without MetS Citation[24]. Somatisation, which is the expression of physical symptoms in the absence of medically explained physical illness, might be viewed as a part of maladaptive thought or behavior – or just as a dysfunctional response – to the sexual dysfunction itself, which is often unaccepted or unrecognised in its organic basis. The National Epidemiologic Survey on Alcohol and Related Conditions Citation[25] in a representative sample of the US population found an increased risk of panic disorder in overweight men. On the other hand, somatisation and social phobia were the most common psychiatric symptoms among patients attending a psychological consultation for bariatric surgery Citation[26]. We recently reported that both phobic and somatised anxiety were significantly associated with the degree of obesity after adjustment for age, although these associations were lost when adjusted for the presence of other factors of MetS Citation[27]. It can be speculated that the severity of organic conditions associated with obesity could render the effect of intra-psychic components less relevant. Whether somatised or phobic anxiety were the determinants or the result of both obesity and MetS is difficult to know from our data. However, it is interesting to note that a decreased central serotoninergic tone is associated with MetS Citation[28],Citation[29] and with pre-clinical carotid artery atherosclerosis Citation[30]. Repeated stressful conditions can, in turn, activate the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system. Björntorp Citation[31] hypothesised that long-term activation of the HPA axis is involved in the pathogenesis of abdominal obesity and its co-morbidities. In population studies, adrenal hormones have showed statistical associations to centralisation of body fat as well as to obesity Citation[31],Citation[32]. There is evidence from clinical to cellular and molecular studies that elevated cortisol is causing accumulation of fat in visceral adipose tissues as well as other metabolic abnormalities, which characterises MetS Citation[31],Citation[32].
MetS and hypogonadism: epidemiological features
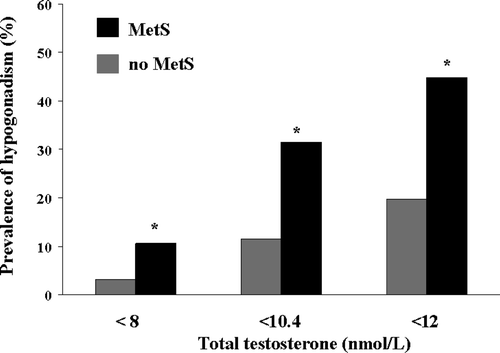
MetS is often associated with testosterone deficiency Citation[24],Citation[33],Citation[34], whereas hypogonadism represents a risk factor for diabetes mellitus and MetS Citation[35-37] and vice-versa Citation[38],Citation[39]. The association between hypogonadism and MetS has also been described in ED patients by our study and other groups' Citation[24],Citation[40]. ED is the most commonly referred symptom of male hypogonadism Citation[41],Citation[42] and represents a warning sign of MetS Citation[22], hence studying MetS in ED subjects might give further insights on their inter-relationships. We found that in subjects with ED the prevalence of hypogonadism was three times higher in those with MetS than in those without, despite that different criteria for diagnosing hypogonadism was applied (). Accordingly, patients with MetS, however defined, reported lower free testosterone levels and higher ANDROTEST scores Citation[43]. ANDROTEST is a recently validated structured interview specifically designed for the screening of hypogonadism in a sexual dysfunction population Citation[44] with similar sensitivity, but higher specificity, than other available inventories Citation[45-47]. The addition of the hypogonadal symptoms (such as lower sexual desire and mood disturbances) to the impaired penile blood flow determined by metabolic alterations has a detrimental effect, further decreasing self-esteem and increasing anxiety Citation[24]. Male hypogonadism might then be considered another MetS-associated condition. Although NCEP-ATPIII attributes identical relevance to all the diagnostic criteria for MetS, it is well-known that each component of the syndrome has a different impact on the aforementioned conditions. As mentioned before, we observed that elevated blood pressure and hyperglycemia, which are well-known CV risk factors, are associated with impaired penile vascular flow to a greater extent than the other components of MetS Citation[24],Citation[43],Citation[48]. Conversely, hypogonadism is associated with abdominal adiposity and hyperglycemia Citation[24],Citation[43],Citation[48].
Figure 3. Prevalence of hypogonadism (as derived form different criteria) in a consecutive series of 1491 subjects with or without metabolic syndrome (MetS), attending our Unit for sexual dysfunction. *p < 0.0001 vs. no MetS.
In one of our studies, we found that IDF criteria identified a higher number of subjects than the NCEP-ATPIII classification Citation[48]. However, the NCEP-ATPIII definition resulted to be a better predictor of low testosterone, than the IDF one. In fact, subjects fulfilling IDF, but not NCEP-ATPIII criteria, did not appear to have a greater chance of being affected by hypogonadism, in comparison with those without MetS. There are important differences into the two MetS definitions reported in that may explain the weaker association of IDF criteria with hypogonadism. First, lowering the cut-off value for waist circumference and hyperglycemia leads to the inclusion of patients with a relatively lower level of insulin resistance in the category of MetS. Low testosterone has been associated with a reduction of insulin sensitivity in samples from the general population Citation[49],Citation[50], and a more efficient identification of subjects with insulin resistance could be of greater help in the screening of hypogonadism. Furthermore, Zumoff et al. Citation[51] and Corona et al. Citation[27] showed that total and free testosterone is decreased in obese males in proportion to the degree of their obesity. Hence, lowering thresholds of waist circumference reduce the ability of this MetS category to identify hypogonadal patients. In addition, the mandatory status of the waist criterion in IDF definition results in a relatively lower prevalence of other (potentially stronger) risk factors in patients with MetS such as diabetes mellitus Citation[52-57]. Not only NCEP-ATP-III criteria result as the best predictor of hypogonadism, but they also remain the main predictor of arteriogenic ED Citation[48].
Type 2 diabetes is characterised by insulin resistance and is often associated with MetS. In addition, hyperglycemia is one of the conditions used for the diagnostic approach of MetS. Diabetes per se, independently of MetS, was not associated with hypogonadism Citation[43], although T2DM patients are usually more insulin-resistant than the rest of the population Citation[58]. It could be speculated that some factors associated with visceral adiposity, and upstream of insulin resistance, could contribute to hypogonadism. Out data show that MetS is clearly superior to diabetes in the identification of hypogonadal patients among those referring for sexual dysfunction. Interestingly, similar results were previously published for coronary heart disease (CHD) in the Third National Health and Nutritional Examination Survey (NHANES III): compared with individuals with MetS, those with T2DM, but without MetS, did not have an increased CHD prevalence Citation[59]. This is tantamount in saying that healthcare professionals involved in sexual medicine should carefully investigate visceral adiposity (abdominal increase in waistline + hypertriglyceridemia), with the aim to prevent obesity-related ED and hypogonadism.
MetS and hypogonadism: physiopathological features
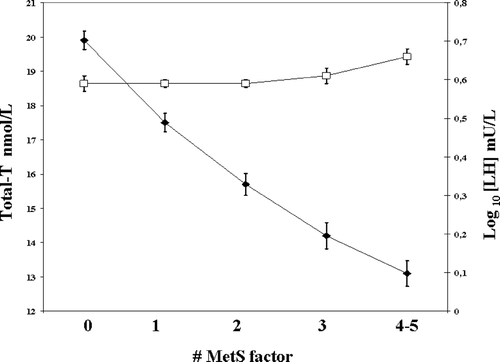
All these findings indicate that adipose tissue is not an inactive depot of reserve energy but it is able to secrete or elaborate molecules with regulatory potential on the hypothalamus-pituitary-testis axis. As shown in , the MetS-related decline in testosterone levels is not supported by a parallel increase in pituitary LH, suggesting impairment in gonadotrophin secretion. Which are the molecules that smooth the compensatory increase of GnRH/LH to MetS-induced testosterone decline is still unknown, but reasonable candidates are estrogens, insulin and leptin, TNFα or other adipokines. In a recent study, it has been demonstrated that a weekly low dose (2.5 mg) of the aromatase inhibitor letrozole can restore testosterone levels and increase LH levels in severely obese hypogonadal men Citation[60]. Hence, increased aromatisation of androgens by fat stores will increase estrogen levels, which, in turn, will decrease LH secretion. Insulin can directly signal to the hypothalamus by increasing GnRH synthesis and release Citation[61]. Accordingly, in a mouse model of central depletion of the insulin receptor the phenotype recapitulates MetS and hypogonadotropic hypogonadism Citation[62].
Figure 4. Total testosterone (T; black circles) and LH (log. transformed; open squares) levels as function of metabolic syndrome (MetS) factors in a consecutive series of 1535 patients attending our Unit for sexual dysfunction. Data are expressed as mean ± SE. p < 0.0001 for trend for testosterone levels. p = NS for trend for LH levels.
Androgens, in turn, can signal to adipocytes trough the androgen receptor (AR) or through the estrogen ones. In vivo studies in men have shown that testosterone affects triglyceride metabolism in visceral fat depots and regulates insulin sensitivity Citation[63]. In fact, withdrawing of testosterone supplementation in subjects with hypogonadotropic hypogonadism for only 2 weeks increases insulin resistance, without changing body composition Citation[64]. Accordingly, AR gene deletion in mice closely resembles MetS in humans Citation[65]. However, also mice deficient for the farnesoid X receptor (FXR), a member of the nuclear receptor superfamily of ligand-activated transcription factors, develop a phenotype closely related to human MetS. FXR−/– mice displayed elevated plasma and hepatic cholesterol and triglyceride levels with an accelerated hepatic response to eating a high carbohydrate diet and peripheral insulin resistance Citation[66]. Interestingly, androsterone, one of the main metabolites of DHT, a steroid without previously known biological activities, is now considered, beside bile acid, a natural ligand for FXR Citation[67].
On the other hand, treating hypogonadal diabetic subjects with testosterone improves insulin resistance, although only limited series of patients were reported in controlled studies Citation[68-71].
How androgens can signal to fat depots and lessen them is still a matter of active research, but this is the core of the so-called andropause, or, at least, of the ameliorable part of this age-related process. ED is another key component of this story, because it can reflect, and alert, the presence of co-morbidities. In some way, ED subjects are ‘lucky’, because they have the opportunity to unmask other metabolic derangements. MetS and diabetes mellitus are often associated with ED and hypogonadism, which, per se, further exacerbates the sexual problem. In fact, in animal models, the presence of a diabetes mellitus-induced hypogonadism can alter all the major pathways involved in mediating penile erection such as NOS, PDE5 and RhoA/ROCK Citation[72],Citation[73]. Recognising, through ED, underlying conditions such as hypogonadism and/or diabetes/ MetS might be a useful motivation for men to improve their health-related lifestyle choices. Encouraging changes in life style, such as diet or physical exercise, can improve not only overall men's health but also sexual health, delaying the age-related androgen decline.
Conclusions
ED, hypogonadism and MetS are closely associated with each other. The presence of ED should become an opportunity – for the patient and for the doctor – to screen for the presence of co-morbidities. Hence, ED subjects are ‘lucky’, because ED offers a unique chance to undergo medical examination and therefore, to improve not only their sexual but, most importantly, their overall health. The correction of concomitant risk factors such as MetS and hypogonadism could reduce CV morbidity and mortality.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Despres J P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887
- Phillips G B. Sex hormones, risk factors and cardiovascular disease. Am J Med 1978; 65: 7–11
- Traish A M, Guay A T, Feeley R, Saad F. The dark side of testosterone deficiency: I. metabolic syndrome & erectile dysfunction. J Androl 2008, [Epub ahead of print]
- Reaven G M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37: 1595–1607
- Reaven G M. The metabolic syndrome: requiescat in pace. Clin Chem 2005; 51: 931–938
- Grundy S M. Point: the metabolic syndrome still lives. Clin Chem 2005; 51: 1352–1354
- Eckel R H, Grundy S M, Zimmet P Z. The metabolic syndrome. Lancet 2005; 365: 1415–1428
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497
- Alberti K G, Zimmet P, Shaw J. Metabolic syndrome a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med 2006; 23: 469–480
- Marin P, Arver S. Androgens and abdominal obesity. Baillieres Clin Endocrinol Metab 1998; 12: 441–451
- Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men. Circulation 2000; 102: 179–184
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi M G, Commerford P, Lang C C, Rumboldt Z, Onen C L, Lisheng L, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005; 366: 1640–1649
- Kragelund C, Omland T. A farewell to body-mass index. Lancet 2005; 366: 1589–1591
- Kahn R, Buse J, Ferrannini E, Stern M, American Diabetes Association; European Association for the Study of Diabetes. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005; 28: 2289–2304
- Grundy S M, Cleeman J I, Daniels S R, Donato K A, Eckel R H, Franklin B A, Gordon D J, Krauss R M, Savage P J, Smith SC Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–2752
- Cameron A J, Shaw J E, Zimmet P Z. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am 2004; 33: 351–375
- Ogden C L, Carroll M D, Curtin L R, McDowell M A, Tabak C J, Flegal K M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006; 295: 1549–1555
- Alley D E, Chang V W. The changing relationship of obesity and disability, 1988–2004. JAMA 2007; 298: 2020–2027
- Flegal K M, Graubard B I, Williamson D F, Gail M H. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 2007; 298: 2028–2037
- Gallus S, Colombo P, Scarpino V, Zuccaro P, Negri E, Apolone G, La Vecchia C. Overweight and obesity in Italian adults 2004, and an overview of trends since 1983. Eur J Clin Nutr 2006; 60: 1174–1179
- Esposito K, Giugliano D. Obesity, the metabolic syndrome, and sexual dysfunction. Int J Impot Res 2005; 17: 391–398
- Esposito K, Giugliano F, Martedi E, Feola G, Marfella R, D'Armiento M, Giugliano D. High proportions of erectile dysfunction in men with the metabolic syndrome. Diabetes Care 2005; 28: 1201–1203
- Kupelian V, Shabsigh R, Araujo A B, O'Donnell A B, McKinlay J B. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging Study. J Urol 2006; 176: 222–226
- Corona G, Mannucci E, Schulman C, Petrone L, Mansani R, Cilotti A, Balercia G, Chiarini V, Forti G, Maggi M. Psycho-biologic correlates of the metabolic syndrome and associated sexual dysfunction. Eur Urol 2006; 50: 595–604
- Pickering R P, Grant B F, Chou S P, Compton W M. Are overweight, obesity, and extreme obesity associated with psychopathology? Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry 2007; 68: 998–1009
- Rosik C H. Psychiatric symptoms among prospective bariatric surgery patients: rates of prevalence and their relation to social desirability, pursuit of surgery, and follow-up attendance. Obes Surg 2005; 15: 677–683
- Corona G, Mannucci E, Fisher A D, Lotti F, Petrone L, Balercia G, Bandini E, Forti G, Maggi M. Low levels of androgens in men with erectile dysfunction and obesity. J Sex Med 2008; 5: 2454–2463
- Muldoon M F, Mackey R H, Korytkowski M T, Flory J D, Pollock B G, Manuck S B. The metabolic syndrome is associated with reduced central serotonergic responsivity in healthy community volunteers. J Clin Endocrinol Metab 2006; 91: 718–721
- Muldoon M F, Mackey R H, Williams K V, Korytkowski M T, Flory J D, Manuck S B. Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity. J Clin Endocrinol Metab 2004; 89: 266–271
- Muldoon M F, Mackey R H, Sutton-Tyrrell K, Flory J D, Pollock B G, Manuck S B. Lower central serotonergic responsivity is associated with preclinical carotid artery atherosclerosis. Stroke 2007; 38: 2228–2233
- Björntorp P. Do stress reactions cause abdominal obesity and comorbidities. Obes Rev 2001; 2: 73–86
- Wallerius S, Rosmond R, Ljung T, Holm G, Björntorp P. Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J Endocrinol Invest 2003; 26: 616–619
- Laaksonen D E, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen T P, Salonen R, Rauramaa R, Salonen J T. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol 2003; 149: 601–608
- Kapoor D, Malkin C J, Channer K S, Jones T H. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol (Oxf) 2005; 63: 239–250
- Stellato R K, Feldman H A, Hamdy O, Horton E S, McKinlay J B. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care 2000; 23: 490–494
- Oh J Y, Barrett-Connor E, Wedick N M, Wingard D L, Rancho Bernardo Study. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care 2002; 25: 55–60
- Kupelian V, Page S T, Araujo A B, Travison T G, Bremner W J, McKinlay J B. Low Sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in non-obese men. J Clin Endocrinol Metab 2006; 91: 843–850
- Laaksonen D E, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen T P, Valkonen V P, Salonen J T. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab 2005; 90: 712–719
- Derby C A, Zilber S, Brambilla D, Morales K H, McKinlay J B. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts male ageing study. Clin Endocrinol (Oxf) 2006; 65: 125–131
- Kaplan S A, Meehan A G, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men?. J Urol 2006; 176: 1524–1527
- Nieschlag E, Swerdloff R, Behre H M, Gooren L J, Kaufman J M, Legros J J, Lunenfeld B, Morley J E, Schulman C, Wang C, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. ISA, ISSAM, and EAU recommendations. Eur Urol 2005; 48: 1–4
- Maggi M, Schulman C, Quinton R, Langham S, Uhl-Hochgraeber K. The burden of testosterone deficiency syndrome in adult men: economic and quality-of-life impact. J Sex Med 2007; 4(Pt 1)1056–1069
- Corona G, Mannucci E, Petrone L, Balercia G, Paggi F, Fisher A D, Lotti F, Chiarini V, Fedele D, Forti G, et al. NCEP-ATPIII-defined metabolic syndrome, type 2 diabetes mellitus, and prevalence of hypogonadism in male patients with sexual dysfunction. J Sex Med 2007; 4(Pt 1)1038–1045
- Corona G, Mannucci E, Petrone L, Balercia G, Fisher A D, Chiarini V, Forti G, Maggi M. ANDROTEST: a structured interview for the screening of hypogonadism in patients with sexual dysfunction. J Sex Med 2006; 3: 706–715
- Morley J E, Charlton E, Patrick P, Kaiser F E, Cadeau P, McCready D, Perry H M, III. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49: 1239–1242
- Heinemann L A, Saad F, Heinemann K, Thai D M. Can results of the aging males' symptoms (AMS) scale predict those of screening scales for androgen deficiency. Aging Male 2004; 7: 211–218
- Smith K W, Feldman H A, McKinlay J B. Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol (Oxf) 2000; 53: 703–711
- Corona G, Mannucci E, Petrone L, Schulman C, Balercia G, Fisher A D, Chiarini V, Forti G, Maggi M. A comparison of NCEP-ATPIII and IDF metabolic syndrome definitions with relation to metabolic syndrome-associated sexual dysfunction. J Sex Med 2007; 4: 789–796
- Pitteloud N, Mootha V K, Dwyer A A, Hardin M, Lee H, Eriksson K F, Tripathy D, Yialamas M, Groop L, Elahi D, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005; 28: 1636–1642
- Pitteloud N, Hardin M, Dwyer A A, Valassi E, Yialamas M, Elahi D, Hayes F J. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab 2005; 90: 2636–2641
- Zumoff B, Strain G W, Miller L K, Rosner W, Senie R, Seres D S, Rosenfeld R S. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab 1990; 71: 929–931
- Barrett-Connor E, Khaw K T, Yen S S. Endogenous sex hormone levels in older adult men with diabetes mellitus. Am J Epidemiol 1990; 132: 895–901
- Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med 1992; 117: 807–811
- Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004; 89: 5462–5468
- Corona G, Mannucci E, Petrone L, Ricca V, Balercia G, Mansani R, Chiarini V, Giommi R, Forti G, Maggi M. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res 2006; 18: 190–197
- Corona G, Mannucci E, Mansani R, Petrone L, Bartolini M, Giommi R, Forti G, Maggi M. Organic, relational and psychological factors in erectile dysfunction in men with diabetes mellitus. Eur Urol 2004; 46: 222–228
- Zhang X H, Filippi S, Morelli A, Vignozzi L, Luconi M, Donati S, Forti G, Maggi M. Testosterone restores diabetes-induced erectile dysfunction and sildenafil responsiveness in two distinct animal models of chemical diabetes. J Sex Med 2006; 3: 253–264
- Kahn S E, Hull R L, Utzschneider K M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846
- Alexander C M, Landsman P B, Teutsch S M, Haffner S M, Third National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003; 52: 1210–1214
- Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol 2008; 158: 741–747
- Burcelin R, Thorens B, Glauser M, Gaillard R C, Pralong F P. Gonadotropin-releasing hormone secretion from hypothalamic neurons: stimulation by insulin and potentiation by leptin. Endocrinology 2003; 144: 4484–4491
- Brüning J C, Gautam D, Burks D J, Gillette J, Schubert M, Orban P C, Klein R, Krone W, Müller-Wieland D, Kahn C R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000; 289: 2122–2125
- Mårin P, Odén B, Björntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab 1995; 80: 239–243
- Yialamas M A, Dwyer A A, Hanley E, Lee H, Pitteloud N, Hayes F J. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 2007; 92: 4254–4259
- Lin H Y, Xu Q, Yeh S, Wang R S, Sparks J D, Chang C. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes 2005; 54: 1717–1725
- Sinal C J, Tohkin M, Miyata M, Ward J M, Lambert G, Gonzalez F J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000; 102: 731–744
- Wang S, Lai K, Moy F J, Bhat A, Hartman H B, Evans M J. The nuclear hormone receptor farnesoid X receptor (FXR) is activated by androsterone. Endocrinology 2006; 147: 4025–4033
- Boyanov M A, Boneva Z, Christov V G. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 2003; 6: 1–7
- Kapoor D, Goodwin E, Channer K S, Jones T H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154: 899–906
- Kapoor D, Clarke S, Stanworth R, Channer K S, Jones T H. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2007; 156: 595–602
- Saad F, Gooren L, Haider A, Yassin A. Effects of testosterone gel followed by parenteral testosterone undecanoate on sexual dysfunction and on features of the metabolic syndrome. Andrologia 2008; 40: 44–48
- Zhang X H, Filippi S, Morelli A, Vignozzi L, Luconi M, Donati S, Forti G, Maggi M. Testosterone restores diabetes-induced erectile dysfunction and sildenafil responsiveness in two distinct animal models of chemical diabetes. J Sex Med 2006; 3: 253–264
- Vignozzi L, Morelli A, Filippi S, Ambrosini S, Mancina R, Luconi M, Mungai S, Vannelli G B, Zhang X H, Forti G, et al. Testosterone regulates RhoA/Rho-kinase signaling in two distinct animal models of chemical diabetes. J Sex Med 2007; 4: 620–630
