Abstract
Aims
The objective of this research is to evaluate the cost-effectiveness of zuranolone, the first oral treatment indicated for postpartum depression (PPD) in adults approved by the United States Food and Drug Administration.
Methods
Zuranolone and selective serotonin reuptake inhibitor (SSRI) trial-based efficacy was derived from an indirect treatment comparison. Long-term efficacy outcomes were based on a large longitudinal cohort study. Maternal health utility values were derived from trial-based, short-form 6-D responses. Other inputs were derived from literature and economic data from the US Bureau of Labor Statistics. We estimated costs (2023 US dollars) and quality-adjusted life-years (QALYs) for patients with PPD treated with zuranolone (14-day dosing) or SSRIs (chronic dosing). The indirect costs and QALYs of the children and partners were also estimated.
Results
The incremental cost-effectiveness ratio for zuranolone versus SSRIs was $94,741 per QALY gained over an 11-year time horizon. Maternal total direct medical costs averaged $84,318 in the zuranolone arm, compared to $86,365 in the SSRI arm. Zuranolone-treated adults averaged 6.178 QALYs compared to 6.116 QALYs for the SSRI arm. Costs and utilities for the child and partner were also included in the base case. Drug and administration costs for zuranolone averaged $15,902, compared to $30 for SSRIs over the studied time horizon. Results were sensitive to the model time horizon.
Limitations
As head-to-head trials were not available to permit direct comparison, efficacy inputs were derived from an indirect treatment comparison which can be confounded by cross-trial differences. The data used are reflective of a general PPD population rather than marginalized individuals who may be at a greater risk for adverse PPD outcomes. The model likely excludes unmeasured effects for patient, child, and partner.
Conclusions
This economic model’s results suggest that zuranolone is a more cost-effective therapy compared to SSRIs for treating adults with PPD.
KEY POINTS
Question: Is zuranolone cost-effective compared to selective serotonin-reuptake inhibitors for the treatment of postpartum depression (PPD) in adults in a United States (US) health care setting?
Findings: The model, which incorporated clinical trial data, long-term longitudinal cohort data, US Bureau of Labor Statistics data on compensation, and other peer-reviewed literature, projects that zuranolone is cost-effective compared to selective serotonin-reuptake inhibitors for the treatment of PPD at a willingness-to-pay threshold of $150,000 (USD).
Meaning: For adults with PPD requiring pharmacological intervention, zuranolone may be a cost-effective treatment option with the potential to confer quality-of-life benefits for these patients and their families as well as economic benefits for society.
Introduction
Postpartum depression (PPD) is one of the most common medical complications in the perinatal period, with symptoms reported in 13.2% of new mothers across the United StatesCitation1,Citation2. PPD symptoms are heterogenous, but can include depressed mood, anxiety, markedly diminished interest or pleasure in activities, clinically significant weight loss or gain, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue, feelings of worthlessness or guilt, diminished ability to think or concentrate or indecisiveness, and recurrent thoughts of death or suicidal ideationCitation3. PPD can also lead to maternal suicide, a major contributor of pregnancy-related mortality in the United StatesCitation4–11.
PPD is inconsistently screened for and diagnosed, which may result in missed cases and undertreatmentCitation1,Citation12–16. Underdiagnosis can lead to increased long-term repercussions, as research has found unresolved PPD to be associated with elevated depressive symptoms until at least 11 years after childbirthCitation17,Citation18. Additionally, significant racial-ethnic and socioeconomic differences in PPD-related care postpartum have been shown to exacerbate the societal burden associated with PPDCitation19.
PPD can also affect partners, caregivers, and children. Partners of patients experiencing PPD can face higher levels of stress, anxiety, and depressive symptoms compared to partners of patients without PPDCitation20,Citation21. PPD symptoms have also been associated with negative impacts on maternal–infant interactionCitation17,Citation22–27, which may include short-term and long-term deleterious effects on physical and mental development of the childCitation17,Citation22–24,Citation28. Indeed, children exposed early in life to maternal depression may experience long-term disruptions in neural activity and heightened stress responsesCitation29,Citation30. A large 18-year longitudinal cohort study showed that the children of patients with persistent and severe PPD were at higher risk of behavioral problems at age 3.5 years (OR = 4.84; 95% CI = 2.94–7.98), lower mathematical grades at age 16 years (OR = 2.65; 95% CI = 1.26–5.57), and depression at age 18 years (OR = 7.44; 95% CI = 2.89–19.11) compared with children of adults with an Edinburgh Postnatal Depression Scale (EPDS)Citation31 of less than 13 points in the postnatal yearCitation17. Other cohort studies have reported associations between maternal depression and pediatric injuries, incidence of attention-deficit/hyperactivity disorder (ADHD), and poor academic performance in children whose birthing parent experienced depression around the time of birthCitation32–34. Early, effective intervention to address maternal depression has been found to improve mother–baby bonding and child developmental and behavioral outcomesCitation35–37.
The economic burden associated with maternal depression, especially among untreated and sub-optimally treated patients, is substantial and impacts patients, their families, employers, and healthcare payersCitation38–42. Women with PPD have more hospital admissions and overall higher health care resource utilization and health expenditures than women who do not have PPDCitation39,Citation41. A 2017 model found that the multi-year average cost of untreated perinatal mood and anxiety disorders per affected mother–child pair was approximately $32,000 USDCitation40. There are also substantial indirect costs associated with maternal depression, such as absenteeism and presenteeism in the workplaceCitation38,Citation42.
There are two Food and Drug Administration (FDA) approved pharmacotherapy treatment options specifically indicated for PPD (FDA Press Announcement, March 19, 2019; FDA Press Announcement, August 4, 2023). BrexanoloneCitation43 is a neuroactive steroid (NAS) ɣ-aminobutyric acid type A (GABAA) positive allosteric modulator (PAM) that is chemically identical to endogenous allopregnanolone and is administered as a continuous IV infusion over 60 h in an inpatient setting. Brexanolone is hypothesized to help restore normal signaling in brain networksCitation44.
Zuranolone is a PAM of both synaptic and extrasynaptic GABAA receptors and a NAS approved as an oral, once-daily, 14-day treatment course for adults with PPD in the United StatesCitation45. The safety and efficacy of zuranolone were evaluated in two phase 3, double-blind, randomized, placebo-controlled clinical trials. In the ROBIN Study, 151 patients with PPD (17-item Hamilton Rating Scale for Depression [HAMD-17] ≥ 26) were randomized in a 1:1 ratio to receive zuranolone 30 mg (once daily in the evening), or placebo for a 14-day treatment course and were followed for a month after treatment (day 45). Patients with PPD who received zuranolone demonstrated a significantly greater HAMD-17 change from baseline (CFB) at day 15 (least squares mean [LSM] CFB: −17.8 versus −13.6, respectively; LSM difference = −4.2; 95% CI = −6.9 to −1.5; p = 0.003) compared with those who received placeboCitation46. The most common (≥5% and greater than placebo) adverse reactions in zuranolone-treated patients were somnolence, nasopharyngitis, dizziness, fatigue, and diarrheaCitation47. In the SKYLARK Study, 196 patients with PPD (HAMD-17 ≥ 26) were randomized in a 1:1 ratio to receive zuranolone (50 mg, once daily in the evening) or placebo for the 14-day treatment course and were followed for a month after treatment (day 45). Patients with PPD who received zuranolone demonstrated statistically significant HAMD-17 CFB at day 15 (LSM CFB: −15.6 versus −11.6, respectively; LSM difference = −4.0; 95% CI = −6.3, −1.7; p = 0.001) compared with those who received placeboCitation48. The most common adverse reactions (≥5% and greater than placebo) in the zuranolone-treated patients were somnolence, dizziness, diarrhea, fatigue, and urinary tract infectionCitation47.
Selective serotonin reuptake inhibitors (SSRIs) and other antidepressants are common treatment options that are used in clinical practice for PPD; however, these therapies have not been evaluated in robust randomized clinical trials for PPD nor are they approved by the US FDA for the treatment of PPDCitation49–52. A Cochrane systematic review reported that the evaluation of evidence of SSRIs for use in PPD is characterized by studies with a substantial risk of bias due to high dropout rates, selective reporting, and small sample sizesCitation51. SSRIs can take 6 to 12 weeks to reach peak efficacy for most patientsCitation53. Use of SSRIs may be limited by subtherapeutic dosing and inadequate clinician follow-upCitation54–56. SSRIs have also been associated with a range of adverse events that may affect treatment outcomesCitation57,Citation58.
To date, the cost-effectiveness of zuranolone to treat adults with PPD has not been established. This study aimed to estimate the cost-effectiveness of oral pharmacologic treatments used for PPD (zuranolone [14-day treatment course] and chronic SSRI treatment) from the perspectives of patients with PPD, family members, physicians, payers, and society in the United States.
Methods
Model overview
The model structure is similar to that used in an assessment of the cost-effectiveness of brexanolone versus SSRIs for patients with PPD. Health states were defined by the EPDS, consistent with the zuranolone PPD clinical trialsCitation46,Citation48,Citation59. The clinical and economic effects of PPD on patients, their children and their partners/caregivers were modeled over an 11-year time horizon with a closed-cohort state transition modelCitation59. EPDS trajectories were modeled using clinical trial data for the initial treatment periods followed by published data from a large, 11-year longitudinal cohort study, which is described in more detail in the model inputs sectionCitation17. The model compared two treatment strategies, including a zuranolone arm, where patients initiated a single 14-day treatment (retreatment was not considered in this model) of zuranolone at baseline (patients stable on SSRIs at baseline were allowed to remain on their antidepressant), and a comparator arm in which all patients initiated SSRIs at baseline. Outcomes were calculated from a societal perspective, such that costs and utilities incurred by stakeholders in addition to the patient were included in the model. Inputs to the model were based on PPD-specific studies whenever possible.
Model structure
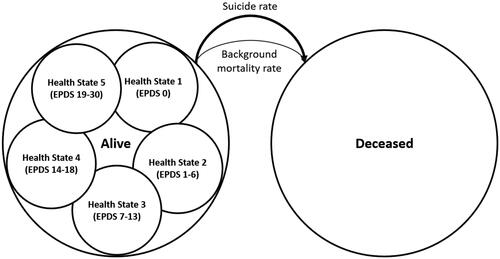
Patients enter the model at the point of a PPD pharmacological treatment decision between initiating therapy on zuranolone or chronic SSRI utilizationCitation60. The model tracked patients in five depressive symptom ranges based on the EPDS scores and included mortalityCitation61. The five depressive symptom ranges were divided into the following EPDS categories: None (EPDS 0; Health State 1); Minimal (EPDS 1–6; Health State 2); Mild (EPDS 7–13; Health State 3); Moderate (EPDS 14–18; Health State 4); and Severe (EPDS 19–30; Health State 5). EPDS scores between 0 and 13 were classified as non-depressed, while scores above 13 were classified as depressed. EPDS scores were related to child and partner outcomes. Patients could move between health states based on a weekly cycle for an 11-year duration in the base case, with 5- and 18-year durations in scenario analysis. No retreatment was considered in the model. illustrates the model structure.
Patient population and perspective
The modeled patient population used inputs from the zuranolone 50 mg SKYLARK Study, which reflected dosing consistent with the US prescribing informationCitation47. The SKYLARK Study included patients who were on average 31 years old and had an average HAMD-17 score of 28.6 at baselineCitation48. An unanchored matching-adjusted indirect comparison (MAIC) was conducted to match the zuranolone treatment arm to the placebo arm of an SSRI trial to provide an assessment of the relative effect of zuranolone vs. placebo and was used for the baseline distribution in EPDS health statesCitation62,Citation63. The MAIC results were incorporated into a network meta-analysis (NMA) such that the link for zuranolone into the network was based on the matched comparison between zuranolone and the selected placebo arm, allowing indirect treatment comparisons (ITCs) to be conductedCitation62,Citation63. Model inputs appear in . The model includes direct medical costs and indirect costs, including absenteeism, presenteeism, unemployment, and quality-adjusted life-year (QALY) changes for the PPD-affected patient, child whose birth is associated with the PPD episode, and the patient’s partner/caregiver.
Table 1. Model inputs.
Model inputs
Short-term efficacy
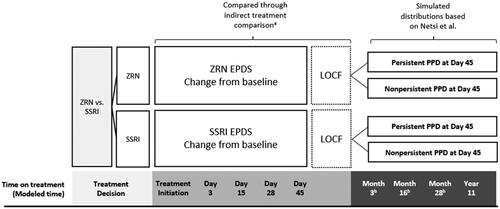
No head-to-head trials comparing zuranolone and SSRIs have been conducted. Results from an NMA, with incorporated efficacy inputs from the MAIC, were used to model the change in EPDS scores over the first 45 days of the model for the zuranolone and SSRI treatment strategiesCitation62. Specifically, EPDS CFB scores and related distributions for the five symptom EPDS categories at day 3, day 15 (week 2), day 28 (week 4) and last observation (day 45 for zuranolone and week 12/18 for SSRIs, applied at day 45) were used, along with linear interpolation between interim time points. The model carried the day 45 scores forward until week 12 post-treatment initiation (equivalent to the 8-month post-birth timepoint in Netsi et al.)Citation17. Sensitivity analysis of short-term efficacy was conducted by varying EPDS distributions based on the standard errors of the change from baseline values estimated in the ITC. Scenario analysis also used a Bucher ITC with a randomized controlled trial of antidepressant therapy for PPDCitation62,Citation63.
Long-term efficacy
We used the results of a large, longitudinal cohort as described in Netsi et al. to characterize EPDS trajectories for 8 months to 11 years postpartum after the trial observation periodCitation17. Netsi et al. designated patients into three severity categories of PPD (EPDS score of 13–14, 15–16, 17 or higher at 2 months postpartum) and defined patients as “persistent” if their EPDS score remained at 13, 15, or 17 or higher, respectively, at 8 months postpartum, and “not persistent” if their EPDS score at 8 months postpartum fell below 13, 15, or 17, respectively, for a total of six unique trajectoriesCitation17. Based on the EPDS last observation from the ITC, we calculated the proportion of patients in each arm in each of these categories and modeled their long-term outcomes to 11 years after treatment initiation. In the sensitivity analysis, we examined outcomes using last observation carried forward (LOCF) from the ITC rather than the outcome trajectory approach using Netsi et al. categorizationsCitation17. summarizes data sources for EPDS-based efficacy.
Figure 2. Efficacy sources for zuranolone and SSRIs.
aITC reference: Meltzer-BrodyCitation62. b Observation time points correspond to months 8, 21, and 33, respectively, from Netsi et al.
Abbreviations: EPDS, Edinburgh Postnatal Depression Scale; LOCF, last observation carried forward; PPD, postpartum depression; SSRI, selective serotonin reuptake inhibitor; ZRN, zuranolone; ITC, indirect treatment comparison.
Suicide and background mortality
Background mortality was based on the US life tablesCitation64. Patients suffering from moderate-to-severe PPD were assumed to experience an elevated suicide rateCitation65.
Health utility
We estimated patient health utility for each EPDS category using short-form 6D (SF-6D) data based on clinical trial SF-36 dataCitation66. Specifically, the ROBIN study data for all arms were pooled and mean and standard errors were estimatedCitation46.
Owing to the empirical challenges of measuring the health utility of infants, we did not model differences in utility for children (ages 0 to < 2), regardless of maternal PPD severity. Health utilities for children aged 2 and higher were derived from the relative risk of behavioral disorders for children of patients with PPD. Children of patients in health states 4 and 5 had decreased health utilities based on the increased risk for behavioral disorders and a study of the utilities for children with attention deficit hyperactivity disorder (ADHD). Children of patients in health states 1–3 were calculated as a weighted average of ADHD and non-ADHD utilities for children, based on the risk for behavioral issues in the general population. All children under 2 were also assumed to have utilities equivalent to those of the non-ADHD childrenCitation27,Citation67.
Partners of patients with PPD were assumed to have an elevated risk of major depressive disorder (MDD), but otherwise utilities were estimated from the general population. Partners’ utilities were based on the prevalence of MDD among all menCitation68, adjusted by the odds ratio of higher MDD among men who are partners of patients with postpartum depressionCitation69. The excess risk of MDD among men whose partner is suffering from PPD experienced a utility weight decrement for the duration of the time that the patient spent in a PPD stateCitation70. To account for the impact on a patient’s support system, who could be a partner or another relative or caregiver, the utility decrement was applied to 100% of partners.
Drug costs
The wholesale acquisition cost of a full 14-day treatment course of zuranolone is $15,900. We assumed that a portion of zuranolone patients, consistent with the proportion of patients in SKYLARK receiving background ADTs, also incurred the cost of SSRI treatment ($1.17 per week for 26 weeks), reflecting the cost of generic sertraline (average mg cost of sertraline [Redbook, accessed April 19,2023] × Zoloft daily dose for MDD [50 mg])Citation48. Although real-world use of SSRIs varies substantially based on response rates and adverse events, we assumed for the purposes of modeling that all patients received SSRI treatment for 26 weeks.
Direct medical costs
Health care costs, aside from the costs of zuranolone and SSRIs (modeled separately), were included for the patient based on a private insurance claims analysis. Costs for the patient from treatment initiation to 1-year postpartum were based on Epperson et al. estimated from total all-cause spend, pharmaceutical costs, and health care resource utilizationCitation39. SSRI costs were removed to avoid double-counting. The patient’s health care costs after 1-year postpartum in the model were based on estimates for MDD, derived from a claims analysis performed by Greenberg et al.Citation71. From both sources, the costs for PPD or MDD were applied to health states 4 and 5, while the costs for non-depressed individuals were applied to health states 1–3.
Children’s direct medical costs
Children (ages 0 to < 2) of patients with moderate-to-severe PPD were assumed to be at increased likelihood of health care system use based on a claims analysis (N = 135,678) measuring the incremental (PPD minus non-PPD) all-cause health care costs of children during their first 24 months post-birthCitation41. All-cause health care costs among children were estimated over a 24-month follow-up period among matched PPD and non-PDD exposure cohorts, and costs from the PPD cohort were applied to health states 4 and 5, while costs from the non-PPD cohort were applied to health states 1–3. Costs after age 2 years were based on a cost-of-illness model that estimated the impacts of untreated perinatal mood and anxiety disorders on childrenCitation40. Specifically, we calculated the incremental costs due to PPD from childhood obesity and behavioral and developmental disorders due to the increased prevalence of these conditions amongst children of patients with PPD and applied the incremental costs to health states 4 and 5 for children over the age of 2 years.
Partners’ direct medical costs
Partners’ medical costs were based on the average total all-cause costs for partners in households in which the mother had PPD compared to households in which the mother did not have PPDCitation39. We assumed that 63% of mothers lived with a partner based on a cohort of mothers caring for a child (< age 18) who self-reported being married or cohabitating for the purposes of calculating partners’ direct medical costsCitation72.
Indirect costs: productivity losses
Absenteeism and presenteeism were sourced from a PPD model by Luca et al. based on research from Evans-Lacko and Knapp, Ammerman et al., Rost et al., and Greenberg et al. on the incremental cost of absenteeism due to maternal or general depressionCitation38,Citation40,Citation42,Citation73,Citation74. Given that productive time spent in the formal labor market should be valued in an economic model, using wages plus fringe benefits at the margin, wages related to absenteeism and presenteeism were inflated to account for the total value of compensationCitation75,Citation76. To account for the value of labor for those who work in the home along with those in formal labor markets, costs of absenteeism and presenteeism were applied to all patients, using the average wages plus fringe benefitsCitation77. Patients with PPD also face an excess risk of unemployment; we assumed their entire compensation was lost while experiencing moderate-to-severe PPDCitation40.
An income decrement was also applied to partners of patients experiencing moderate-to-severe PPD. The data for this input were derived from a study that used the Medical Expenditure Panel Survey (MEPS) to estimate the difference in income (including self-reported wages, government aid, business income, gifts, and capital gains) of adults without depressive symptoms. The income of such adults was compared between those who lived in a household that contained another adult with depressive symptoms versus those who lived in a household without adults with depressive symptomsCitation78. As with direct costs, indirect costs for partners were only applied to the 63% of partners assumed to be cohabitating with the patient. Note that indirect costs due to the potential for lower labor force participation of a partner as well as a partner’s absenteeism and presenteeism were not separately considered, as all such effects were conservatively assumed to be included in the income decrement described above.
Medical costs were inflation-adjusted to 2023 USD based on the medical care component of the Consumer Price IndexCitation79. Wages and income were inflated to 2023 USD based on the US Bureau of Labor Statistics wage indexCitation80.
Adverse events
Specific treatment-related adverse events that were not captured in the general utility function derived from trial patients were included in the model. Adverse events experienced upon cessation of SSRI therapy were associated with an additional utility decrementCitation81,Citation82 applied to all patients in the SSRI arm and the share of patients using concomitant SSRIs in the zuranolone arm. In addition, incidence of somnolenceCitation47 was also associated with an additional utility decrementCitation83. Somnolence was assumed to persist during the 2-week treatment course for zuranolone, while withdrawal symptoms from SSRIs were modeled to occur during the week following conclusion of treatment (model week 26) and the week afterCitation81.
Deterministic and probabilistic sensitivity analysis
Deterministic sensitivity analysis (DSA) varied each parameter individually and assessed the impact on the results. Probabilistic sensitivity analysis (PSA) simultaneously varied all model parameters in a Monte Carlo simulation of 1,000 iterations. Distributions for each assumption appear in . Results are presented as cost-effectiveness acceptability curves (CEAC).
Results calculation
Total and incremental costs and QALYs were estimated for the zuranolone and SSRI treatment strategies over the 11-year time horizon of the model. The incremental cost-effectiveness ratio (ICER) and net monetary benefits (NMB) were also estimated. Costs and benefits were discounted at 3% per yearCitation84. Calculations were performed in Microsoft Excel.
Results
presents base case costs and QALYs. Treatment costs associated with zuranolone averaged $15,902, compared to $30 for SSRIs. Over an 11-year time horizon, direct medical costs for PPD patients, excluding treatment, were $84,318 with zuranolone, and $86,365 for SSRIs. Zuranolone-treated patients accrued more health on average (6.178 QALYs) than SSRI-treated patients (6.116 QALYs) over an 11-year time horizon. On average, the children of zuranolone-treated patients incurred $28,982 of direct medical costs and accrued 8.112 QALYs, while the children of SSRI-treated patients incurred costs of $29,276 and accrued 8.103 QALYs. On average, the partners of zuranolone-treated patients incurred $20,726 of direct medical costs and accrued 7.690 QALYs while the partners of SSRI-treated patients incurred costs of $20,953 and accrued 7.679 QALYs. The model also demonstrates that there would be 150.9 suicides per 10,000 zuranolone-treated patients, compared to 166.2 suicides per 10,000 SSRI-treated patients.
Table 2. Summary of base case results (11-year time horizon).
Patients treated with zuranolone incurred absenteeism costs of $4,227 and presenteeism costs of $13,761. Patients treated with SSRIs incurred absenteeism costs of $4,683 and presenteeism costs of $15,245. The difference in absenteeism and presenteeism costs for patients taking zuranolone versus SSRIs were −$456 and −$1,484, respectively. For patients treated with zuranolone, lost earnings due to suicide were found to be $20,728. For patients treated with SSRIs, lost earnings due to suicide were $23,034 per patient. This is a −$2,306 difference between zuranolone and SSRIs for lost earnings due to suicide.
Compared to SSRIs, zuranolone treatment costs were higher, while patient, child, and partner health care costs were lower. Incremental QALY gains were 0.062 for mothers, 0.009 for children, and 0.012 for partners. The ICER for zuranolone compared to SSRIs was $94,741.
presents scenario analyses results. A LOCF approach, instead of the long-term trajectories derived from Netsi et al., resulted in zuranolone being the dominant treatment. Using the Bucher ITC method instead of the network meta-analysis changed results negligibly. Shorter time horizons decreased cost-effectiveness, and longer horizons increased the cost-effectiveness of zuranolone versus SSRIs. Limiting the model to the perspective of the mother only yielded a higher ICER but results were still cost-effective at the $150,000 willingness-to-pay threshold per QALY.
Table 3. Scenario analysis.
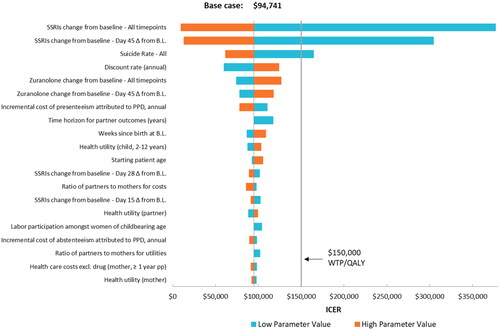
The one-way sensitivity analysis appears in . Varying each parameter independently shows that zuranolone is consistently cost-effective except when varying the efficacy parameter from the indirect treatment comparison for SSRIs or using the lower bound input for suicide rate.
Figure 3. One-way sensitivity analysis.
Note: Change from baseline input values were negative, such that the high value represented the smaller numerical change.
Abbreviations: WTP, willingness-to-pay; QALY, quality-adjusted life year; BL, baseline; PPD, postpartum depression; SSRI, selective serotonin reuptake inhibitor.
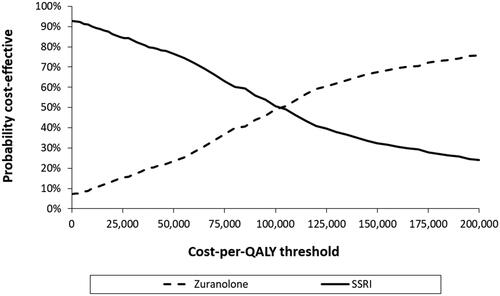
The probabilistic sensitivity analysis showed that at a $150,000 willingness-to-pay threshold per QALY, 68% of simulations indicate that zuranolone was cost-effective versus SSRIs ().
Discussion
PPD is a significant public health issue that can have an impact on patients, their families, and society. There have been limited pharmacological treatment options indicated for PPD. In this study, we evaluated the cost-effectiveness of the first oral pharmacological treatment approved for PPD, zuranolone, compared to SSRIs, from a societal perspective in the United States. The findings of this model indicate that zuranolone has an ICER below $150,000 per QALY compared to SSRIs, suggesting that zuranolone achieves favorable cost-effectiveness. The $150,000 per QALY threshold has become more routinely used over time and is about 2.5-times the mean per capita income in the United States in 2022Citation85–88.
The model utilized an 11-year time horizon, beginning from the point of the treatment decision and consistent with previous modeling of PPD conducted by Eldar-Lissai et al.Citation59. The 11-year base case in the zuranolone model relies on evidence from a large longitudinal study suggesting that long-term outcomes are dependent on the severity and duration of the PPD episode. PPD episodes that persist for 8 months postpartum are associated with a higher propensity for maternal depression 11 years after childbirth. The patient’s future depressive episodes are associated with higher direct medical costs and lower quality-of-life (QoL). In fact, research has found statistically significant adverse outcomes for the children born of patients with PPD; PPD episodes that persist for 8 months postpartum can also impact a child’s educational performance and propensity for depression up to 16 and 18 years later, respectivelyCitation17.
While direct medical costs incurred by patients with PPD and their family are substantial, the zuranolone model also examined value from the societal perspective to comprehensively capture the broader impact a PPD episode can have on the patient and the entire household, expanding on prior modelsCitation40,Citation59,Citation78,Citation89. Research has demonstrated that societal costs are particularly high in PPD compared to other conditions, specifically the indirect costs incurred from functional limitations of the individual, effects on child development, the need for additional supportive care from a partner or support person, and the overall well-being of the familyCitation40,Citation71.
The impact of PPD on personal productivity is a core part of the overall economic burden. This analysis included a long-term productivity model for patients that estimates PPD-only indirect costs related to labor market participation, absenteeism, presenteeism, and home production. The societal cost of suicide is substantial, owing to the lifetime of expected QALYs and future income to be lost. This research also demonstrates that the impact on the PPD patient’s partner is substantial, similar to results previously reportedCitation89.
Previous research suggests that shorter PPD episodes may improve maternal–child bonding, thereby improving long-term outcomes for the patient, child, and partnerCitation90,Citation91. These findings suggest that early intervention and treatment to mitigate PPD symptoms could potentially prevent long-term adverse outcomes for the entire family. Consistent with this, the American College of Obstetricians and Gynecologists’ (ACOG) updated 2023 guidance calls for timely screening, diagnosis, and early effective treatment for PPDCitation92,Citation93.
Limitations
This analysis has limitations that should be noted. Published cost-effectiveness research that examines cost per QALY within the PPD disease area is limited and subject to differences in treatment, time horizon, and perspective. Nevertheless, as is common in economic modeling, particularly for novel therapies, uncertainty arises due to limited data availability, which includes the absence of longer-term clinical data for zuranolone, head-to-head efficacy or safety comparisons with existing treatments, and inclusion of real-world zuranolone data. Only data derived from a randomized controlled trial can reliably identify causal effects and, even then, features like attribution bias or external validity can limit their interpretation. Published literature on the economic and resource burden of PPD in the US is limited; therefore, we used data from the most relevant studies to fill evidence gaps, which also included data from similar conditions (e.g. ADHD for offspring of patients with PPD). This model assumes causality of the effect of treatment of PPD on episode reduction based on an ITC between zuranolone and SSRIs, designed to simulate a randomized controlled trial. With the exception of the randomized controlled trial presented by Sharp et al. the external randomized controlled trials for SSRIs generally had small sample sizes and short durations, used a variety of antidepressants, allowed open-label treatment, and had substantial risk of biasCitation63. It is also important to note that the results of this model were sensitive to the assumed efficacy of the comparator SSRIs included. Additionally, the model was most sensitive to the difference between using two trajectory scenarios: the long-term EPDS trajectories in Netsi et al. for persistent and non-persistent cases (base case); and conversely, carrying forward the last efficacy observation from the clinical trials. This model uses Netsi et al. in the base case, but we recognize the limitations of utilizing these data, which require an assumption of an immediate reduction in treatment effect at month 3Citation17. Additionally, given the distribution of EPDS scores at the end of the trial data and the distribution implied in the Netsi et al. cohort, migrating from the trial data to the cohort data led to an instantaneous and potentially implausible large shift towards higher severity. Future research should explore this assumption with analysis of long-term trial extension data.
It is important to note that the zuranolone model omitted other impactful aspects of PPD societal burden, such as the effect on other offspring or the potential for long-term complications related to education, risk-taking, anti-social behavior, and criminality. The model also does not sufficiently capture health utility effects related to behavioral, developmental, and physical diagnosis in offspring of patients with PPD, likely leading to an understatement of the value of zuranolone in the treatment of PPD.
Somnolence, experienced by some patients taking zuranolone, is included as an adverse event in the model, but other adverse events are excluded owing to lack of data. Patients treated with SSRIs experience adverse events throughout the course of treatment, the most common of which are sexual dysfunction and weight gainCitation94,Citation95. Patients may experience some negative effects while discontinuing SSRI useCitation81. The zuranolone model conservatively assumes that, beyond the AEs associated with the symptoms of SSRI withdrawal, AEs related to SSRI use are already captured in the health state utility values derived from trial data, in which patients could remain on SSRIs if stable.
A potential benefit of zuranolone not expressly captured in the model is the shorter duration of treatment (14-day treatment course), which creates a shorter window for on-treatment adverse events, compared to SSRI treatments which require chronic daily dosing. It should be noted that, while zuranolone 50 mg/day rapidly demonstrated improvement in depressive symptoms compared with placebo, demonstrating a statistically significant improvement as early as day 3 in the SKYLARK StudyCitation48, the benefit of this rapid effect is not well captured in this cost-effectiveness model. The model accounts for this advantage of zuranolone over SSRIs for only a few weeks in the 11-year model, omitting some of the main potential benefits of zuranolone. These benefits may include the rapid feedback of the efficacy of treatment, the ability to switch treatments earlier in the episode if needed, and the short-term treatment course, including potential for better adherence and persistence, a challenge for many new parents which can be further exacerbated by racial and ethnic disparities. The study also imputed observations to assess treatment effects when all treatments had been administered for sufficient time to observe results, which was day 45 in the SKYLARK Study and ranging up to approximately 18 weeks across comparator studies. As a conservative means to compare results at day 45, and as to not underestimate the effectiveness of SSRIs which often require 6–12 weeks to reach maximum efficacyCitation53, the last observation in the comparator studies was applied at day 45, potentially underestimating the true EPDS CFB mean difference between zuranolone and SSRIs.
The value of zuranolone in the model may also be underestimated given that QoL in children and the impact of the child’s QoL on the parent is difficult to measure; therefore, conservative assumptions were used in the model. This model also conservatively applied cost inputs for partners who were married or cohabitating while neglecting the significantly increased cost PPD poses on single parents. While the model did account for utilities of a caregiver in all cases, this research omitted the specific impact of other caregivers, including the patient’s parents, grandparents, siblings, or other family members who may also be playing a role in taking care of the infant, and also be affected by the patient’s PPD episode. Although the Second Panel on Cost-effectiveness AnalysisCitation84 recommends including the costs and health impacts of all caregivers, there are not sufficient data to incorporate the complete map of the patient’s relationships. The broader consequences of maternal suicide for the family unit were also not fully captured in this model. While the model accounts for the cost of suicide in terms of maternal QALYs and economic productivity, it did not capture the full impact of maternal suicide associated with persistent PPD for the entire family.
Finally, important health equity considerations are not assessed in our model. Inputs from this model reflect an average patient population with PPD; however, understanding the effects of PPD in marginalized populations that may be disproportionately impacted is paramount. The utilization of a fast-acting oral treatment with a 2-week duration could potentially help alleviate issues associated with chronic therapy, the need for extended caregiver support, stigma, and continuity of care posed by chronic SSRI utilization.
Future research should address the limitations identified in this study, such as incorporating a broader perspective on the societal burden of PPD and considering the broader impact on family members and marginalized populations. By doing so, we can better understand the value of treatment options that aim to rapidly address PPD symptoms. The cost-effectiveness of fast-acting pharmacological PPD treatment options should be re-evaluated in the future as more data accumulate on real-world treatment effectiveness and long-term outcomes. In addition, these cost-effectiveness estimates will likely change over time, for example, decreasing upon generic entry to the market.
Conclusion
The economic model’s results suggest zuranolone is a more cost-effective therapy compared to SSRIs for treating adults with PPD in the US. The analysis shows that the use of zuranolone to treat adult patients with PPD for whom pharmacologic therapy is warranted could improve short- and long-term outcomes for patients, children, and partners, and provide broader economic benefits to society.
Transparency
Declaration of funding
This study was funded by Sage Therapeutics, Inc. (Cambridge, MA) and Biogen, Inc. (Cambridge, MA). Medical writing support was provided by Medicus Economics LLC (funded by Sage Therapeutics, Inc. and Biogen, Inc.). Logistical support was provided by Boston Strategic Partners (funded by Sage Therapeutics, Inc. and Biogen, Inc.).
Declaration of financial/other relationships
KMD serves as a consultant to Brii Biosciences, Inc.; Gerbera Therapeutics; GH Research Ltd.; Neuroscience Software, Inc.; Reunion Neuroscience; and Sage Therapeutics, Inc.; reports grants from Sage Therapeutics, Inc., awarded to Zucker Hillside Hospital/Feinstein Institutes for Medical Research during the conduct of the brexanolone injection and zuranolone clinical trials; and received grants from the NIH, Premier Healthcare, and Woebot Health and royalties from an NIH employee invention outside of the submitted work. EF is an employee of Sage Therapeutics, Inc., and may own stock or stock options in the company. LOC is a former employee of Sage Therapeutics, Inc, and may own stock or stock options in the company. SJJ and EC are employees of Medicus Economics LLC, which was paid fees by Sage Therapeutics, Inc. to conduct the research in the manuscript.
Author contributions
EC and SJJ had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: KMD, EF, LOC, SJJ, EC. Acquisition, analysis, or interpretation of data: EF, LOC, SJJ, EC, KMD. Drafting of the manuscript: EF, LOC, SJJ, EC. Critical revision of the manuscript for important intellectual content: KMD, EF, LOC, SJJ, EC. Statistical analysis: EF, LOC, SJJ, EC. Obtained funding: EF, LOC. Administrative, technical, or material support: EF, LOC, SJJ, EC. Study supervision: EF, LOC.
Acknowledgements
The authors would like to thank Margaret E. Gerbasi (Sage Therapeutics, Inc.) for her input and guidance during model development. The authors would also like to thank Rebecca Straubing (Medicus Economics LLC) for medical writing support.
Role of the funder/sponsor
The funding source was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. The publication of study results was not contingent on the funding source’s approval or censorship of the manuscript.
Reviewer disclosures
The peer reviewers on this manuscript have received an honorarium from JME for their review work.
One of the reviewers has received manuscript or speaker’s fees from Astellas, Eisai, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Kyowa Yakuhin, Lundbeck Japan, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Nihon Medi-Physics, Novartis, Otsuka Pharmaceutical, Shionogi, Shire, Sumitomo Pharma, Takeda Pharmaceutical, Tsumura, Viatris, Wiley Japan, and Yoshitomi Yakuhin, and research grants from Eisai, Mochida Pharmaceutical, Meiji Seika Pharma, Shionogi, and Sumitomo Pharma.
Additional information
Clinical trials and natural history study data are described in peer-reviewed literature; patient-level data were provided by Sage Therapeutics, Inc. Other data were collected from publicly available sources.
References
- Bauman BL, Ko JY, Cox S, et al. Vital signs: postpartum depressive symptoms and provider discussions about perinatal depression—United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(19):575–581. doi: 10.15585/mmwr.mm6919a2.
- Depression during and after pregnancy [Internet]. Reproductive Health: Centers for Disease Control and Prevention [cited 2023 Oct 10].
- Stewart DE, Vigod S. Postpartum depression. N Engl J Med. 2016;375(22):2177–2186. doi: 10.1056/NEJMcp1607649.
- Appleby L, Mortensen PB, Faragher EB. Suicide and other causes of mortality after post-partum psychiatric admission. Br J Psychiatry. 1998;173(3):209–211. doi: 10.1192/bjp.173.3.209.
- Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219–1224. doi: 10.1089/jwh.2015.5346.
- Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13(2):153–160. doi: 10.1002/wps.20128.
- Johannsen BM, Larsen JT, Laursen TM, et al. All-cause mortality in women with severe postpartum psychiatric disorders. Am J Psychiatry. 2016;173(6):635–642. doi: 10.1176/appi.ajp.2015.14121510.
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77–87. doi: 10.1007/s00737-005-0080-1.
- Mangla K, Hoffman MC, Trumpff C, et al. Maternal self-harm deaths: an unrecognized and preventable outcome. Am J Obstet Gynecol. 2019;221(4):295–303. doi: 10.1016/j.ajog.2019.02.056.
- Moses-Kolko EL, Hipwell AE. First-onset postpartum psychiatric disorders portend high 1-year unnatural-cause mortality risk. Am J Psychiatry. 2016;173(6):559–561. doi: 10.1176/appi.ajp.2016.16030317.
- Yu H, Shen Q, Bränn E, et al. Perinatal depression and risk of suicidal behavior. JAMA Netw Open. 2024;7(1):e2350897. doi: 10.1001/jamanetworkopen.2023.50897.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment Cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77(9):1189–1200. doi: 10.4088/JCP.15r10174.
- Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265–282. doi: 10.1007/s40263-019-00605-7.
- Sherman LJ, Ali MM. Diagnosis of postpartum depression and timing and types of treatment received differ for women with private and medicaid coverage. Womens Health Issues. 2018;28(6):524–529. doi: 10.1016/j.whi.2018.08.007.
- Ukatu N, Clare CA, Brulja M. Postpartum depression screening tools: a review. Psychosomatics. 2018;59(3):211–219. doi: 10.1016/j.psym.2017.11.005.
- Wang Z, Liu J, Shuai H, et al. Mapping global prevalence of depression among postpartum women. Transl Psychiatry. 2021;11(1):543. doi: 10.1038/s41398-021-01663-6.
- Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247–253. doi: 10.1001/jamapsychiatry.2017.4363.
- Vliegen N, Casalin S, Luyten P. The course of postpartum depression: a review of longitudinal studies. Harv Rev Psychiatry. 2014;22(1):1–22. doi: 10.1097/HRP.0000000000000013.
- Kozhimannil KB, Trinacty CM, Busch AB, et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011;62(6):619–625. doi: 10.1176/ps.62.6.pss6206_0619.
- Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26–35. doi: 10.1046/j.1365-2648.2003.02857.x.
- Vismara L, Rollè L, Agostini F, et al. Perinatal parenting stress, anxiety, and depression outcomes in first-time mothers and fathers: a 3- to 6-months postpartum follow-up study. Front Psychol. 2016;7:938. doi: 10.3389/fpsyg.2016.00938.
- Koutra K, Chatzi L, Bagkeris M, et al. Antenatal and postnatal maternal mental health as determinants of infant neurodevelopment at 18 months of age in a mother-child cohort (Rhea Study) in Crete, Greece. Soc Psychiatry Psychiatr Epidemiol. 2013;48(8):1335–1345. doi: 10.1007/s00127-012-0636-0.
- Pearson RM, Evans J, Kounali D, et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry. 2013;70(12):1312–1319. doi: 10.1001/jamapsychiatry.2013.2163.
- Surkan PJ, Ettinger AK, Hock RS, et al. Early maternal depressive symptoms and child growth trajectories: a longitudinal analysis of a nationally representative US birth cohort. BMC Pediatr. 2014;14(1):185. doi: 10.1186/1471-2431-14-185.
- Valla L, Wentzel-Larsen T, Smith L, et al. Association between maternal postnatal depressive symptoms and infants’ communication skills: a longitudinal study. Infant Behav Dev. 2016;45(Pt A):83–90. doi: 10.1016/j.infbeh.2016.10.001.
- Verkuijl NE, Richter L, Norris SA, et al. Postnatal depressive symptoms and child psychological development at 10 years: a prospective study of longitudinal data from the South African Birth to Twenty cohort. Lancet Psychiatry. 2014;1(6):454–460. doi: 10.1016/S2215-0366(14)70361-X.
- Woolhouse H, Gartland D, Mensah F, et al. Maternal depression from pregnancy to 4 years postpartum and emotional/behavioural difficulties in children: results from a prospective pregnancy cohort study. Arch Womens Ment Health. 2016;19(1):141–151. doi: 10.1007/s00737-015-0562-8.
- Eastwood JG, Jalaludin BB, Kemp LA, et al. Relationship of postnatal depressive symptoms to infant temperament, maternal expectations, social support and other potential risk factors: findings from a large Australian cross-sectional study. BMC Pregnancy Childbirth. 2012;12(1):148. doi: 10.1186/1471-2393-12-148.
- Behrendt HF, Scharke W, Herpertz-Dahlmann B, et al. Like mother, like child? Maternal determinants of children’s early social-emotional development. Infant Ment Health J. 2019;40(2):234–247. doi: 10.1002/imhj.21765.
- Center on the Developing Child at Harvard University. Maternal depression can undermine the development of young children. Working Paper No. 8. 2009. Available from: https://www.developingchild.harvard.edu.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782.
- Mulraney M, Giallo R, Efron D, et al. Maternal postnatal mental health and offspring symptoms of ADHD at 8-9 years: pathways via parenting behavior. Eur Child Adolesc Psychiatry. 2019;28(7):923–932. doi: 10.1007/s00787-018-1254-5.
- Murray L, Arteche A, Fearon P, et al. The effects of maternal postnatal depression and child sex on academic performance at age 16 years: a developmental approach. J Child Psychol Psychiatry. 2010;51(10):1150–1159. doi: 10.1111/j.1469-7610.2010.02259.x.
- Siqueira Barcelos R, da Silva Dos Santos I, Matijasevich A, et al. Maternal depression is associated with injuries in children aged 2-4 years: the Pelotas 2004 Birth Cohort. Inj Prev. 2019;25(3):222–227. doi: 10.1136/injuryprev-2017-042641.
- Gilden J, Molenaar NM, Smit AK, et al. Mother-to-infant bonding in women with postpartum psychosis and severe postpartum depression: a clinical cohort study. J Clin Med. 2020;9(7):2291. doi: 10.3390/jcm9072291.
- Hare MM, Kroll-Desrosiers A, Deligiannidis KM. Peripartum depression: does risk versus diagnostic status impact mother-infant bonding and perceived social support? Depress Anxiety. 2021;38(4):390–399. doi: 10.1002/da.23121.
- Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–1819. doi: 10.1016/S0140-6736(14)61277-0.
- Ammerman RT, Chen J, Mallow PJ, et al. Annual direct health care expenditures and employee absenteeism costs in high-risk, low-income mothers with major depression. J Affect Disord. 2016;190:386–394. doi: 10.1016/j.jad.2015.10.025.
- Epperson CN, Huang MY, Cook K, et al. Healthcare resource utilization and costs associated with postpartum depression among commercially insured households. Curr Med Res Opin. 2020;36(10):1707–1716. doi: 10.1080/03007995.2020.1799772.
- Luca DL, Margiotta C, Staatz C, et al. Financial toll of untreated perinatal mood and anxiety disorders among 2017 births in the United States. Am J Public Health. 2020;110(6):888–896. doi: 10.2105/AJPH.2020.305619.
- Moore Simas TA, Huang MY, Packnett ER, et al. Matched cohort study of healthcare resource utilization and costs in young children of mothers with postpartum depression in the United States. J Med Econ. 2020;23(2):174–183. doi: 10.1080/13696998.2019.1679157.
- Rost K, Smith JL, Dickinson M. The effect of improving primary care depression management on employee absenteeism and productivity. A randomized trial. Med Care. 2004;42(12):1202–1210. doi: 10.1097/00005650-200412000-00007.
- Zulresso [package insert]. Cambridge (MA): Sage Therapeutics Inc; 2022.
- Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–489. doi: 10.1016/S0140-6736(17)31264-3.
- FDA News Release: FDA approves first oral treatment for postpartum depression [Internet]. US Food and Drug Administration; 2023 [cited 2023 Apr 8]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of Zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951–959. doi: 10.1001/jamapsychiatry.2021.1559.
- Zurzuvae [package insert]. Cambridge (MA): Sage Therapeutics and Biogen; 2023.
- Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180(9):668–675. doi: 10.1176/appi.ajp.20220785.
- di Scalea TL, Wisner KL. Pharmacotherapy of postpartum depression. Expert Opin Pharmacother. 2009;10(16):2593–2607. doi: 10.1517/14656560903277202.
- Kim DR, Epperson CN, Weiss AR, et al. Pharmacotherapy of postpartum depression: an update. Expert Opin Pharmacother. 2014;15(9):1223–1234. doi: 10.1517/14656566.2014.911842.
- Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;2014(9):Cd002018. doi: 10.1002/14651858.CD002018.pub2.
- Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157–163. doi: 10.1177/1060028019873320.
- Frazer A, Benmansour S. Delayed pharmacological effects of antidepressants. Mol Psychiatry. 2002;7 Suppl 1(S1):S23–S28. doi: 10.1038/sj.mp.4001015.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33(4):143–161. doi: 10.3109/0167482X.2012.728649.
- Jones LE, Turvey C, Carney-Doebbeling C. Inadequate follow-up care for depression and its impact on antidepressant treatment duration among veterans with and without diabetes mellitus in the Veterans Health Administration. Gen Hosp Psychiatry. 2006;28(6):465–474. doi: 10.1016/j.genhosppsych.2006.08.002.
- Lingam R, Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164–172. doi: 10.1034/j.1600-0447.2002.1r084.x.
- Anagha K, Shihabudheen P, Uvais NA. Side effect profiles of selective serotonin reuptake inhibitors: a cross-sectional study in a naturalistic setting. Prim Care Companion CNS Disord. 2021;23(4):20m02747. doi: 10.4088/PCC.20m02747.
- Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3(1):22–27. doi: 10.4088/pcc.v03n0105.
- Eldar-Lissai A, Cohen JT, Meltzer-Brody S, et al. Cost-effectiveness of brexanolone versus selective serotonin reuptake inhibitors for the treatment of postpartum depression in the United States. J Manag Care Spec Pharm. 2020;26(5):627–638. doi: 10.18553/jmcp.2020.19306.
- Meltzer-Brody S, Howard LM, Bergink V, et al. Postpartum psychiatric disorders. Nat Rev Dis Primers. 2018;4(1):18022. doi: 10.1038/nrdp.2018.22.
- McCabe-Beane JE, Segre LS, Perkhounkova Y, et al. The identification of severity ranges for the Edinburgh Postnatal Depression Scale. J Reproductive Infant Psychol. 2016;34(3):293–303. doi: 10.1080/02646838.2016.1141346.
- Meltzer-Brody S., Gerbasi ME, Mak C, et al. Indirect comparisons of relative efficacy estimates of zuranolone and selective serotonin reuptake inhibitors for postpartum depression. J Med Econ. 2024.
- Sharp DJ, Chew-Graham C, Tylee A, et al. A pragmatic randomised controlled trial to compare antidepressants with a community-based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial. Health Technol Assess. 2010;14(43):iii, iii–iv, ix–xi, 1–153. doi: 10.3310/hta14430.
- Statistics NCfH. Mortality table 3. Life table for females: United States, 2020. In: LifeExpectancyTables2020, editor. PDF: National Vital Statistics System; 2022. Available from: https://stacks.cdc.gov/view/cdc/118055
- Wilkinson A, Anderson S, Wheeler SB. Screening for and treating postpartum depression and psychosis: a cost-effectiveness analysis. Matern Child Health J. 2017;21(4):903–914. doi: 10.1007/s10995-016-2192-9.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. doi: 10.1016/s0167-6296(01)00130-8.
- Peasgood T, Bhardwaj A, Biggs K, et al. The impact of ADHD on the health and well-being of ADHD children and their siblings. Eur Child Adolesc Psychiatry. 2016;25(11):1217–1231. doi: 10.1007/s00787-016-0841-6.
- Prevalence of major depressive episode in young adults: National Institute of Mental Health; 2021 [cited 2023 Dec 5].
- Nishimura A, Fujita Y, Katsuta M, et al. Paternal postnatal depression in Japan: an investigation of correlated factors including relationship with a partner. BMC Pregnancy Childbirth. 2015;15(1):128. doi: 10.1186/s12884-015-0552-x.
- Sapin C, Fantino B, Nowicki ML, et al. Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health Qual Life Outcomes. 2004;2(1):20. doi: 10.1186/1477-7525-2-20.
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653–665. doi: 10.1007/s40273-021-01019-4.
- Ertel KA, Rich-Edwards JW, Koenen KC. Maternal depression in the United States: nationally representative rates and risks. J Womens Health (Larchmt). 2011;20(11):1609–1617. doi: 10.1089/jwh.2010.2657.
- Evans-Lacko S, Knapp M. Global patterns of workplace productivity for people with depression: absenteeism and presenteeism costs across eight diverse countries. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1525–1537. doi: 10.1007/s00127-016-1278-4.
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162. doi: 10.4088/JCP.14m09298.
- Employer costs for employee compensation—June 2023 [press release]. Washington (DC): U.S. Bureau of Labor Statistics; 2023.
- Neumann PJ, Sanders GD, Russell LB, et al., editors. Cost-effectiveness in health and medicine. New York (NY): Oxford University Press; 2016.
- Lakdawalla DN, Doshi JA, Garrison LP, Jr, et al. Defining elements of value in health care—a health economics approach: an ISPOR special task force report [3]. Value Health. 2018;21(2):131–139. doi: 10.1016/j.jval.2017.12.007.
- Greenberg P, O’Callaghan L, Fournier AA, et al. Impact of living with an adult with depressive symptoms among households in the United States. J Affect Disord. 2023;349:107–115. doi: 10.1016/j.jad.2023.12.040.
- U.S. Bureau of Labor Statistics. Consumer price index for all urban consumers: medical care. CPIMEDSL; 2023. Available from: https://fred.stlouisfed.org/
- U.S. Bureau of Labor Statistics. Median usual weekly earnings (second quartile), employed full time, wage and salary workers, women. LEU0252882700; 2023. Available from: https://www.bls.gov/cps/data.htm
- Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guidelines evidence-based? Addict Behav. 2019;97:111–121. doi: 10.1016/j.addbeh.2018.08.027.
- Single Technology Appraisal Esketamine for treatment-resistant depression [ID1414]. London (UK): National Institute for Health and Care Excellence; 2020.
- Cambron-Mellott MJ, Mettam S, Li VW, et al. Examining the impact of excessive daytime sleepiness on utility scores in patients with obstructive sleep apnoea and/or narcolepsy in five European countries. BMC Neurol. 2022;22(1):317. doi: 10.1186/s12883-022-02827-7.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. Jama. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158.
- Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165–178. doi: 10.1586/14737167.8.2.165.
- Garrison LP, Jackson T, Paul D, et al. Value-based pricing for emerging gene therapies: the economic case for a higher cost-effectiveness threshold. J Manag Care Spec Pharm. 2019;25(7):793–799. doi: 10.18553/jmcp.2019.18378.
- Mean per capita income in the United States: St. Louis Federal Reserve; 2022 [cited 2024 Feb 2022].
- Ride J. Setting the boundaries for economic evaluation: investigating time horizon and family effects in the case of postnatal depression. Value Health. 2018;21(5):573–580. doi: 10.1016/j.jval.2017.10.016.
- Goodman JH. Influences of maternal postpartum depression on fathers and on father-infant interaction. Infant Ment Health J. 2008;29(6):624–643. doi: 10.1002/imhj.20199.
- Milgrom J, Holt C. Early intervention to protect the mother-infant relationship following postnatal depression: study protocol for a randomised controlled trial. Trials. 2014;15(1):385. doi: 10.1186/1745-6215-15-385.
- Screening and diagnosis of mental health conditions during pregnancy and postpartum. ACOG Committee on Clinical Practice Guidelines-Obstetrics; June 2023. Contract No.: 6.
- Treatment and management of mental health conditions during pregnancy and postpartum. ACOG Committee on Clinical Practice Guidelines-Obstetrics; June 2023. Contract No.: 6.
- Bushnell GA, Stürmer T, Swanson SA, et al. Dosing of selective serotonin reuptake inhibitors among children and adults before and after the FDA black-box warning. Psychiatr Serv. 2016;67(3):302–309. doi: 10.1176/appi.ps.201500088.
- Kelly K, Posternak M, Alpert JE. Toward achieving optimal response: understanding and managing antidepressant side effects. Dialogues Clin Neurosci. 2008;10(4):409–418. doi: 10.31887/DCNS.2008.10.4/kkelly.