Abstract
Objective: We investigated the effects of testosterone replacement therapy (TRT) on bone mineral density (BMD) among hypogonadal men with osteopenia/osteoporosis.
Methods: From our previous EARTH study population, 74 patients with a clinical diagnosis of osteopenia or osteoporosis and hypogonadism were included in this study, as the TRT (n = 35) and control (n = 34) groups. The TRT group was administered 250 mg of testosterone enanthate injection every 4 weeks for 12 months. The BMD, waist circumference, body mass index, body fat percentage, and muscle volume were measured at baseline and at 12 months. Blood biochemical data, including total cholesterol, triglycerides, HDL-cholesterol, hemoglobin A1c, and adiponectin values were also evaluated.
Results: At the 12-month visit, BMD significantly increased in both groups. However, comparisons on changes of parameter values from baseline to the 12-month visit between the TRT and control groups were significantly different in BMD (5.0 ± 5.0 vs. 3.0 ± 3.2; p = .0434) and in adiponectin value (−0.90 ± 3.33 vs. 0.10 ± 2.04; p = .0192). There were no significant changes in other parameters.
Conclusions: TRT for 12 months could improve BMD with a decrease in adiponectin levels among hypogonadal men with osteopenia/osteoporosis.
Introduction
Decreased bone mineral density (BMD), such as in osteopenia and osteoporosis, is considered a serious public health concern, which may contribute to compression spine fractures and femoral neck fractures, resulting in a decrease of activities of daily living and quality of life (QOL). It is estimated that over 12 million people suffer from osteoporosis in Japan, and it is generally more common in elderly women than men [Citation1,Citation2]. A Japanese community-based epidemiological investigation demonstrated that in men and women over 40 years of age, the prevalence rate of osteoporosis was 3.4% and 19.2% in lumbar vertebrae and 12.4% and 26.5% in the femoral neck, respectively [Citation1,Citation2]. Conversely, the incidence of femoral neck fracture in men is four-fold the frequency in women with osteoporosis [Citation3]. Various risk factors for osteoporosis have been identified, including aging, female sex, low body weight, lack of exercise, decreased muscle volume, decreased sex hormone concentrations, and some medications [Citation4–6]. Particularly, it is widely accepted that there is a significant relationship between estrogen deficiency and the development of osteopenia among menopausal women aged over 50 years [Citation4].
Similar to the established mechanisms of female osteopenia, it is currently known that decline in testosterone levels with age can contribute to decrease in BMD among men aged over 50 years [Citation6–8]. Serum testosterone levels are known to decline with age in men by 2–3% annually, which is associated with specific symptoms of late-onset hypogonadism (LOH) syndrome [Citation8]. The LOH syndrome is characterized by a cluster of clinical symptoms, including decreased libido and sexual desire, muscle weakness, and increased visceral fat, obesity, deterioration of insulin resistance, and dyslipidemia, in addition to osteoporosis [Citation8–11]. Testosterone replacement therapy (TRT) is a widely accepted tool to improve these clinical conditions in hypogonadal men, and its clinical use has increased substantially over the past several years [Citation8–11].
Indeed, some previous studies suggested an ameliorative effect of TRT on osteoporosis/osteopenia [Citation12–17], whereas the other studies failed to find a positive effect on BMD [Citation18–20]. Therefore, whether TRT is effective to treat BMD remains unclear. A recent randomized controlled study (the EARTH study) in Japan demonstrated that TRT during 1 year contributed to a significant decrease in waist circumference and serum triglycerides (TG), a significant increase in muscle mass volume and serum hemoglobin, and some degree of improvement in erectile function and QOL among 334 hypogonadal men [Citation20]. However, the efficacy of TRT on BMD was not shown in the EARTH study. Therefore, the objective of the present subanalysis of EARTH study data was to investigate the effects of TRT on BMD among hypogonadal men with osteopenia/osteoporosis.
Methods
Study subjects
Data of hypogonadal patients with osteopenia/osteoporosis were obtained from the EARTH study population. The biochemical diagnosis of hypogonadism was based on the Japanese biochemical criteria as follows: free testosterone (FT) ≤8.5 pg/ml (TRT is the first choice of treatment for hypogonadism) and FT 8.5–11.8 pg/ml (TRT is a relative choice of treatment) [Citation21]. According to the Japanese osteoporosis diagnostic criteria established by the Japanese Society for Bone and Mineral Metabolism in 2012, osteopenia was defined as <–1.0 standard deviation (SD) (approximately 88%) of BMD/young adult mean (YAM) ratio, and osteoporosis was defined as <70% of BMD/YAM ratio, based on BMD measured by dual-energy X-ray absorptiometry at the AP lumbar spine (L2–L4) [Citation22].
The eligibility and exclusion criteria of the EARTH study were described previously [Citation20]. Additionally, we also excluded patients with any medication associated with bone metabolism, such as calcium analogs, vitamin D, and bisphosphonate, patients with a history of spine compression or femoral neck fracture, patients who underwent chemotherapy for any malignancies, and patients with incomplete data.
Finally, data of 74 hypogonadal patients with osteopenia were obtained from the EARTH study. In this study, 35 patients underwent TRT for 12 months and 34 control patients did not receive treatment. Five patients with incomplete data were excluded. The patients provided written informed consent before participation in the study, and the protocol and study procedures were approval by the Kanazawa University Hospital Institutional Review Board.
Study protocols
At the baseline visit, eligible participants underwent physical examination, history of their current medications and medical illnesses was collected, as well as blood samples for blood biochemistry, including FT value. FT levels were measured by radioimmunoassay with a DPC's free-T kit (Mitsubishi Kagaku, Tokyo, Japan). Waist circumference, body mass index (BMI), body fat percentage, BMD, and whole muscle mass volume were examined in all patients at baseline and the 12-month visit. Additionally, blood biochemical data, including total cholesterol (Tchol), TG, high-density lipoprotein cholesterol (HDL-Chol), hemoglobin A1c, and total adiponectin value were measured before and after the treatment. BMD was evaluated based on dual-energy X-ray absorptiometry with a Lunar Prodigy Instrument (GE Healthcare, Madison, WI) at the AP lumbar spine (L2–L4). Body fat percentage and body muscle volume were assessed using the BodyPlanner™ DF800 body fat monitoring system (Yamato Biospace Technology, Hyogo, Japan), and the body muscle volume was measured in eight phases, and quantified in levels from 0 to 7 (the latter indicates large volume).
The TRT group was administered intramuscular testosterone enanthate (250 mg; Enarmon Depot®; ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) every 4 weeks for 12 months, whereas the control group received no treatment.
Statistical analysis
Initially, backgrounds of patients with and without osteopenia, as well as those receiving TRT and those without, were compared using Student’s t-test. For each group, changes in parameters were compared using Wilcoxon’s signed rank test, and changes from baseline were evaluated using the Mann–Whitney U-test. All statistical analyses were performed using SPSS™ statistics 22 (SPSS Inc., Chicago, IL). In all analyses, p values < .05 were considered statistically significant.
Results
Among a total of 74 participants, the mean (SD) ages and mean (SD) FT values were 67.9 ± 8.6 years and 7.03 ± 2.39 pg/ml, respectively (). The mean (SD) BMD was 77.3 ± 7.8%. Waist circumference, BMI, body fat percentage, whole muscle volume, Tchol values were significantly lower in the patients with osteopenia/osteoporosis than in those without. Additionally, patients with osteopenia/osteoporosis had a significantly higher serum adiponectin value (p = .00431). There were no significant differences between the two groups in any other parameters.
Table 1. Comparisons of patient background characteristics between osteopenia and non-osteopenia group.
Patient characteristics in both groups are summarized in . The mean (SD) ages of patients in the TRT and control groups were 66.1 ± 8.4 years and 69.5 ± 7.3 years, respectively. The patients in the TRT group were significantly younger than those in control (p = .0349). The FT value in the TRT group was slightly, but not significantly higher than in the control group (7.52 ± 2.38 vs. 6.71 ± 2.27 pg/ml; p = .0539). No statistically significant differences were observed between the two groups for other baseline characteristics.
Table 2. Comparisons on patients' backgrounds between TRT and control groups.
Changes of each parameter from baseline to 12-month visit between TRT and control groups are shown in and . BMD showed a significant increase by 12-month treatment in the TRT group (from 77.3 ± 7.8 to 82.4 ± 7.7%; p < .001) and the control group (from 76.8 ± 8.7 to 79.9 ± 9.3%; p < .001) (, ). A significant decrease in HDL-Chol was observed in the TRT group at the 12-month visit (from 57 ± 15 to 54 ± 12; p = .0133), which was not observed in the control group (p = .0871). From baseline to the 12-month visit, there were no significant changes in adiponectin levels, waist circumference, BMI, body fat percentage, whole muscle volume, HbA1c, Tchol, and TG values between both groups.
Figure 1. Changes of adiponectin, waist circumference, body mass index, body fat percentage, muscle volume, HbA1c, total cholesterol, triglyceride, and HDL-cholesterol, from baseline to 12-month visit between TRT and control groups were shown. A significant decrease in HDL-Chol was observed in the TRT group at the 12-month visit, whereas it was not observed in the control group. There were no significant changes in adiponectin levels, waist circumference, BMI, body fat percentage, whole muscle volume, HbA1c, Tchol, and TG values between both groups. *Significant difference; ♦, TRT group; ▪, control group.
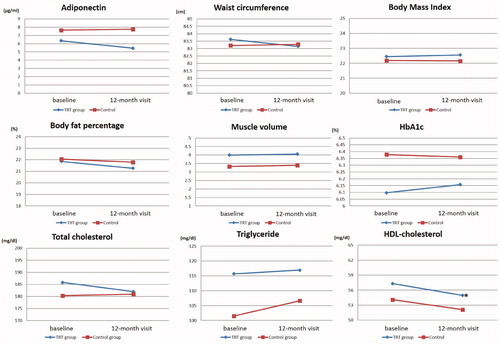
Figure 2. Changes of BMD from baseline to 12-month visit between TRT and control groups were shown. BMD showed a significant increase by 12-month treatment in both groups. *Significant difference; ♦, TRT group; ▪, control group.
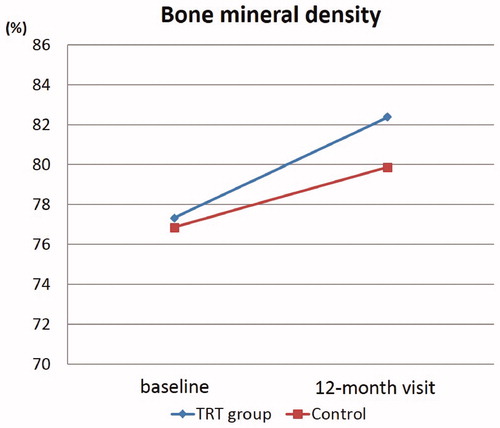
Table 3. Comparisons on changes from baseline to 12-month visit in each parameter.
Comparison of changes of parameters values from baseline at 12-month visit between the TRT and control groups showed a significant difference in BMD (5.0 ± 5.0 vs. 3.0 ± 3.2; p = .0434) and adiponectin value (−0.90 ± 3.33 vs. 0.10 ± 2.04; p = .0192) (). Thus, TRT for 12 months contributed to a significant improvement of BMD with a decrease in adiponectin levels. Waist circumference, BMI, body fat percentage, whole muscle volume, HbA1c, Tchol, TG, and HDL-Chol showed no significant differences in changes from baseline to the 12-month visit.
Table 4. Comparisons on changes from baseline to 12-months visit between TRT and control groups.
Discussion
Concomitantly with the worldwide gradual extension of the average life span, the incidence of osteopenia and osteoporosis is expected to increase in the future, especially in men compared with women. Falls and bone fractures can potentially result in a decrease in activities of daily living and QOL for elderly men. Thus, the prevention of such events is an important issue in the aging society. The incidence of bone fracture is significantly associated with deterioration of physical functions, such as a decrease in BMD, muscle volume, and muscular strength, and these clinical conditions are closely linked with testosterone deficiency for men [Citation23,Citation24]. In particular, testosterone plays a major role in maintaining BMD. Additionally, the prevalence of osteoporosis among men with testosterone deficiency is double that of those with normal testosterone levels [Citation8,Citation24]. Therefore, TRT is expected to be useful for the prevention and management of osteoporosis and the improvement of BMD among hypogonadal men. However, very few randomized control studies have focused on the effects of TRT on BMD. Thus, we conducted the present subanalysis of the EARTH study to address this knowledge gap. As a result, we found some notable effects of TRT on hypogonadal symptoms among Japanese men [Citation20]. This study is the first study to demonstrate a beneficial effect of TRT on BMD among hypogonadal men with osteopenia in Japan.
In the present study, the FT levels were not significantly different between the two groups stratified by BMD. For this reason, our study subjects consisted only of a population of hypogonadal men. However, patients with osteopenia/osteoporosis had significantly lower waist circumference, BMI, body fat percentage, and whole muscle volume compared with those without osteopenia/osteoporosis. One meta-analysis reported that lower BMI was significantly associated with a decrease in BMD, which confers a risk of substantial importance for bone fractures independent of age and sex [Citation25]. A cross-sectional study including 358 men aged 50 years and older described that decline in BMI was an independent risk factor of osteoporosis or osteopenia, while an increase in BMI was a protective factor for decreased BMD in elderly men [Citation26]. Conversely, some reports suggest that obesity and metabolic syndrome can also have a negative effect on BMD in elderly men [Citation27,Citation28]. The association between BMI and BMD is conflicting and complex, and is likely to be modified by the interaction between BMI and various metabolic and lifestyle factors, such as diabetes mellitus, exercise loss, and lifestyle habits.
We found that serum adiponectin was significantly higher in patients with osteopenia/osteoporosis. Adiponectin is a circulating peptide hormone secreted by adipocytes; low serum adiponectin is significantly associated with central adiposity, insulin resistance, and cardiovascular morbidity [Citation29,Citation30]. Recent studies demonstrated that adiponectin receptors were also present in bone tissues including osteoblasts and osteoclasts [Citation31], and higher serum adiponectin was associated with lower BMD value and increased incidence of osteoporotic fracture in elderly men [Citation32–34]. A cross-sectional study indicated that adiponectin correlated negatively with body fat percentage and BMD in older men [Citation33], which was substantially consistent with our results. Additionally, another longitudinal cohort study, including 999 elderly men and a median observation of 5.2 years showed that the risk of osteoporotic fractures increased with increasing serum adiponectin values with a hazard ratio of 1.30 (95% CI, 1.09–1.55) after adjusting for several other risk factors [Citation34].
Although adiponectin is secreted from adipocytes, circulating adiponectin increases in lean conditions, but decreases with increasing visceral fat, which seems to be a paradoxical phenomenon [Citation29]. Some recent studies have identified bone marrow adipose tissue (MAT) as an alternative endocrine organ of adiponectin [Citation35,Citation36]. Increased MAT with aging is known to be associated with the development of osteopenia and osteoporosis, and may also contribute to an increase in adiponectin levels among men with osteopenia/osteoporosis. Interestingly, the present study demonstrated that TRT for 12 months could contribute to a significant decrease in adiponectin values along with a significant increase in BMD. This finding suggests that a decrease in MAT by TRT could lead to a slight decline of adiponectin. However, the current data on MAT are limited, and further studies are required to reach a more definite conclusion in this regard.
We found that 12-month TRT could contribute to the improvement of BMD for hypogonadal men. Some previous randomized clinical trials suggested a beneficial effect of TRT on BMD [Citation12–16]. A previous study, including 15 asthmatic men who were receiving long-term glucocorticoid treatment, revealed that the TRT group had a significant improvement in lumbar BMD by 250 mg testosterone depot injection monthly for 12 months compared with controls [Citation12]. Transdermal testosterone (5 mg/day for 12 months) prevented bone loss at the femoral neck, in addition to decreased body fat, and increased lean body mass in elderly healthy men with low bioavailable testosterone levels [Citation13]. Crawford et al. reported that lumbar spine BMD increased significantly in men who required long-term systemic glucocorticoid treatment and were treated with 12 months of testosterone (200 mg every 2 weeks) [Citation14]. Additionally, long-term effects of TRT on BMD were also reported by a nonrandomized cross-sectional study [Citation15,Citation16]. A previous study, including 72 hypogondal men under a long-term observation of up to 16 years, while receiving TRT, showed that TRT increased BMD in hypogonadal men regardless of age. Further, the greatest increase was seen during the first year of treatment in previously untreated patients with initially low BMD. Furthermore, BMD can be continuously maintained within the normal range by TRT for a long-term observation [Citation15]. The pooled results of a meta-analysis suggested that testosterone had a beneficial effect on BMD, and it produced a consistent reduction in bone resorption markers [Citation17].
Conversely, the other randomized studies failed to find a positive effect of TRT on BMD. A testosterone patch of 6 mg/day for 36 months could contribute to a significant improvement in BMD, but the mean BMD changes did not differ between TRT and control groups [Citation18]. Christmas et al. mentioned that BMD and urinary excretion of deoxypyridinoline showed no significant changes from baseline between TRT and control groups [Citation19]. These conflicting data can be explained by differences between the study population background characteristics, including baseline BMD, testosterone levels, and the presence of various metabolic parameters. Further studies are required for a more accurate understanding of the effects of TRT on BMD. Furthermore, data on the clinical effects of TRT on the prevention of falls and bone fractures, which are associated with morbidity and mortality for elderly people, are still unavailable.
The present study has several limitations. The main limitations were the small sample (35 patients with TRT and 34 controls) and the short-term observation period. The subjects should be followed for a longer period. Additionally, our subjects consisted of hypogonadal men with mean of FT levels of 7.03 ± 2.39 pg/ml, 74 (31.4%) patients, with a mean age of 67.9 ± 9.4 years, were selected from the EARTH study. This is a relatively higher prevalence than that in the age-matched community-based population in Japan [Citation2]. Furthermore, the present study includes lack of data of estradiol, which is well known to be associated with bone metabolism. In addition, this study was a retrospective subanalysis of the EARTH study. Indeed, there was a statistically significant difference in participants’ age at baseline between the two groups. Therefore, prospective double blind placebo controlled studies, including a large number of participants, long-term observation, and estradiol evaluation, are certainly required to validate our conclusions.
Disclosure statement
The authors report no declarations of interest.
References
- Yoshimura N, Muraki S, Oka H, et al. Cohort profile: research on osteoarthritis/osteoporosis against disability study. Int J Epidemiol. 2010;39:988–995.
- Yoshimura N, Muraki S, Oka H, et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. 2009;27:620–628.
- van Staa TP, Dennison EM, Leufkens HG, et al. Epidemiology of fractures in England and Wales. Bone. 2001;29:517–522.
- Greenspan SL, Myers ER, Maitland LA, et al. Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA. 1994;271:128–133.
- De Laet CE, Van Hout BA, Burger H, et al. Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J Bone Miner Res. 1998;13:1587–1593.
- Basurto L, Zarate A, Gomez R, et al. Effect of testosterone therapy on lumbar spine and hip mineral density in elderly men. Aging Male. 2008;11:140–145.
- Köhn FM. Testosterone and body functions. Aging Male. 2006;9:183–188.
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5:427–448.
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15.
- Lunenfeld B, Arver S, Moncada I, et al. How to help the aging male? Current approaches to hypogonadism in primary care. Aging Male. 2012;15:187–197.
- Reid IR, Wattie DJ, Evans MC, et al. Testosterone therapy in glucocorticoid-treated men. Arch Intern Med. 1996;156:1173–1177.
- Kenny AM, Prestwood KM, Gruman CA, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–M272.
- Crawford BA, Liu PY, Kean MT, et al. Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab. 2003;88:3167–3176.
- Behre HM, Kliesch S, Leifke E, et al. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2386–2390.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of long-acting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 36 months controlled study. Aging Male. 2012;15:96–102.
- Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63:280–293.
- Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966–1972.
- Christmas C, O'Connor KG, Harman SM, et al. Growth hormone and sex steroid effects on bone metabolism and bone mineral density in healthy aged women and men. J Gerontol A Biol Sci Med Sci. 2002;57:M12–M18.
- Konaka H, Sugimoto K, Orikasa H, et al. Effects of long-term androgen replacement therapy on the physical and mental statuses of aging males with late-onset hypogonadism: a multicenter randomized controlled trial in Japan (EARTH Study). Asian J Androl. 2016;18:25–34.
- Namiki M, Akaza H, Shimazui T, et al. Clinical practice manual for late-onset hypogonadism syndrome. Int J Urol. 2008;15:377–388.
- Hagino H. Revised osteoporosis diagnostic criteria and Japanese practice guideline on osteoporosis. Clin Calcium. 2014;24:11–18.
- Lunenfeld B, Nieschlag E. Testosterone therapy in the aging male. Aging Male. 2007;10:139–153.
- Fink HA, Ewing SK, Ensrud KE, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91:3908–3915.
- De Laet C, Kanis JA, Odén A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338.
- Jiang Y, Zhang Y, Jin M, et al. Aged-related changes in body composition and association between body composition with bone mass density by body mass index in Chinese Han men over 50-year-old. PLoS One. 2015;10:e0130400.
- Zhou J, Zhang Q, Yuan X, et al. Association between metabolic syndrome and osteoporosis: a meta-analysis. Bone. 2013;57:30–35.
- Muka T, Trajanoska K, Kiefte-de Jong JC, et al. The association between metabolic syndrome, bone mineral density, hip bone geometry and fracture risk: the Rotterdam study. PLoS One. 2015;10:e0129116.
- Li S, Shin HJ, Ding EL, et al. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188.
- Gable DR, Hurel SJ, Humphries SE. Adiponectin and its gene variants as risk factors for insulin resistance, the metabolic syndrome and cardiovascular disease. Atherosclerosis. 2006;188:231–244.
- Shinoda Y, Yamaguchi M, Ogata N, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208.
- Johansson H, Odén A, Lerner UH, et al. High serum adiponectin predicts incident fractures in elderly men: osteoporotic fractures in men (MrOS) Sweden. J Bone Miner Res. 2012;27:1390–1396.
- Song HJ, Oh S, Quan S, et al. Gender differences in adiponectin levels and body composition in older adults: Hallym aging study. BMC Geriatr. 2014;14:8.
- Basurto L, Galván R, Cordova N, et al. Adiponectin is associated with low bone mineral density in elderly men. Eur J Endocrinol. 2009;160:289–293.
- Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375.
- Sulston RJ, Cawthorn WP. Bone marrow adipose tissue as an endocrine organ: close to the bone? Horm Mol Biol Clin Investig. 2016;28:21–38.

