Abstract
Background
Atrial fibrillation (AF) is a common treatable risk factor for stroke. Screening for paroxysmal AF in general practice is difficult, but biomarkers might help improve screening strategies.
Objectives
We investigated six blood biomarkers for predicting paroxysmal AF in general practice.
Methods
This was a pre-specified sub-study of the SCREEN-AF RCT done in Germany. Between 12/2017-03/2019, we enrolled ambulatory individuals aged 75 years or older with a history of hypertension but without known AF. Participants in the intervention group received active AF screening with a wearable patch, continuous ECG monitoring for 2x2 weeks and usual care in the control group. The primary endpoint was ECG-confirmed AF within six months after randomisation. High-sensitive Troponin I (hsTnI), brain natriuretic peptide (BNP), N-terminal pro-B-type natriuretic peptide (NT-pro BNP), N-terminal pro atrial natriuretic peptide (NT-ANP), mid-regional pro atrial natriuretic peptide (MR-pro ANP) and C-reactive protein (CRP) plasma levels were investigated at randomisation for predicting AF within six months after randomisation.
Results
Blood samples were available for 291 of 301 (96.7%) participants, including 8 with AF (3%). Five biomarkers showed higher median results in AF-patients: BNP 78 vs. 41 ng/L (p = 0.012), NT-pro BNP 273 vs. 186 ng/L (p = 0.029), NT-proANP 4.4 vs. 3.5 nmol/L (p = 0.027), MR-pro ANP 164 vs. 125 pmol/L (p = 0.016) and hsTnI 7.4 vs. 3.9 ng/L (p = 0.012). CRP levels were not different between groups (2.8 vs 1.9 mg/L, p = 0.1706).
Conclusion
Natriuretic peptide levels and hsTnI are higher in patients with AF than without and may help select patients for AF screening, but larger trials are needed.
KEY MESSAGES
BNP, NT-pro BNP, NT-ANP and MR-pro ANP and hsTnI levels are higher in patients with AF than without AF
With a sensitivity at 100%, BNP had the highest specificity of 60% (BNP level 50.1ng/L), followed by NT-pro BNP with a specificity of 53% (179ng/l)
Trial Registration:
- ClinicalTrials.gov Identifier: NCT02392754
- www.DZHK.de: SCREEN-AF DZHK15
Introduction
Atrial fibrillation (AF) is one of the most common cardiac arrhythmias. Its prevalence increases with age [Citation1]. Risk factors such as hypertension increase the likelihood of its occurrence [Citation2]. AF is a major risk factor for stroke. It can be challenging to detect since it is often paroxysmal and clinically unnoticed. A disabling or fatal ischaemic stroke can be the first manifestation of previously undetected AF [Citation3]. When AF is diagnosed, oral anticoagulant therapy (OAC) is highly effective for stroke prevention [Citation4].
Screening to improve the early detection of AF seems an attractive strategy for stroke prevention but currently its role is controversial. According to the European Society of Cardiology, a ‘systematic ECG screening should be considered to detect AF in individuals aged 75 years or older or those at high risk of stroke’ [Citation5]. However, the US Preventive Services Task Force recommends against routine ECG screening for AF, citing insufficient evidence [Citation6]. And although evidence is arising that AF screening in the primary care setting is feasible [Citation7–9], strategies to improve the efficiency, effectiveness and number needed to screen (NNS) are required.
Several plasma biomarkers have been shown to be predictive of future AF, including natriuretic peptides [Citation10], troponin and C-reactive protein (CRP) [Citation11,Citation12]. However, most studies investigated brain natriuretic peptide (BNP) or N-terminal pro-B-type BNP (NT-pro BNP) but not N-terminal pro atrial natriuretic peptide (NT-ANP) or mid-regional pro atrial natriuretic peptide (MR-pro ANP) In vivo, NT-pro ANP, can be further cleaved into smaller fragments, making the mid-regional pro-ANP, respectively amino acids 53-90, (MR-pro ANP) the preferred detection site of this natriuretic peptide [Citation13]. After thoracic surgery or in post-stroke patients [Citation14,Citation15], BNP has been shown to identify patients who are more likely to have AF detected by ECG monitoring.
We hypothesised that natriuretic peptides would identify patients who have a higher probability of AF detection with prolonged – that is, at least 24h or longer – ECG monitoring.
Methods
Study design
The SCREEN-AF trial was an investigator-initiated, multicentre, open-label, randomised clinical trial investigating non-invasive home-based AF screening interventions among primary care patients aged 75 years or older. Participants were recruited from general practices in Canada and Germany. The current pre-specified sub-study analysis on blood biomarkers was conducted only in the German study population. The SCREEN-AF main trial’s study protocol and primary results have been published [Citation9]. This biomarker sub-study involved additional informed consent to provide a blood sample at baseline. The SCREEN-AF trial and sub-study were approved by ethics committees representing all sites. The SCREEN-AF trial was registered under ClinicalTrials.gov Identifier (NCT02392754) and DZHK.de (SCREEN-AF DZHK15).
Recruitment of general practitioners and patients
General practitioners were recruited via telephone using pre-existing contact lists at the Department of General Practice (University Medical Centre Göttingen) and the Department of General Practice and Primary Care (University Medical Centre Hamburg-Eppendorf). Practices were located in Lower Saxony (n = 17), Thuringia (n = 1), North Rhine-Westphalia (n = 1) or Hamburg (n = 2). All participating practices screened their electronic medical records for potentially eligible patients and sent invitation letters to everyone meeting eligibility criteria.
Eligible to participate were community-dwelling male and female individuals aged 75 years or older without known AF or atrial flutter who were not receiving OAC but were considered potential OAC candidates if AF was diagnosed based on their CHADS2 (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke) score ≥ 2, indicating moderate or high risk for stroke, and no OAC contraindications. All participants had a history of hypertension requiring antihypertensive medication and were in sinus rhythm at the time of enrolment (assessed by 30-second pulse palpation and heart auscultation by the enrolling study physician). Exclusion criteria were previously documented AF or atrial flutter, a pacemaker, defibrillator, implanted loop recorder or life expectancy of less than six months. Participants provided written informed consent and were not paid to participate.
If a patient was interested, a mobile study team (physician, nurse and medical student) arranged an individual appointment at the general practice to enrol the patient and perform study procedures at baseline and again at 3 months; the patients’ general practitioner conducted 6-month visits.
Study interventions
Participants were randomly allocated (1:1) to the screening or control group via web-based randomisation using computer-generated random block sizes of 4 and 6 [Citation16].
The control group received standard primary care (no AF screening) and a follow-up exam by the general practitioner at six months. AF in this group was detected if a patient reported cardiac symptoms and received diagnostic (12-lead ECG) or as an incidental finding if the ECG was done for other reasons, e.g. prior to elective surgery.
The screening group received a 2-week ambulatory continuous ECG patch monitor (cECG) at baseline and again at three months, in addition to standard clinical care and follow-up exam at six months. The Zio XT (iRhythm Technologies) was used, a small adhesive patch applied to the chest providing single-lead cECG and allowing the patient to shower [Citation17,Citation18]. Further details are reported elsewhere [Citation9].
In Germany, all – control and screen - participants of the main SCREEN-AF trial were offered participation in a blood biomarker sub-study. At baseline, all blood samples were taken and pre-processed by a member of the flying study team in the rooms of each general practice, before being transported to the lab. Biomarkers were measured by commercially available immunoassays from Abbott (BNP, NT-pro BNP, hsTnI, CRP), Biomedica (NT-ANP) and BRAHMS (MR-pro ANP). A creatinine level was also measured. The patient´s characteristics () were assessed at baseline by self-report and extracted from the patient´s medical record provided by the general practitioner.
Table 1. Characteristics of all patients included in the biomarker analysis (n = 291).
Outcomes
Biomarker levels – four natriuretic peptides (BNP, NT-pro BNP, NT-ANP, MR-pro ANP), hsTnI and CRP – were correlated with the primary outcome of the SCREEN-AF main trial (AF detection at six months post-randomisation). In the intervention and control group, AF was defined as one or more episodes of continuous AF or atrial flutter lasting more than five minutes on continuous ECG or diagnosed by 12-lead ECG or other source documentation. All AF events underwent central adjudication by two arrhythmia physicians (authors of the main trial) blinded to a randomisation group. Using the 25%, 50% and 75% quantiles of biomarker levels as cut-off values, the proportion of correctly classified patients (either with or without AF) was calculated, as well as the highest specificity for each of the six biomarkers while keeping sensitivity at 100%.
Statistical analysis
All analyses of this sub-study were performed using SAS software version 9.4 (TS1M7) (SAS Institute, Cary NC). Further details are described elsewhere [Citation9].
The descriptive patient characteristics are presented as mean ± standard deviation for normally distributed continuous variables, median and interquartile range for non-normally distributed continuous variables and categorical variables and as count and percentage for categorical variables with few categories. The six biomarkers were compared between patients with and without AF using the Wilcoxon test. The predictive ability for each of the six biomarkers was estimated using the receiver operating characteristic (ROC) curve and the area under the curve (AUC). All analyses are explorative and the corresponding P-values are considered descriptive measures.
Results
Between 11 December 2017 and 26 March 2019, 301 German patients from 21 general practices were randomised (151 in the screening and 150 in the control group). Blood samples were available for 291 patients. These patients were included in this biomarker analysis (143 from the screening and 148 from the control group) ().
Figure 1. Flow diagram SCREEN-AF sub-trial (*1 withdrew consent for biomarker analysis, 3 did not give consent for biomarker analysis, 6 blood samples not available).
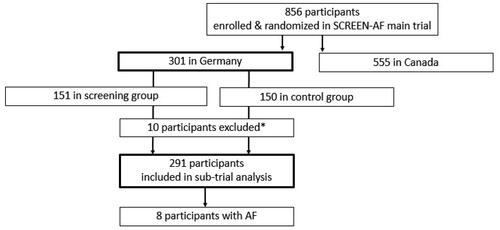
Sixteen practices kept a screening log and sent out a total of 669 invitation letters to eligible patients, which resulted in a response rate of 37% (248 patients enrolled and randomised). Fifty-three patients were recruited by the five practices that did not keep a log. Therefore, the number of invitation letters from these practices is unknown.
Baseline characteristics
Patients´ baseline characteristics in the intervention and control groups were similar () and comparable to the characteristics of the entire SCREEN-AF study population, including average age, prevalence of comorbidities and average CHA2DS2-VASc score [Citation9]. All German patients were of Caucasian ethnicity.
Occurrence of atrial fibrillation
At six months, 8 of 291 (2.7%) German patients were newly diagnosed with AF, 7 of 143 (4.9%) in the screening group, 1 of 148 (0.7%) in the control group, similar to the entire SCREEN-AF study population (5.3% and 0.5%) [Citation9]. In the control group patient, a 12-lead-ECG established the diagnosis. In the screening group 6 AF cases were detected by the ZIO patch with a duration of the most extended episode ranging from 50 min to 11 days. In one case, a Holter monitor outside the study intervention was used to establish the diagnosis. Therefore, seven of the eight AF cases in this analysis were rated as paroxysmal AF and one case as persistent AF, lasting longer than seven days.
Biomarker plasma levels
Blood levels for all four natriuretic peptides and hsTnI were higher in patients with AF compared to patients without AF CRP levels were not different between groups ().
Table 2. Biomarker levels in patients with and without AF.
Predicting atrial fibrillation using biomarkers
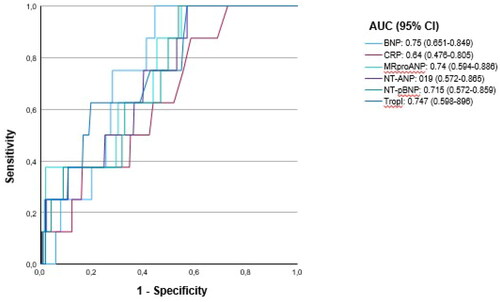
shows a receiver operating characteristic (ROC) curve analysis for different biomarkers for identifying patients developing atrial fibrillation. The area under the curve was between 0.64 5 and 0.75.
Cut-off values
Excluding CRP, all biomarkers are allowed to correctly classify 75-100% of AF patients at the 50% quantile. Approximately 51% of patients without AF were correctly classified at the same cut-off. If sensitivity was kept at 100%, BNP and NT-pro BNP showed the highest specificity of 60%, respectively 53% ().
Table 3. Proportion of correctly classified patients according to the biomarker cut-off values for the 25%, 50% and 75% quantiles.
Discussion
Main findings
Natriuretic peptide and high-sensitive Troponin I levels were higher in community-dwelling primary care patients with paroxysmal AF and persistent AF lasting longer than seven days. If the sensitivity was to remain 100%, BNP and NT-pro BNP showed a specificity of 60% and 53%, respectively.
Biomarkers to improve screening for AF
Multiple studies have shown that natriuretic peptides are not only elevated in patients with congestive heart failure but also in patients with AF and therefore might be helpful in patient selection for prolonged rhythm monitoring for AF detection [Citation19,Citation20, Citation21]. However, most studies focused on selected populations, such as hospitalised patients who had suffered a recent stroke, myocardial infarction or underwent surgery [Citation14,Citation15,Citation22]. For example, Karolina et al. found that MR-proANP might be a good predictor of new-onset atrial fibrillation in patients with acute myocardial infarction [Citation22]. More similar to the primary care setting, Schnabel et al. focused on a community setting using the data of 3,120 Framingham cohort participants to show that BNP can improve AF risk stratification [Citation22].
In a secondary analysis of the randomised LOOP Study, patients with an NT-pro BNP level > 125 pg/mL had an increased risk of AF diagnosis [Citation23]. The population-based cohort study STROKESTOP II demonstrated that NT-pro BNP could help to identify patients at higher risk of AF using the same cut-off level of > = 125 ng/L to offer extended ECG screening to patients [Citation24]. In a similar diagnostic setting but a different patient population (stroke patients), Wasser et al. showed that using a BNP cut-off level of 30 ng/L detected 83% of AF cases [Citation15]. We extend these findings to a population of elderly patients in primary care with hypertension as the leading risk factor.
Since a triage test needs to have a very high sensitivity, BNP and NT-pro BNP showed the most favourable results, with the highest specificity at 100%-sensitivity levels. Comparing these two markers, half-life length does not play a role. BNP with a half-life of 20 min even had a higher specificity than NT-pro BNP with a half-life of 60-120 min [Citation25].
As congestive heart failure is also a common co-morbidity among primary care patients, it might be challenging to use biomarkers to screen for AF in patients with CHF and vice versa, respectively, to determine heart failure in patients with AF. However, compared to high-sensitive Troponin T (hsTnT), galectin-3, ST2, fibrinogen, urate and CRP, NT-pro BNP had the best accuracy [Citation26]; compared to MR-pro ANP, NT-pro BNP had a similar diagnostic value to establish the diagnosis of acute heart failure in AF-patients [Citation27].
Strengths and limitations
The main limitation is the small number of participants and, therefore, the small number of AF events in this analysis, which was due to a delayed recruitment start in Germany in the international SCREEN-AF trial. Our biomarker analysis was too small to compare the diagnostic power of the different natriuretic peptides. However, despite the limited power, our results showed statistically significant differences in median values for high-sensitive Troponin I and natriuretic peptide levels investigated between patients with and without developing AF.
We also did not investigate the biomarkers at different points in time. Patients developing AF may have had higher levels during the follow-up at three months and serial measurements might improve the sensitivity of a biomarker (but also increase costs if done in clinical practice).
The follow-up in the SCREEN-AF main trial, including all patients in this biomarker analysis was longer (six months) than the screening period (2 × 2 weeks) [Citation9]. We aimed to show that two short episodes of continuous ECG-monitoring for 2 weeks are more effective in finding AF than six months of usual care. Longer follow-up is necessary to determine whether the EGC patch detects AF earlier than usual care or detects episodes that escape usual care monitoring. For comparison, in stroke patients, 3 × 10-day Holter-ECG-monitoring detects AF earlier than usual care during three years of follow-up [Citation28]. Additionally, the value of repeated screening (e.g. annual 2 weeks ECG monitoring) deserves future study.
Clinical implications
Several factors, such as age, chronic kidney disease or congestive heart failure (CHF), influence natriuretic peptide and/or troponin levels. Patients in which CHF is clinically suspected should receive an echocardiogram. If the patient has a known history of CHF and no signs of an acute exacerbation, an elevated BNP or NT-pro BNP level might be challenging to interpret. In the future, established cut-off levels might provide guidance since natriuretic peptide levels are elevated in patients with CHF and AF. But levels are even higher if both diseases are present [Citation29].
Conclusion
Natriuretic peptide levels and hsTnI are higher in patients with AF than without. In the future, biomarkers may help to select ambulatory patients at high risk for AF for AF-screening using prolonged ECG monitoring. More extensive studies, including multivariable prediction model trials are needed to assess if biomarkers add value when combined with baseline characteristics. And if so, to define optimal cut-off levels and to determine if combinations of biomarkers will provide improved yield.
Acknowledgements
The authors thank all participating primary care physicians and their teams.
Disclosure statement
JH has research grants and speaking fees from Medtronic, Boston Scientific, BMS/Pfizer, Servier and consulting fees from Bayer. DG reported receiving an operating grant from the Canadian Stroke Prevention Intervention Network (C-SPIN) during the conduct of the main SCREEN-AF trial (C-SPIN is a peer-reviewed national network grant funded by the Canadian Institutes of Health Research [CIHR]). He was supported by a Mid-Career Investigator Award from the Heart and Stroke Foundation (Canada) and the Department of Medicine and Bastable-Potts Chair at Sunnybrook Health Sciences Centre, Toronto. He serves as a Canadian national co-leader for the ARCADIA trial. He received an honorarium for participation in ad hoc advisory board for HLS Therapeutics Inc. He has had no personal financial relationships with cardiac monitoring device manufacturers or pharmaceutical companies related to AF in the past 6+ years. RW reports receiving grants from Deutsches Zentrum für Herz-/Kreislaufforschung for the conduct of the study, Deutsche Forschungsgemeinschaft, European Union, Bundesministerium für Bildung und Forschung, Boehringer Ingelheim and Medtronic; personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Daiichi-Sankyo, BMS, Medtronic, Novartis, Pfizer, Pharmacosmos, sciarc and Servier outside the submitted work.
Additional information
Funding
References
- Riley AB, Manning WJ. Atrial fibrillation: an epidemic in the elderly. Expert Rev Cardiovasc Ther. 2011;9(8):1–8. doi: 10.1586/erc.11.107.
- Weng L-C, Preis SR, Hulme OL, et al. Genetic predisposition, clinical risk factor burden, and lifetime risk of atrial fibrillation. Circulation. 2018;137(10):1027–1038. doi: 10.1161/CIRCULATIONAHA.117.031431.
- Borowsky LH, Regan S, Chang Y, et al. First diagnosis of atrial fibrillation at the time of stroke. Cerebrovasc Dis. 2017;43(3-4):192–199. doi: 10.1159/000457809.
- Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377–387. doi: 10.1016/S1474-4422(15)70027-X.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;42(5):373–498. doi: 10.1093/eurheartj/ehaa612.
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for atrial fibrillation: US preventive services task force recommendation statement. J Am Med Assoc. 2022;327(4):360–367. doi: 10.1001/jama.2021.23732.
- Atlas SJ, Ashburner JM, Chang Y, et al. Screening for undiagnosed atrial fibrillation using a single-lead electrocardiogram at primary care visits: patient uptake and practitioner perspectives from the VITAL-AF trial. BMC Prim Care. 2023;24(1):135. doi: 10.1186/s12875-023-02087-5.
- Lowres N, Neubeck L, Redfern J, et al. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110(2):213–222. doi: 10.1160/TH13-02-0165.
- Gladstone DJ, Wachter R, Schmalstieg-Bahr K, et al. Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol. 2021;6(5):558–567. doi: 10.1001/jamacardio.2021.0038.
- Sepehri Shamloo A, Bollmann A, Dagres N, et al. Natriuretic peptides: biomarkers for atrial fibrillation management. Clin Res Cardiol. 2020;109(8):957–966. doi: 10.1007/s00392-020-01608-x.
- Ardhianto P, Yuniadi Y. Biomarkers of atrial fibrillation: which one is a true marker? Cardiol Res Pract. 2019;2019:1–8. doi: 10.1155/2019/8302326.
- Fu Y, Pan Y, Gao Y, et al. Predictive value of CHA2DS2-VASc score combined with hs-CRP for new-onset atrial fibrillation in elderly patients with acute myocardial infarction. BMC Cardiovasc Disord. 2021;21(1):175. doi: 10.1186/s12872-021-01978-8.
- Yagmur E, Sckaer JH, Koek GH, et al. Elevated MR-proANP plasma concentrations are associated with sepsis and predict mortality in critically ill patients. J Transl Med. 2019;17(1):415. doi: 10.1186/s12967-019-02165-2.
- Amar D, Zhang H, Tan KS, et al. A brain natriuretic peptide-based prediction model for atrial fibrillation after thoracic surgery: development and internal validation. J Thorac Cardiovasc Surg. 2019;157(6):2493–2499.e1. doi: 10.1016/j.jtcvs.2019.01.075.
- Wasser K, Weber-Krüger M, Gröschel S, et al. Brain natriuretic peptide and discovery of atrial fibrillation After stroke: a subanalysis of the Find-AFRANDOMISED trial. Stroke. 2020;51(2):395–401. doi: 10.1161/STROKEAHA.119.026496.
- ROME. [cited 2024 Feb 28]. Available from: https://rome.phri.ca/SCREEN-AF/.
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(1):95.e11-7–95.e17. doi: 10.1016/j.amjmed.2013.10.003.
- Turakhia MP, Hoang DD, Zimetbaum P, et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013;112(4):520–524. doi: 10.1016/j.amjcard.2013.04.017.
- Sramko M, Wichterle D, Melenovsky V, et al. Independent effect of atrial fibrillation on natriuretic peptide release. Clin Res Cardiol. 2019;108(2):142–149. doi: 10.1007/s00392-018-1332-1.
- Kara K, Geisel MH, Möhlenkamp S, et al. B-type natriuretic peptide for incident atrial fibrillation-The Heinz Nixdorf recall study. J Cardiol. 2015;65(6):453–458. doi: 10.1016/j.jjcc.2014.08.003.
- Seegers J, Zabel M, Grüter T, et al. Natriuretic peptides for the detection of paroxysmal atrial fibrillation. Open Heart. 2015;2(1):e000182. doi: 10.1136/openhrt-2014-000182.
- Karolina I, Michał K, Marzenna Z. MR-proANP level predicts new onset atrial fibrillation in patients with acute myocardial infarction. Biomarkers. 2020;25(7):573–577. doi: 10.1080/1354750X.2020.1814414.
- Schnabel RB, Larson MG, Yamamoto JF, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121(2):200–207. doi: 10.1161/CIRCULATIONAHA.109.882241.
- Xing LY, Diederichsen SZ, Højberg S, et al. Effects of atrial fibrillation screening According to N-terminal pro-B-Type natriuretic peptide: a secondary analysis of the randomized LOOP study. Circulation. 2023;147(24):1788–1797. doi: 10.1161/CIRCULATIONAHA.123.064361.
- Kemp Gudmundsdottir K, Fredriksson T, Svennberg E, et al. Stepwise mass screening for atrial fibrillation using N-terminal B-type natriuretic peptide: the STROKESTOP II study. Europace. 2020;22(1):24–32. doi: 10.1093/europace/euz255.
- Gaggin HK, Januzzi JL. The past, the present, and the future of natriuretic peptides in the diagnosis of heart failure. European Heart Journal Supplements. 2018;20(suppl_G):G11–G20. doi: 10.1093/eurheartj/suy024.
- Merino-Merino A, Saez-Maleta R, Salgado-Aranda R, et al. Biomarkers in atrial fibrillation and heart failure with non-reduced ejection fraction: diagnostic application and new cut-off points. Heart Lung. 2020;49(4):388–392. doi: 10.1016/j.hrtlng.2020.02.043.
- Eckstein J, Potocki M, Murray K, et al. Direct comparison of mid-regional pro-atrial natriuretic peptide with N-terminal pro B-type natriuretic peptide in the diagnosis of patients with atrial fibrillation and dyspnoea. Heart. 2012;98(20):1518–1522. doi: 10.1136/heartjnl-2012-302260.
- Wachter R, Weber-Krüger M, Hamann GF, et al. Long-Term follow-up of enhanced Holter-Electrocardiography monitoring in acute ischemic stroke. J Stroke. 2022;24(1):98–107. doi: 10.5853/jos.2021.01207.
- Werhahn SM, Becker C, Mende M, et al. NT-proBNP as a marker for atrial fibrillation and heart failure in four observational outpatient trials. ESC Heart Fail. 2022;9(1):100–109. doi: 10.1002/ehf2.13703.
- Hoffmann J, Hanß S, Kraus M, et al. The DZHK research platform: maximisation of scientific value by enabling access to health data and biological samples collected in cardiovascular clinical studies. Clin Res Cardiol. 2023;112(7):923–941. doi: 10.1007/s00392-023-02177-5.