Abstract
Echinacea. (Asteraceae) extracts have been advocated traditionally for use in individuals suffering from sore throats, coughs, and various other respiratory symptoms that could be due to bacterial infections. We therefore evaluated six different commercial Echinacea. extracts, with defined composition of standard marker compounds, for their ability to inactivate 15 different human pathogenic bacteria and two pathogenic fungi. The extracts were derived from E. angustifolia. roots or mixtures of E. purpurea. roots and aerial parts and contained different relative amounts of alkylamides and polysaccharides and similar overall concentrations of caffeic acid derivatives. Five bacteria, Streptococcus pyogenes, Haemophilus influenzae, Legionella pneumophila, Clostridium difficile,. and Propionibacterium acne., were very sensitive to one or more of the extracts, but the patterns of sensitivity were quite different for the various extracts. Furthermore, there were no correlations between bacterial sensitivity and the concentrations of marker compounds in the extracts. The other bacteria and fungi were either slightly sensitive to one or more extracts or were totally resistant. In conclusion, certain preparations of Echinacea., especially ethanol formulations, could provide useful protection or symptom alleviation in cases of various upper and lower respiratory infections, such as sinusitis, bronchitis, pharyngitis, tonsillitis, and pneumonia, as well as cutaneous infections, by means of their selective bactericidal activities, although we do not know which components of the extracts are responsible for these activities.
Introduction
Different species and parts of Echinacea. (Asteraceae) have been used traditionally in North America for the treatment of various symptoms of “colds” and “flu,” as well as for other applications such as treatment of candidiasis, lung diseases, and wound healing (Bergner, Citation1997; Barrett, Citation2003). Chemical analysis has led to the identification and characterization of a number of well-known marker compounds, including polysaccharides, specific caffeic acid derivatives, and alkylamides (Bauer, Citation1998; Binns et al. Citation2002). Extracts containing different combinations of these compounds have all demonstrated biological activities in various bioassay tests, such as immune-modulating activities, antiviral activities, antifungal activities, antioxidant activities, and effects on cannabinoid receptors (Burger et al., Citation1997; Bauer, Citation1998; Rininger et al., Citation2000; Goel et al., Citation2002; Merali et al., Citation2003: Randolph et al., Citation2003; Gertsch et al., Citation2004; Brovelli et al., Citation2005; Hudson et al., Citation2005; Vimalanathan et al., Citation2005; Raduner et al., Citation2006; Sharma et al,. Citation2006a, Citation2006b; Matthias et al., Citation2007).
Many clinical trials have been conducted in individuals suffering from natural or experimentally induced rhinovirus infections, but with variable results (Barrett et al., Citation2003; Turner et al., Citation2005; Schoop et al., Citation2006). This variability may be due to differences in the therapeutic products and experimental protocols. Thus, the question of clinical efficacy will remain unresolved until some of the variables have been considered systematically.
There is considerable early literature, particularly from Europe, to suggest that Echinacea. exerts useful antibacterial activities both in clinical situations and laboratory studies (Bauer Citation1998; Blumenthal et al., Citation2000). In a recent study, a standardized Echinacea. preparation administered daily to normal human subjects resulted in a few significant changes in the aerobic bacterial composition of fecal samples (Hill et al., Citation2006), but the subject of direct antibacterial effects was not addressed.
To better define which products might be useful for clinical study, we decided to carry out a comprehensive quantitative evaluation of the effects of several chemically defined Echinacea. preparations from commercial sources that are currently used in North America. They were tested on 15 different human pathogenic bacteria, which included a number of species associated with respiratory and gastrointestinal infections. Because Echinacea is known to have light-activated antifungal (Merali et al., Citation2003) and antiviral (Vimalanathan et al., Citation2005) activity, the bacteria were tested using both light-activated and dark protocols.
Materials and Methods
Extracts
A total of six commercial extracts on the Canadian and U.S. markets were selected for this study, representing two lots from each of three types of preparation with distinct phytochemical profiles. Detailed specifications and lot numbers are on file. The composition of specific chemical markers was determined by HPLC analysis, and these data are summarized in . The extracts were stored at −20°C for the duration of the study. For each experiment, the extracts were diluted in phosphate-buffered saline 1:20, so that they were compared under conditions of equivalent consumption.
Table 1 Composition of extracts.
Quantitative and qualitative phytochemical analysis
Extracts were characterized by analysis of five replicate subsamples for the three recognized (Bauer et al., Citation1998) biologically active ingredients of Echinacea., specifically alkylamides, polysaccharides, and caffeic acids. Validated methods for standard preparation, quantitative extraction, and HPLC analysis of alkylamide and caffeic acid components have been published (Binns et al., Citation2002). An Agilent model 1100 HPLC system (Agilent Technologies, Santa Clara, CA, USA) with diode array detection was used for these analyses. Compounds determined were cichoric acid, caftaric acid, caffeic acid, cynarin, echinacoside, and chlorogenic acid. For alkylamides, the method provided quantification of dodeca-2E., 4E., 8Z., 10E./Z. tetraenoic acid isobutylamide amide, designated as alkylamide 8/9 according to Bauer (Citation1998). For species confirmation (whether E.. pallida var angustifolia. (DC) Cronq or E. purpurea. (L) Moench), the profile of 25 isobutyl amides was compared with authentic reference profiles as described in Binns et al. (Citation2002).
Polysaccharides were determined by removing any residual alcohol from samples by rotary evaporation. To a 20-mL liquid sample, 30 mL 99% ethanol was added, vortex mixed, and refrigerated for 15 min to allow polysaccharide precipitation. The sample was then centrifuged for 15 min at 4000 rpm and supernatant was discarded. The pellet was then resuspended in 8 mL water and then 32 mL 99% ethanol added. The sample was vortex mixed and then refrigerated for 15 min. The supernatant was discarded and the pellet dried at 65°C (approx. 15 h). The dry weight was then recorded as the crude polysaccharide extract.
Bacteria
A total of 15 strains of common pathogenic bacteria were used. Most of them were standard ATCC strains, purchased form PML Microbiologicals (Wilsonville OR, USA). The others were clinical isolates acquired from the Clinical Microbiology Proficiency Testing laboratory at University of British Columbia. Most of the aerobes were propagated and assayed on sheep blood agar plates at 35°C. Anaerobes were cultivated in anaerobic chambers at 35°C. Legionella pneumophila. was cultivated on charcoal agar plates, Mycobacterium smegmatis. on Middlebrook agar plates with a 5% CO2 atmoshere, and Haemophilus influenzae. on chocolate agar with a 5% CO2 atmosphere.
In addition, specimens of Candida albicans., a wild strain and an ATCC strain, and a wild strain of Trichoderma viride. (courtesy Eduardo Jovel, University of British Columbia), were grown and assayed on Sabouraud-dextrose agar.
All organisms, plates and related reagents were obtained from PML Microbiologicals unless specified otherwise. Spore preparations were obtained by the ethanol method of Wullt et al. (Citation2003).
Treatment protocol
The standard method was as follows. Several isolated colonies of each bacterium were removed and dispensed into PBS (phosphate-buffered saline) by vortex mixing to give a homogeneous suspension of approximately 1 × 108 CFU (colony-forming units) per mL. Aliquots of suspension were mixed 1:1 with the diluted extract in transparent sterile plastic tubes and either exposed to light or covered in aluminum foil (dark exposure) for a period of 60 min with incubation at room temperature (20°C) on a rocker platform. Light exposure comprised a combination of UVA (long-wave ultraviolet) and visible (fluorescent lamps). The rationale for including light exposure was our numerous publications indicating the existence of photoactive ingredients in Echinacea. extracts (Towers et al., Citation1997; Merali et al., Citation2003; Hudson et al., Citation2005).
After the treatment, each mixture was serially diluted (10x dilutions) in PBS, and 2.5-μ L aliquots were spotted onto appropriate agar plates divided into sectors for each dilution and spread uniformly with sterile plastic loops, allowed to dry, and the plates incubated under the conditions stated above. After incubation of the plates for 24–72 h, depending on the system, colonies were counted manually and compared with untreated samples. In some cases, bacteria were treated with PBS only and irradiated, to examine for possible antibacterial effects of the radiation. The radiation alone gave no significant reduction in CFU; consequently, control values in the tables are given for dark-exposed samples only.
Results
The six extracts represented three groups of two, as indicated in . Extracts A1 and A2 were rich in alkylamides and low in polysaccharides, B1 and B2 had moderate levels of alkylamides with no polysaccharides, and C1 and C2 were devoid of alkylamides but rich in polysaccharides. Their total contents of caffeic acid derivatives were similar among the groups, although the patterns of individual compounds differed considerably, as discussed below.
Five of the bacteria tested showed substantial sensitivity to various extracts. However, because the patterns were quite different between them, they are discussed separately. shows the results for Streptococcus pyogenes.. In this case, all A and B extracts were very active, giving rise to more than 3 log10 decrease in CFU, both in light and dark. To distinguish between light and dark effects, dose-response curves were done for B1, as shown in . This clearly shows the photoactive effect. In contrast C1 and C2 showed no effect on S. pyogenes., in light or dark. Additional experiments showed that titers of control bacteria, that is, without exposure to Echinacea., were not significantly affected by light irradiation.
Figure 1 Dose-response curve for S. pyogenes. incubated with extract B1. Aliquots of S. pyogenes. were treated with different 10-fold dilutions of extract B1, according to the standard protocol described in “Materials and Methods,” with or without exposure to light. The treated samples were then plated out for colony formation.
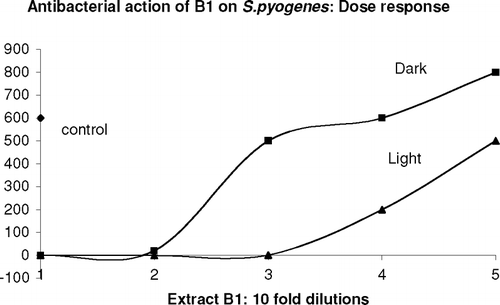
Table 2 Streptococcus pyogenes. susceptibility.
In the case of Haemophilus infuenzae. (), B1 and B2 showed the greatest effect, more in light than dark, while A1 and A2 showed only small levels of inactivation, and C1 and C2 were again inactive.
Table 3 Haemophilus influenzae. susceptibility.
Table 4 Legionella pneumophila. susceptibility.
Legionella pneumophila. displayed a totally different pattern (). All extracts were equally effective, giving approximately 3 log10 reductions, both in light and dark.
Propionibacterium acne. was very sensitive to B1 and B2 in light, slightly sensitive in the dark, but this organism was essentially resistant to A and C extracts, both in light and dark ().
B1 and B2 showed impressive activity against Clostridium difficile., particularly in light (> 3 log10 reduction), A extracts were moderately active, with 1.25 to 1.85 log reductions, but C1 and C2 showed very little activity (). Purified spore preparations, made according to Wullt et al. (Citation2003), were also very sensitive to B extracts.
Table 5 Propionibacterium acne. susceptibility.
Table 6 Clostridium difficile. susceptibility.
The other test organisms, listed in , were relatively resistant to all extracts, in light and dark, although in a few cases some organisms, such as C. albicans., P, aeruginosa., and S. aureus., showed reductions of up to 1 log10; but these were insignificant compared with the sensitive organisms shown above.
Table 7 Species relatively insensitive to Echinacea. extracts (<1 log10 CFU inactivated).
Discussion
Several conclusions can be drawn from these results: (1) The Echinacea. extracts were selective in their antibacterial activities. (2) Different organisms showed significant differences in their patterns of sensitivity, and in some cases photosensitizers were clearly involved. (3) There were no correlations between chemical composition of the extracts, in terms of known marker compounds, and their corresponding antibacterial activities. (4) Different preparations of Echinacea. show markedly different effects on bacteria.
Echinacea. extracts have often been advocated for control of various respiratory infections, including those with bacterial implications, such as colds, flu, coughs (Bergner, Citation1997), usually based on anecdotal reports. Our data show considerable selectivity in antibacterial activities. Five important human pathogens were very sensitive to one or more extracts: S. pyogenes., frequently associated with pharyngitis and impetigo; H. influenzae., associated with pneumonia and meningitis; L. pneumophila., the cause of Legionnaire's disease; C. difficile., associated with epidemics of diarrhea (CDAD) and pseudomembranous colitis, and P. acne., the causative agent of acne vulgaris. This selectivity should be considered as a positive feature as it suggests that such extracts could be useful in controlling these pathogens while sparing other beneficial bacteria.
S. pyogenes. and H. influenzae. were particularly susceptible to the B extracts from E. purpurea., whereas the A extracts from E. angustifolia. roots inactivated S. pyogenes. (Gram positive) but not H. influenzae. (Gram negative). The dose-response curves for the former () indicated the presence of photoactivity.
The Gram-negative L. pneumophila. was very sensitive to all the extracts, both in light and dark. This suggests a different mechanism of action, and different active compounds, from those active against the other organisms. Nevertheless, there is no reason, a priori., to assume a similar mode of action for all the sensitive but unrelated bacteria.
The Gram-positive organism P. acne. was sensitive only to B extracts in the presence of light. These extracts must therefore contain a photosensitizer that was absent in A and C extracts. We cannot say, however, whether this compound required UVA or visible light, as our experimental setup contained both sources of light. The Gram-positive C. difficile. was sensitive, to varying degrees, to all extracts, particularly to A1 and A2, but in all cases activity was enhanced somewhat by light. Thus, the distinct patterns of activity shown by the different extracts suggests that there are distinct mechanisms for the five bacteria.
We also examined 12 other organisms, 10 bacteria and two fungi. Most were resistant to all extracts, although Candida albicans. (previously reported as sensitive to Echinacea.; Merali et al., 2003), Staphylococcus aureus., and Pseudomonas aeruginosa. displayed consistently low levels of inactivation by one or more extracts (at most 1 log10 reduction in CFU). Mycobacterium smegmatis. was barely affected, which suggests that the purported benefits of Echinacea. in tuberculosis patients (Bergner, Citation1997; Barrett, Citation2003) cannot be due to direct antibacterial effects.
In general, the B extracts were much more active than the others, and C1 and C2 were almost devoid of activity, except for their impressive activity against P. acne.. But there was no correlation between these patterns of antibacterial activity and the composition of the marker compounds shown in . Thus, the A extracts were derived from E. angustifolia. roots, and contained high concentrations of alkylamides, little polysaccharide, and normal levels of caffeic acid derivatives (CADs). In contrast, the very active ethanol B extracts from E. purpurea. roots and aerial parts contained moderate levels of alkylamides, no polysaccharides, and normal levels of CADs. The relatively inactive aqueous C extracts from E. purpurea. aerial parts were rich in polysaccharides and contained normal level levels of CADs, but were devoid of alkylamides. None of these three classes of compound correlated with antibacterial activity. Furthermore, when the six individual CADs were individually compared, that is, concentrations of caftaric acid, chlorogenic acid, caffeic acid, cynarin, echinacoside, and cichoric acid, there was still no correlation. Consequently, the Echinacea. components previously held responsible for various bioactivities (see introduction), namely, alkylamides and polysaccharides, are not individually responsible for direct, bactericidal activities, although some of these and the phenolic compounds could conceivably contribute to these activities.
In conclusion, certain preparations of Echinacea., especially ethanol formulations, could provide useful protection or symptom alleviation in cases of pharyngitis, bronchitis, pneumonia, and various cutaneous lesions, including acne vulgaris, wound infections, and soforth, by means of their selective bactericidal activities, although at present we do not know which components of the extracts are responsible for these activities.
Notes
*Dedicated to Professor John Thor Arnason of the University of Ottawa, Department of Biology, on the occasion of his sixtieth birthday.
References
- Barrett B. Medicinal properties of Echinacea.: A critical review. Phytomedicine 2003; 2: 66–86
- Bauer R. Echinacea.: Biological effects and active principals. Phytomedicines of Europe: Chemistry and biological activity, L D Lawson, R Bauer. ACS Symposium Series, 691, American Chemical Society, Washington, DC 1998; 140–157
- Bergner P. The Healing Power of Echinacea & Goldenseal. Prima Lifestyle Publishing, Roseville, California 1997
- Binns S E, Livesey J F, Arnason J T, Baum B R. Phytochemical variation in Echinacea. from roots and flowerheads of wild and cultivated populations. J Agric Food Chem 2002; 50: 3673–3687
- Blumenthal M, Golberg A, Brinckmann J. Herbal Medicine: Expanded Commission E Monographs. Integrative Medicine Communications, Newton, MA 2000; 88–102
- Brovelli E A, Rua D, Roh-Schmidt H, Chandra A, Lamont E, Noratto G D. Human gene expression as a tool to determine horticultural maturity in a bioactive plant. J Agric Food Chem 2005; 53: 8156–8161
- Burger R A, Torres A R, Warren R P, Caldwell V D, Hughes B D. Echinacea.-induced cytokine production by human macrophages. Int J Immunopharmacol 1997; 19: 371–379
- Gertsch J, Schoop R, Kuenzle U, Suter A. Echinacea. alkylamides modulate TNF-α gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Letters 2004; 577: 563–568
- Goel V, Chang C, Slama J V, Barton R, Bauer R, Gahler R. Echinacea. stimulates macrophage function in the lung and spleen of normal rats. J Nutr Biochem 2002; 13: 487–492
- Hill L L, Foote J C, Erickson B D, Cerniglia C E, Denny G S. Echinacea purpurea. supplementation stimulates select groups of human gastrointestinal tract microbiota. J Clin Pharm Ther 2006; 31: 599–604
- Hudson J B, Towers G HN. Phytomedicines as antivirals. Drugs of the Future 1999; 24: 295–320
- Hudson J, Vimalanathan S, Kang L, Treyvaud Amiguet V, Livesey J, Arnason J T. Characterization of antiviral activities in Echinacea. root preparations. Pharm Biol 2005; 43: 790–796
- Matthias A, Banbury L, Stevenson L M, Bone K M, Leach D N, Lehmann R P. Alkylamides from Echinacea. modulate induced immune responses in macrophages. Immunol Inv 2007; 36: 117–130
- Merali S, Binns S, Paulin-Levasseur M, Ficker C, Smith M, Baum B R, Arnason J T. Antifungal and anti-inflammatory activity of the genus Echinacea.. Pharm Biol 2003; 41: 412–420
- Raduner S, Majewska A, Chen J Z, Xie X Q, Hamon J, Faller B, Altmann K H, Gertsch J. Alkylamides from Echinacea. are a new class of cannabinomimetics. J Biol Chem 2006; 281: 14192–14206
- Randolph R K, Gellenbeck K, Stonebrook K, Brovelli E, Qian Y, Bankaitis-Davis D, Cheronis J. Regulation of human gene expression as influenced by a commercial blended Echinacea. product: Preliminary studies. Exp Biol Med 2003; 228: 1051–1056
- Rininger J A, Kickner S, Chigurupati P, McLean A, Franck Z. Immunopharmacological activity of Echinacea. preparations following simulated digestion on murine macrophages and human peripheral blood cells. J Leuk Biol 2000; 68: 503–510
- Sharma M, Arnason J T, Burt A, Hudson J B. Echinacea. extracts modulate the pattern of chemokine and cytokine secretion in rhinovirus-infected and uninfected epithelial cells. Phytotherapy Res 2006a; 20: 147–152
- Sharma M, Arnason J T, Hudson J B. Echinacea. extracts modulate the production of multiple transcription factors in uninfected cells and rhinovirus-infected cells. Phytotherapy Res 2006b; 20: 1074–1079
- Schoop R, Klein P, Suter A, Johnston S L. Echinacea. in the prevention of induced rhinovirus colds: A meta-analysis. Clin. Therapeutics 2006; 28: 174–183
- Sloley B D, Urichuk L J, Tywin C, Coutts R T, Pang P KT, Shan J J. Comparison of chemical components and antioxidant capacity of different Echinacea. species. Pharmacy Pharmacol 2001; 53: 849–857
- Towers G HN, Page J E, Hudson J B. Light-mediated biological activities of natural products from plants and fungi. Curr Org Chem 1997; 1: 395–414
- Turner R B, Bauer R, Woelkart K, Hulsey T C, Gangemi J D. An evaluation of Echinacea angustifolia. in experimental rhinovirus infections. N Engl J Med 2005; 353: 341–348
- Vimalanathan S, Kang L, Treyvaud Amiguet V, Livesey J, Arnason J T, Hudson J. Echinacea purpurea. aerial parts contain multiple antiviral compounds. Pharm. Biol. 2005; 43: 740–745
- Wullt M, Odenholt I, Walder M. Activity of three disinfectants and acidified nitrite against Clostridium difficile. spores. Infection Control Hosp Epidem 2003; 24: 765–768
