ABSTRACT
Background
Homocystinuria (HCU) is a rare metabolic disease that affects many organs, including the eyes. Aims: to assess visual functions, ocular characteristics, visual quality of life and time from the onset of ocular manifestations to HCU-diagnosis in patients with HCU.
Material and methods
Eighteen patients underwent ophthalmological examinations and visual quality of life questionnaires.
Results
Best corrected decimal visual acuity was median 1.0 (range amaurosis - 1.3) right eye and 1.0 (range amaurosis -1.3) left eye. Five patients presented with severe myopia as first HCU manifestation, duration to HCU diagnosis was mean 13.6 years (range 2-25). Two patients had suffered ectopia lentis as first HCU manifestation, HCU diagnosis was established mean 8.0 years (range 7-9) later. One patient had suffered both from thrombosis and ectopia lentis prior to diagnosis. Another four patients suffered thromboembolic events before diagnosis. Median VFQ-25 composite score was 93 (68-98).
Conclusions
The prevalence of myopia, ectopia lentis and monocular blindness was high in HCU-patients, which was reflected in their visual quality of life. Diagnosis was often delayed after the first ocular manifestation, increasing the risk of other severe non-ocular complications.
1. Introduction
Classic homocystinuria (HCU) is a rare metabolic disease in which severe eye manifestations are common (Citation1,Citation2). The autosomal recessive disease is caused by abnormal metabolism of the amino acid homocysteine (Hcy), due to pathogenic variants in the CBS gene. The CBS gene codes for the vitamin B6-dependent enzyme cystathionine beta-synthase (CBS), which converts homocysteine (Hcy) to cystathionine. Other defects in homocysteine metabolism includes the remethylation disorders (Citation1,Citation3).
The worldwide prevalence of HCU has been estimated as 1:200,000 to 1:335,000 (Citation4), with higher estimated prevalence in Ireland (1:67,000) (Citation5), Italy (1:55,500) (Citation5) and Qatar (1:1800) (Citation6). HCU was added to the Swedish screening program for newborns in 2010 (Citation7), but all the patients included in this study were born prior to its introduction.
Typical non-ocular manifestations of HCU include skeletal ones, which can impact bone mineral density and cause a marfanoid body structure (Citation8,Citation9), an increased risk of thrombosis including sinus thrombosis (Citation10,Citation11), and nervous system manifestations that include varying degrees of intellectual disability, anxiety, depression, obsessive-compulsive disorders, and personality disorders (Citation12).
Ocular manifestations may aid early diagnosis. Ectopia lentis (subluxation or dislocation of the crystalline lens secondary to dysfunction or disruption of zonular fibers) and high myopia (≤-6 Diopters) are among the most common manifestations associated with HCU (Citation2). Other ocular manifestations include retinal degeneration and detachment (Citation13), occlusion of the retinal vessels (Citation14) and glaucoma (Citation15). The underlying pathophysiology and cause of ocular manifestations is not fully known. However, one theory is that a local deficiency of cysteine, a semi-essential amino acid that counteracts protein precipitation (Citation16), leads to the thickening and disruption of zonulae fibers that normally provide a suspensory system for the lens. This results in ectopia lentis (Citation2). Even though ocular manifestations are common in HCU, research on ocular findings in patients diagnosed with HCU is scarce (Citation2). Few studies have used modern devices like optical coherence tomography (OCT), and no studies have used ultra-widefield photography (UWFI) with autofluorescence to examine the retina in patients with HCU. OCT and UWFI can provide valuable information regarding retinal changes, for example, retinal vessel occlusions (Citation17–22).
Treatment for HCU is aimed at lowering the concentration of Hcy. Patients with HCU require lifelong treatment and follow-up to maintain acceptable Hcy levels (Citation23), which plays a crucial role in preventing severe manifestations (Citation2). The most important factor when designing a treatment strategy is whether the patient carries a variant in the CBS gene that is vitamin B6 responsive. B6 non-responsive patients are more challenging to treat, and a combination of betaine and a methionine-restricted diet is often used (Citation23). Vitamin B6 non-responsive patients typically present with more severe manifestations than vitamin B6-responsive patients (Citation2,Citation24).
The objectives of the current study were to assess visual outcomes and anterior and posterior segment findings in patients with HCU, grouped by B6 responsiveness. Further objectives were to study the time that elapsed between the onset of visual or ocular problems and diagnosis and the visual quality of life in patients with HCU.
2. Material and methods
Patients who participated in this regional clinical cohort study were recruited from the Department of Endocrinology at Karolinska University Hospital. The inclusion criterion was the biochemical diagnosis of homocystinuria and homozygous or compound heterozygous pathogenic variants in the CBS gene. A total of 19 patients fulfilled the inclusion criterion and 18 patients consented to participation. Information regarding patients’ disease manifestations, sex, age, and prior visits to ophthalmologists was obtained through medical records. The patients were grouped by responsiveness to vitamin B6 using the general guideline by Morris et al. (Citation2). B6-responsiveness was defined as a total plasma concentration of homocysteine (tHcys) ≤50 µmol/L after treatment with pyridoxine was initiated. Partial B6-responsiveness was defined as having a tHcys of ≥50 µmol/L after starting treatment with pyridoxine, but a reduction of tHcys by >20% from the baseline. Patients with a reduction of tHcys by <20% were defined as B6 non-responsive.
All patients in the study provided informed consent. This study was completed in accordance with the Declaration of Helsinki.
Clinical ophthalmological examinations were performed at St. Erik Eye Hospital by the same ophthalmologist (KTF). They included the medical history of the patient’s subjective symptoms, such as dry eyes and light sensitivity, and the assessment of best-corrected visual acuity (BCVA) (Konstantin Motakis Ortho KM, Lund, Sweden), ocular range of motion and ocular alignment. Refractive errors were quantified using the autorefractor (Triton; Topcon Medical Systems, Tokyo, Japan) in cycloplegia. The anterior and posterior segments of the eye were assessed using a biomicroscope. The intraocular pressure was assessed using iCare (iCare Finland OY, Vanda, Finland).
Mild myopia was defined as 0 diopters (D) to −1.5 D, moderate myopia as −1.5D to −6D, and severe myopia as ≤-6D according to the guidelines by Fredrick (Citation25).
The Topcon SS-OCT (Triton; Topcon Medical Systems, Tokyo, Japan) was used to collect OCT and color fundus images. Optos UWFI (Optos, Dunfermline, Scotland) was used to obtain wide field photographs of the retina. The same retinal specialist (DE) assessed all images.
The VFQ-25, a questionnaire consisting of 25 items designed by the US’ National Eye Institute (NEI) and validated for patients with chronic eye disorders (Citation26,Citation27), was used to evaluate quality of life regarding vision and health. The questionnaire has 12 sub-scales: distance activities, near activities, color vision, ocular pain, peripheral vision, driving, general health, general vision, and vision-specific sub-scales; mental health, social functioning, dependency, and role difficulties. Each sub-scale was scored, and a general VFQ-25 score was estimated, although the general health sub-scale was excluded. VFQ-25 scores were compared in the groups categorized by B6 responsiveness, as well as in groups categorized by surgery.
Student’s T-test was used to compare age at diagnosis in patients grouped by B6 responsiveness.
3. Results
3.1. Demographic data and presentation
A total of 18 patients were included in the study, seven male patients and 11 female patients. The mean age at ophthalmological examination was 42.8 years, range 20.5–77.7 years. The mean age at the time of HCU diagnosis was 25.3 years (range 4–68 years). All patients were receiving treatment at their time of inclusion.
Ten patients were B6 responsive, six patients were partially B6 responsive, and two patients were B6 non-responsive. Among the 18 patients, three sibling pairs were identified. The secondarily diagnosed siblings were excluded from the analysis of age at diagnosis and time to diagnosis, leaving 15 patients. Grouped by B6 responsiveness, with the partially and non-responsive patients in the same group due to the small number of patients, the mean age at HCU diagnosis was 30.9 years (range 15–61 years) in the B6-responsive group (n = 9), compared to 14.2 years (range 4–27 years) in the non/partially B6-responsive group (n = 6) (p = 0.017).
Ocular manifestations typical for HCU like severe myopia or ectopia lentis presented prior to HCU diagnosis in 8/18 patients, based on data from medical records. Five of these patients (5/8) presented with severe myopia as the first manifestation of HCU, four of them later developed ectopia lentis. Two patients (2/8) presented with ectopia lentis as first manifestation of HCU. In one patient (1/8), both severe myopia and ectopia lentis presented prior to HCU diagnosis, but then the patient already had suffered from a thrombosis so neither ocular manifestation was the first manifestation of HCU.
Regardless of first presentation or not, six patients had experienced or suffered from ectopia lentis prior to diagnosis. One single patient developed ectopia lentis after HCU-diagnosis. and .
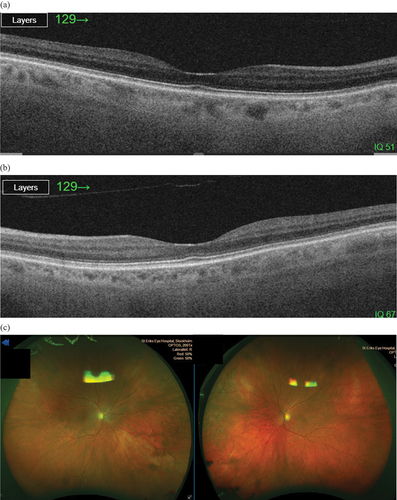
Figure 1. 1a and 1b Patient with homocystinuria, 60 years of age, with bilateral ectopia lentis (subluxated lenses) and severe myopia.
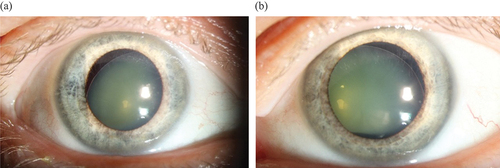
Table 1. Summary of visual and ocular findings in patients with homocystinuria, including symptoms, ectopia lentis and myopia prior to diagnosis, best-corrected visual acuity, strabismus, refraction, surgery due to ectopia lentis, retinal pathology, and other typical homocystinuria manifestations.
3.2. Symptoms and best-corrected visual acuity
Seven patients experienced light sensitivity or dry eyes: six female patients and one male patient. The mean BCVA was 0.99 (range 0.65–1.3 right eye (RE)) and 0.81 (range amaurosis-1.0 left eye (LE)) in the B6-responsive group and 0.71 (range amaurosis-1.0) RE and 0.79 (range 0.15–1.3) LE in the partially or non B6-responsive group.
The mean BCVA for the 7/18 patients who were treated for ectopia lentis or had subluxated lenses was 0.79 (range amaurosis-1.0) RE and 0.64 (amaurosis-1.3) LE. Two patients who underwent surgery for ectopia lentis were blind in one eye. Both had suffered from retinal amotio, and repeated retinal surgery had failed. One patient had been enucleated due to severe secondary glaucoma, and the other patient had developed severe corneal opacities with vascular ingrowth .
Figure 2. Exterior image from 30-year-old patient displaying dense corneal opacities with growth of vessels in the right eye which was blind after repeated surgery for retinal detachment after ectopia lentis.
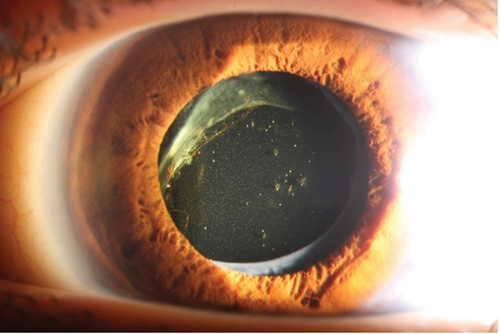
Regular use of eyeglasses was reported by 17/18 patients. A total of 7/18 patients, three in the B6-responsive group and four in the partially or non B6-responsive group had experienced bilateral lens dislocation. Six of these seven had undergone lens surgery, with four patients receiving intraocular lenses (IOL), while two patients were left aphakic. One patient had only slightly subluxated lenses and did not need surgery. For four of the six patients, lens surgery was performed prior to year 2000, and it is likely that many of their complications could have been avoided with modern surgical techniques .
Figure 3. Patient with homocystinuria, 30 years of age, who underwent ocular surgery at 25 years of age because of ectopia lentis, where the crystalline luxated lens was removed and replaced with an intraocular lens (IOL). Several surgical re-operations were needed as the IOL also luxated. Diagnosis of HCU was established three years after the first surgery.
3.3. Ocular coherence tomography (OCT) and ultra-widefield imaging (UWFI)
OCT images and UWF images from both eyes were available for 14 patients and from the one remaining eye in the two patients with monocular prostheses or dense corneal opacities. Pathological findings were present in 4/16 patients examined with OCT: two patients with mild retinal atrophy and two patients with moderate atrophy.
Pathological findings were present in 8/16 patients examined with UWF images, with peripheral atrophy being most common (four patients). A summary of OCT and UWFI findings is shown in and .
3.4. Duration from onset of ophthalmological manifestations to diagnosis
Only two patients were diagnosed immediately after presenting with ocular manifestations (both ectopia lentis), while three patients were diagnosed due to a diagnosed sibling and 13 patients were diagnosed due to non-ocular manifestations. Among the latter, 5/13 patients were diagnosed due to a thrombotic event (four after sinus cavernous thrombosis and one after deep vein thrombosis), 3/13 patients due to fatigue or depression, 3/13 patients due to a marfanoid habitus, one patient was diagnosed due to an intellectual disability and one patient due to suspected connective tissue disease. The mean delay between presenting with severe myopia or ectopia lentis as the possible first manifestation to an established HCU diagnosis for 7/15 patients (three siblings excluded) was 12.0 years (range 2–25 years). The patients’ mean age at diagnosis was 21.3 years (range 8–30 years).
When grouped by B6 responsiveness, the longest mean duration (18.7 years, range 12–25 years) from the onset of eye changes to HCU diagnosis was found in the group of B6-responsive patients (n = 3), while the partially responsive or B6 non-responsive patients (n = 4) had a mean duration of 7.0 years (range 2–10) to diagnosis.
A summary of the type of ocular manifestation as the first manifestation and duration to diagnosis is presented in .
Figure 5. Duration from onset of first ocular manifestation to diagnosis in patients presenting with ocular manifestations, such as ectopia lentis or severe myopia, prior to a diagnosis of homocystinuria. Several patients had both before being diagnosed. Duration from the onset of the first ocular manifestation of homocystinuria to diagnosis is shown in blue. The eye symbol indicates ocular manifestation, and the black box indicates a thrombotic event. “D” indicates time of diagnosis, “EL” indicates ectopia lentis, and “SM” indicates severe myopia.
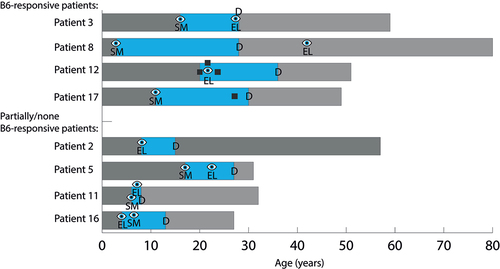
The mean duration from the onset of a thrombotic event to diagnosis for the five patients in which the events led to HCU diagnosis was 5.6 years (range 1–16 years).
3.5. Quality of life, VFQ-25
VFQ-25 was completed by 14/18 patients, nine patients with B6-responsive HCU and five patients with partially B6-responsive HCU. The median composite score for all 14 patients was 93 (range 73–98). The median composite score was 94 (range 73–98) in B6-responsive patients and 92 (range 75–94) in partially or non B6-responsive patients. The median composite score was 93 (75–94) in patients who had undergone surgery (n = 5) and 94 (73–98) in patients who had not undergone surgery (n = 9). The lowest scores were observed in the sub-scales “General vision” (median 80, range 40–100) and “Distance activities” (median 87.5, range 42–100) ().
Table 2. Demographic data, visual and ocular findings, and results of visual quality of life in a group of 18 patients with homocystinuria.
4. Discussion
This clinical cohort study aimed to assess visual functions, ocular manifestations, visual quality of life, and time from eye manifestations to metabolic diagnosis in patients with HCU, grouped by B6 responsiveness. The main findings were a high prevalence of severe myopia (33%) prior to diagnosis and a high percentage of ectopia lentis (39%) experienced at any time during life. Out of the seven patients who had suffered from ectopia lentis, 6/7 (86%) had undergone surgery, 2/6 patients (4/12 eyes; 33%) were left aphakic, and 2/12 eyes were blind due to retinal detachment where repeated retinal surgery had failed.
Mulvihill et al. (Citation28) reported that all 14 patients (100%) included in their study had ectopia lentis at the time of diagnosis, compared to 33% in our study. They also reported a shorter median duration from onset of eye changes to diagnosis (4.0 years) compared to 10.0 years in the current study. The lower frequency of ectopia lentis in the current study could perhaps be explained by the fact that in the study by Mulvihill et al. all patients were B6 non-responsive, while only 2/18 patients (11%) were B6 non-responsive in the current study. These two patients were also the two youngest patients at the time of diagnosis (4 and 5 years of age) and thus received adequate treatment early, probably preventing more severe clinical complications. Supporting this theory is the fact that, when grouped by B6 responsiveness, the prevalence of ectopia lentis was higher in the partially B6-responsive group (67%) than in the B6-responsive group (30%) in our study. Neither of the two patients in the non B6-responsive group in our study had experienced ectopia lentis.
In a study conducted by Al-Dewik et al. (Citation29), ocular manifestations such as myopia and ectopia lentis at the time of diagnosis were reported in 34/69 (49%) in patients with a median age of 8 years at the time of diagnosis. In the current study, ocular manifestations were present in 44% of the patients at the time of diagnosis, which was even later, at 21.5 years.
To the best of our knowledge, the only previous report to use OCT in patients with HCU is that of Gus et al. (Citation30), in which four patients were examined with OCT. Keratoconus was observed in one patient, lens surgery scars in two patients, and peripheral retinal hyperpigmentation in one patient (Citation30). In the current study, 25% of the patients had pathological retinal findings on posterior OCT images. A total of 38% had normal posterior OCT images whilst displaying pathologies on their UWF images. This finding suggests an advantage of including UWFI in the ophthalmological examinations of HCU patients, to detect peripheral retinal findings. However, no specific conclusions could be drawn regarding the probably age-related pathologies detected by OCT and UWFI in these patients.
The mean duration from the onset of ectopia lentis and/or severe myopia, that is typical eye changes related to HCU to diagnosis, varied, but was 12 years (range 2–25 years) in the whole group, when siblings were excluded. When grouped by B6 responsiveness, the longest mean duration (18.7 years, range 12–25 years) from the onset of eye changes as the first manifestation to HCU-diagnosis was found in the group of B6-responsive patients (n = 3), while the partially responsive or B6 non-responsive patients (n = 4) had a mean duration of 7 years (range 2–10) to diagnosis. A possible explanation could be that the partially B6-responsive patients tend to present with other more severe manifestations at an earlier stage (Citation2) and are therefore diagnosed earlier than the B6-responsive patients.
One example of this is the case of one B6-responsive patient who, despite suffering from bilateral ectopia lentis at age 22 and three cavernous sinus thrombosis, was diagnosed 29 years after the first manifestation (severe myopia). Knowledge about early manifestations of HCU combined with early evaluation of homocystinuria could save the patients from other serious disease manifestations, including sinus thrombosis.
The median composite score for the VFQ-25 questionnaire in the current study was 93 (range 73–98). The results, although high when compared to visual quality of life studies on diseases such as diabetic retinopathy (61 in male patients and 53 in female patients) (Citation31), suggest a slight effect on the visual quality of life in HCU patients. When discussing the VFQ-25 results it is important to remember that the questionnaire was only completed by B6-responsive patients and partially B6-responsive patients, not by the B6 non-responsive patients due to their intellectual disabilities.
To our knowledge, the only previous study to evaluate quality of life in patients with HCU was the one conducted by Al-Dewik et al. (Citation29), where a different questionnaire (PedsQL) was used. Although PedsQL 4.0 has been used in studies of several eye diseases (Citation32), it is designed to measure overall quality of life in patients aged from 2 to 18 years of age, whilst VFQ-25 aims to assess visual-related quality of life in adults. Despite the use of a different questionnaire, Al-Dewik et al. (Citation29) found that quality of life was slightly affected in their patients, with a mean total score of 88% compared to 93% in the current study. Another study by our team, on patients with Fabry disease (Citation33), showed a VFQ-25 median composite score of 94%, similar to the current study.
5. Conclusions
The results of this study showed a high prevalence of severe myopia and ectopia lentis prior to diagnosis in patients with HCU. Despite these typical ocular manifestations, delayed diagnosis of HCU was common, and the delay was more pronounced in the B6-responsive group. Knowledge about early ocular manifestations of HCU could prevent HCU patients from developing other severe diseases.
Ocular complications resulted in monocular blindness in two patients. A decreased visual quality of life was experienced by patients with HCU. Furthermore, retinal imaging identified additional pathologies; however, they were probably not related to HCU. Whether early diagnosis can reduce severe ocular complications and whether modern surgical techniques have improved visual outcomes following ectopic lens surgery remains to be evaluated.
Authors’ contributions
Conception and design: KTF and MO
Analysis and interpretation of the data: AK, KTF, MO, MN, DE
Drafting of the article: AK, KTF, MO, MN, DE
Revising it critically for intellectual content: KTF and MO
Final approval of the version to be published: KTF, MO, MN, DE, AK
All authors agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank the patients who participated in this study. The VFQ-25 questionnaire used in this study was designed by the National Eye Institute in the US.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, KTF. The data are not publicly available due to containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Janosík M, Sokolová J, Janosíková B, Krijt J, Klatovská V, Kozich V. Birth prevalence of homocystinuria in Central Europe: frequency and pathogenicity of mutation c.1105C>T (p.R369C) in the cystathionine beta-synthase gene. J Pediatr. 2009;154(3):431–7. doi:10.1016/j.jpeds.2008.09.015.
- Morris AA, Kožich V, Santra S, Andria G, Ben-Omran TI, Chakrapani AB, Crushell E, Henderson MJ, Hochuli M, Huemer M, et al. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis. 2017;40(1):49–74. doi:10.1007/s10545-016-9979-0.
- Huemer M, Diodato D, Schwahn B, Schiff M, Bandeira A, Benoist JF, Burlina A, Cerone R, Couce ML, Garcia‐Cazorla A, et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis. 2017;40(1):21–48. doi:10.1007/s10545-016-9991-4.
- Gerrard A, Dawson C. Homocystinuria diagnosis and management: it is not all classical. J Clin Pathol. 2022;75(11):744–50. doi:10.1136/jcp-2021-208029.
- Weber Hoss GR, Sperb-Ludwig F, Schwartz IVD, Blom HJ. Classical homocystinuria: a common inborn error of metabolism? An epidemiological study based on genetic databases. Mol Genet Genomic Med. 2020;8(6):e1214. doi:10.1002/mgg3.1214.
- Gan-Schreier H, Kebbewar M, Fang-Hoffmann J, Wilrich J, Abdoh G, Ben-Omran T, Shahbek N, Bener A, Al Rifai H, Al Khal AL, et al. Newborn population screening for classic homocystinuria by determination of total homocysteine from Guthrie cards. J Pediatr. 2010;156(3):427–32. doi:10.1016/j.jpeds.2009.09.054.
- Sörensen L, von Döbeln U, Åhlman H, Ohlsson A, Engvall M, Naess K, Backman-Johansson C, Nordqvist Y, Wedell A, Zetterström RH, et al. Expanded screening of one million Swedish babies with R4S and CLIR for post-analytical evaluation of data. Int J Neonatal Screen. 2020;6(2):42. doi:10.3390/ijns6020042.
- Saito M, Marumo K. The effects of homocysteine on the skeleton. Curr Osteoporos Rep. 2018;16(5):554–60. doi:10.1007/s11914-018-0469-1.
- Rahman M, Sharma M, Aggarwal P, Singla S, Jain N. Homocystinuria and ocular complications - a review. Indian J Ophthalmol. 2022;70(7):2272–8. doi:10.4103/ijo.IJO_309_22.
- Magner M, Krupková L, Honzík T, Zeman J, Hyánek J, Kožich V. Vascular presentation of cystathionine beta-synthase deficiency in adulthood. J Inher Metab Dis. 2011;34(1):33–7. doi:10.1007/s10545-010-9146-y.
- Ochoa-Ferraro A, Wanninayake S, Dawson C, Gerrard A, Preece MA, Geberhiwot T. A case series of cerebral venous thrombosis as the first manifestation of homocystinuria. Eur Stroke J. 2021;6(4):420–7. doi:10.1177/23969873211059479.
- Almuqbil MA, Waisbren SE, Levy HL, Picker JD. Revising the psychiatric phenotype of homocystinuria. Genet Med. 2019;21(8):1827–31. doi:10.1038/s41436-018-0419-4.
- Burke JP, O’Keefe M, Bowell R, Naughten ER. Ocular complications in homocystinuria–early and late treated. Br J Ophthalmol. 1989;73(6):427–31. doi:10.1136/bjo.73.6.427.
- Wenzler EM, Rademakers AJ, Boers GH, Cruysberg JR, Webers CA, Deutman AF. Hyperhomocysteinemia in retinal artery and retinal vein occlusion. Am J Ophthalmol. 1993;115(2):162–7. doi:10.1016/S0002-9394(14)73919-4.
- Harrison DA, Mullaney PB, Mesfer SA, Awad AH, Dhindsa H. Management of ophthalmic complications of homocystinuria. Ophthalmol. 1998;105(10):1886–90. doi:10.1016/S0161-6420(98)91035-1.
- Strofaldi A, Khan AR, McManus JJ. Surface exposed free cysteine suppresses crystallization of human γD-Crystallin. J Mol Biol. 2021;433(22):167252. doi:10.1016/j.jmb.2021.167252.
- Midena E, Marchione G, Di Giorgio S, Rotondi G, Longhin E, Frizziero L, Pilotto E, Parrozzani R, Midena G. Ultra-wide-field fundus photography compared to ophthalmoscopy in diagnosing and classifying major retinal diseases. Sci Rep. 2022;12(1):19287. doi:10.1038/s41598-022-23170-4.
- Auger G, Winder S. Spectral domain OCT: an aid to diagnosis and surgical planning of retinal detachments. J Ophthalmol. 2011;2011:725362. doi:10.1155/2011/725362.
- Coscas F, Glacet-Bernard A, Miere A, Caillaux V, Uzzan J, Lupidi M, Coscas G, Souied EH. Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa. Am J Ophthalmol. 2016;161:160–71.e1–2. doi:10.1016/j.ajo.2015.10.008.
- Prasad PS, Oliver SC, Coffee RE, Hubschman JP, Schwartz SD. Ultra wide-field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology. 2010;117(4):780–4. doi:10.1016/j.ophtha.2009.09.019.
- Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, Potsaid B, Liu JJ, Lu CD, Kraus MF, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121(7):1435–44. doi:10.1016/j.ophtha.2014.01.034.
- Kumar V, Surve A, Kumawat D, Takkar B, Azad S, Chawla R, Shroff D, Arora A, Singh R, Venkatesh P, et al. Ultra-wide field retinal imaging: a wider clinical perspective. Indian J Ophthalmol. 2021;69(4):824–35. doi:10.4103/ijo.IJO_1403_20.
- Kumar T, Sharma GS, Singh LR. Homocystinuria: therapeutic approach. Clin Chim Acta. 2016;458:55–62. doi:10.1016/j.cca.2016.04.002.
- Al-Sadeq DW, Thanassoulas A, Islam Z, Kolatkar P, Al-Dewik N, Safieh-Garabedian B, Nasrallah GK, Nomikos M. Pyridoxine non-responsive p.R336C mutation alters the molecular properties of cystathionine beta-synthase leading to severe homocystinuria phenotype. Biochim Biophys Acta Gen Subj. 2022;1866(7):130148. doi:10.1016/j.bbagen.2022.130148.
- Fredrick DR. Myopia. BMJ. 2002;324(7347):1195–9. doi:10.1136/bmj.324.7347.1195.
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–8. doi:10.1001/archopht.119.7.1050.
- Toprak AB, Eser E, Guler C, Baser FE, Mayali H. Cross-validation of the Turkish version of the 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ 25). Ophthalmic Epidemiol. 2005;12(4):259–69. doi:10.1080/09286580590967763.
- Mulvihill A, Yap S, O’Keefe M, Howard PM, Naughten ER. Ocular findings among patients with late-diagnosed or poorly controlled homocystinuria compared with a screened, well-controlled population. J Aapos. 2001;5(5):311–5. doi:10.1067/mpa.2001.118219.
- Al-Dewik N, Ali A, Mahmoud Y, Shahbeck N, Ali R, Mahmoud L, Al‐Mureikhi M, Al‐Mesaifri F, Musa S, El‐Akouri K, et al. Natural history, with clinical, biochemical, and molecular characterization of classical homocystinuria in the Qatari population. J Inherit Metab Dis. 2019;42(5):818–30. doi:10.1002/jimd.12099.
- Gus PI, Donis KC, Marinho D, Martins TF, de Souza CFM, Carloto RB, et al. Ocular manifestations in classic homocystinuria. Ophthalmic Genet. 2021;42(1):71–4. doi:10.1080/13816810.2020.1821384.
- Pawar S, Parkar A, Menon S, Desai N, Namrata D, Dole K. Assessment of quality of life of the patients with diabetic retinopathy using National Eye Institute Visual Functioning Questionnaire (VFQ-25). J Healthc Qual Res. 2021;36(4):225–30. doi:10.1016/j.jhqr.2021.02.004.
- DeCarlo DK, Forte E, Gao L, McGwin G, Owsley C. Reliability and validity of the PedsQL 4.0 generic core scales in pediatric vision impairment. J AAPOS. 2020;24(2):94.1–.7. doi:10.1016/j.jaapos.2020.01.008.
- Nilsson M, Kolagari HT, Epstein D, Samolov B, Olsson M, Naess K, Oscarson M, Teaer Fahnehjelm K. Visual outcome, ocular findings, and visual quality of life in patients with Fabry disease. Ophthalmic Genet. 2022;1–9. doi:10.1080/13816810.2022.2132515.