Abstract
In vitro determinations of the antimicrobial and antioxidant activities of ethanol and aqueous extracts of Disthemonanthus benthamianus Baill. (Caesalpiniaceae) and Zanthoxylum zanthoxyloides Lam. (Rutaceae), which are used as chewing sticks in Nigeria, were investigated. The extracts were screened against eight strains of Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, one methicillin-resistant strain of Staphylococcus epidermidis, twelve strains of Pseudomonas aeruginosa, five strains of Candida albicans and four strains of Bacillus cereus. The in vitro antimicrobial assay was performed by using a Mast Multipoint Inoculator based on the principles of an agar dilution technique. The aqueous extracts had no inhibitory effects on any of the tested microorganisms. The ethanol D. benthamianus extract inhibited P. aeruginosa, E. faecalis, and B. cereus with MIC ≤ 2.64 mg mL−1 and S. aureus and S. epidermidis with MIC ≤ 0.44 mg mL−1. Z. zanthoxyloides ethanol extract was less effective but noteworthy was the MIC ≤ 2.52 mg mL−1 against C. albicans and 0.28 mg mL−1 against some S. aureus strains. D. benthamianus ethanol extract had the best antioxidant activity and Z. zanthoxyloides ethanol extract second best. IC50 for DPPH free radical scavenging of these extracts were 87.76 and 128.28 μg mL−1/mL, respectively; ascorbic acid equivalents were 2.4 and 9.5 mg mg−1 and total antioxidant capacities using the FRAP assay were 4068.06 mM Fe++ mg−1 and 624.86 mM Fe++ mg−1, respectively. The ethanol extracts of D. benthamianus and Z. zanthoxyloides showed significant antimicrobial and antioxidant activities which could be beneficial in oral hygiene.
Introduction
In Nigeria, chewing sticks have been used from time immemorial for maintenance of dental and oral hygiene. The use of chewing sticks is common in the rural areas among the low socioeconomic class. However, some people from higher socioeconomic classes in urban areas also prefer the use of chewing sticks together with toothbrushes because of the desirable effect they derive from it. Chewing-stick usage is now popular in Africa, South America, Asia, and the Middle East as a means of maintaining oral hygiene (CitationAgbelusi et al., 2007).
Disthemonanthus benthamianus Baill. (Caesal- piniaceae) or “orin ayan” is known to be used traditionally as a chewing stick. Primary screens have demonstrated that extracts from many chewing sticks have antimicrobial activity against a broad spectrum of microorganisms, including those commonly implicated in orofacial infections. Some chewing stick extracts have additional biological activities (CitationNdukwe et al., 2004). D. benthamianus has also been used to treat skin rashes. Zanthoxylum zanthoxyloides Lam. (Rutaceae) or “orin ata” is used domestically as chewing stick to clean the mouth. It is used by traditional healers in Nigeria with other plant species for the treatment of a wide range of disorders, including toothache, urinary and venereal diseases, rheumatism and lumbago (CitationAdesina, 2005). Some of the metabolites from Zanthoxylum species have shown cytotoxic, molluscicidal, anticonvulsant, anti-sickling, anesthetic, antibacterial, anti-hypertensive and anti-inflammatory properties (CitationAdesina, 2005). The roots, bark, and leaves of Z. anthoxylum species are used in various medicinal preparations for the treatment of stomachache, toothache, coughs, urinary and venereal diseases, leprous ulcerations, rheumatism, lumbago, etc. They are used as components of antiseptic, antiparasitic, and analgesic preparations for managing small pox, syphilis and related disease conditions. The roots usually give a warm, pungent and benumbing effect on the palate when chewed, and this aromatic warm taste with attendant profuse salivation is believed to be beneficial to the elderly and those with sore gums and other oral disease conditions (CitationAdesina, 2005).
Considering the vast importance of these chewing sticks, this present work focuses on in vitro determination of the antimicrobial and antioxidant activities of the ethanol and aqueous extracts of D. benthamianus and Z. zanthoxyloides.
Materials and methods
Source of plant materials
The plants used for this study are Disthemonanthus benthamianus and Zanthoxylum zanthoxyloides roots. The plant materials were bought from Oja Jagun in Ogbomoso, Oyo State, Nigeria and identified by Dr. A.T.J. Ogunkunle, a botanist in the Department of Pure and Applied Biology, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria.
Preparation of extracts
The plant materials were dried and ground into fine powder separately. The ethanol extracts were obtained by soaking 100 g of each powder in 400 mL 95% ethanol for 4 days at room temperature. This was followed by filtering the ethanol extracts under vacuum and the solvent was evaporated in a water bath at 40°C using a rotatory evaporator (Heidolf, Cole-Parmer Instrument Company, Vernon Hills, IL, USA) The aqueous extracts were also obtained by soaking 100 g of D. benthamianus powder in 400 mL double distilled water and kept at 4°C, while 100 g of Z. zanthoxyloides was soaked in 800 mL double distilled water. Filtrates of the aqueous extracts were obtained every day by sieving and centrifuging at 5,000 g (Beckman, Avanti™ J-25 Centrifuge, Beckman Coulter Inc, Fullerton, CA, USA) for 20 min at 4°C. The supernatant was decanted and filtered through Whatman No. 1 filter paper. Filtrates for each plant were combined and freeze-dried. The aqueous and ethanol extracts were then kept in sample bottles in a dessicator pending usage.
Microbial cultures
The microorganisms used for this bioassay are clinical isolates (except the ATCC strains) collected in semi-solid agars from the National Health Laboratory Service, Port Elizabeth, South Africa. These include eight strains of Escherichia coli (ATCC 35218), eight strains of Staphylococcus aureus, of which one was methicillin-resistant (MRSA) and one was ATCC 43300, one methicillin-resistance strain of Staphylococcus epidermidis (MRSE), twelve strains of Pseudomonas aeruginosa (ATCC 27853), eight strains of Enterococcus faecalis (ATCC 440384 and 29212), five strains of Candida albicans (ATCC 66027) and four strains of Bacillus cereus (ATCC 10876). The strains of E. faecalis, S. epidermidis and S. aureus, and those of E. coli, P. aeruginosa and B. cereus were sub-cultured on blood and nutrient agar plates for 24 h, respectively. Strains of C. albicans were sub-cultured on Sabouraud dextrose agar (SDA) for 48 h. The type culture strains were used for quality control.
Agar plate preparation
Müller-Hinton agar was prepared, autoclaved and cooled to about 50°C before addition of the extracts. The agar medium containing the extracts at final concentrations of 0.44, 0.88, 1.76, and 2.64 mg mL−1 for D. benthamianus extracts, and 0.14, 0.28, 0.56, 0.84, and 2.52 mg mL−1 for Z. zanthoxyloides were added to the Petri dishes and mixed with Müller-Hinton agar by swirling carefully and allowing to set. A Petri dish containing the Müller-Hinton agar only was used as a control for media contamination. Another plate containing a mixture of 100 μL DMSO and the Müller-Hinton agar was used as control to ensure that the DMSO used as solvent for the extracts did not inhibit the growth of the organisms. Each test plate was replicated twice.
Antimicrobial testing
Antimicrobial activity of the tested organisms was carried out by the use of Mast Multipoint Inoculator (Mast Systems, London, UK) based on the principles of agar dilution (CitationAndrews, 2001). The extract was prepared by dissolving in a known amount of DMSO; not exceeding 100 μL DMSO per plate (0.4% V/V). A standardized inoculum was prepared by suspending colonies in saline and adjusting the density to that of a 0.5 McFarland standard. A final volume of 500 μL of the prepared inoculums was transferred into each well of the sterile inoculum pots. The culture medium contained in a Petri dish was inoculated with the sterile pins of the multipoint inoculator. The agar plates were incubated at 37°C for 24 to 48 h. Complete suppression of growth was required for an extract to be declared active.
Scavenging of 2,2-diphenyl-1-picrylhydrazyl
Free radical scavenging activity was determined as described by CitationTang et al. (2004). Each extract (1 g) was dissolved in 100 μL of DMSO and made up to 1 L with Tris buffer. Extracts of each plant at concentrations of 250, 125, 25, 12.5, 2.5, and 1.25 μg mL−1 were mixed with equal volumes of DPPH solution (200 μM in 80% ethanol v/v) and shaken vigorously. The absorbance was measured at 520 nm after incubating for 20 min at room temperature using a microplate reader (Power Wave XS, Bio-tek, Winooski, Vermont, USA). All determinations were performed in triplicate. The radical scavenging activity was obtained from the following equation:
Radical scavenging activity (%) = [(ODcontrol − ODsample) / ODcontrol] × 100.
The control consisted of 120 μL Tris buffer mixed with 120 μL DPPH. The antioxidant activity of plant extracts was expressed as IC50, which is the concentration (in μg mL−1) of extract required to inhibit the formation of DPPH radicals by 50%. Ascorbic acid was used as a standard. The inhibition curve was plotted and IC50 values obtained.
Assessment of total antioxidant capacity using the phosphomolybdenum method
In order to measure total antioxidant capacities of the extracts the phoshomolybdenum method of CitationPrieto et al. (1999) was used. An aliquot of 0.1 mL of sample solution of diluted extracts in water (for aqueous extracts) and in ethanol (for ethanol extracts) was added to 1 mL reagent solution (0.6 M of sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) in an Eppendorf tube. The tubes were capped and placed in a thermal block where incubation was carried out at 95°C for 90 min. The samples were allowed to cool down to room temperature after which the absorbance was read against a blank at 695 nm. The blank solution consisted of 1 mL reagent solution and the appropriate volume of water or ethanol, incubated under the same conditions as the rest of the samples. Antioxidant properties of the extracts were expressed as ascorbic acid equivalent antioxidant capacity, calculated from at least three different concentrations of extract tested in the assay giving a linear response.
Determination of ferric reducing/antioxidant power
Ferric reducing ability of plasma (FRAP) assay of CitationBenzie and Strain (1996) was used to measure total antioxidant potential of the extracts. A standard curve was prepared using different concentrations (100-1000 μmol/L) of FeSO4·7H2O. The results were corrected for dilution and expressed in mmol Fe++/mg dried plant extract. The extracts were first adequately diluted to fit within a linear range. All determinations were performed in triplicate. FRAP reagent (20 mL acetate buffer, 2 mL 2,4,6-tri (2-pyridyl)-S-triazine (TPTZ) solution, 2 mL FeCl3 solution and 2.4 mL distilled water) was prepared and kept in a water bath at 37°C. Aliquots of 10 mL of the diluted extracts, control (ascorbic acid) or FeSO4 standard were transferred into 96-well plates and 250 μL of freshly prepared FRAP reagent was added to each well. The absorbance was measured at 593 nm after 5 min against a blank in which the sample was replaced with water or ethanol. All solutions were used on the day of preparation.
Results
The yields from 100 g of dry D. benthamianus roots were 9.89 g for aqueous and 8.77 g for the ethanol extracts. The corresponding values for Z. zanthoxyloides were 4.15 and 2.80 g, respectively.
In the present study, the aqueous extracts of the two plants used as chewing sticks did not inhibit the growth of any of the tested microbial strains at the various concentrations. The highest concentration tested for D. benthamianus was 2.64 mg mL−1 while that of Z. zanthoxyloides was 2.52 mg mL−1. The ethanol extracts of the plants were not active against any of the strains of E. coli tested. It was observed that D. benthamianus ethanol extract at the highest concentration tested (2.64 mg mL−1) totally inhibited most of the tested microbial strains, except C. albicans, which it partially inhibited (). At a low concentration of 0.44 mg mL−1, the D. benthamianus ethanol extract totally inhibited the methicillin-resistant strains of S. aureus and S. epidermidis, though most of the other tested strains were not sensitive at this concentration.
Table 1. Antimicrobial activity of Disthemonanthus benthamianus ethanol extract against the tested microbial strains.
In , the ethanol extract of Z. zanthoxyloides at 2.52 mg mL−1 showed strong inhibitory effect on all the tested strains of E. faecalis, C. albicans and B. cereus, while three of the tested strains of S. aureus were susceptible to the extract. The ATCC strain of S. aureus and the methicillin-resistant strains of S. aureus and S. epidermidis, were not inhibited by this extract at any of the concentrations used. Also, none of the tested strains of P. aeruginosa used in this study was inhibited by this extract.
Table 2. Antimicrobial activity of Zanthoxylum zanthoxyloides ethanol extract against the tested microbial strains.
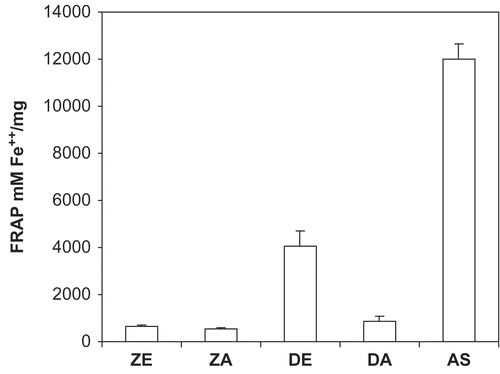
The DPPH radical scavenging activities of the extracts revealed that the ethanol extract of D. benthamianus had the highest radical scavenging activity with an IC50 value of 6.73 μg mL−1, though this value is marginally higher than that of the standard ascorbic acid which had a value of 4.82 μg mL−1 (). The IC50 value of the aqueous extract of D. benthamianus was 87.76 μg mL−1 (), while that of the ethanol extract and aqueous extract of Z. zanthoxyloides was 128.28 μg mL−1 and 191.06 μg mL−1, respectively. The total antioxidant capacity of the extracts using the phosphomolybdenum method showed that the different extracts exhibited various degrees of antioxidant capacity (). This method is based on the reduction of Mo (VI) to Mo (V) by the antioxidant compounds and the formation of a green Mo (V) complex with a maximal absorption at 695 nm. The ascorbic acid equivalent of the ethanol extract of D. benthamianus was 2.4 ± 0.1 mg mg−1, while that of the ethanol extract of Z. zanthoxyloides was 8.0 ± 1.7 mg mg−1. The aqueous extracts of both plants have high values of 13.9 ± 1.3 mg mg−1 and 11.6 ± 0.7 mg mg−1 for DA and ZA respectively. In addition, the FRAP assay () showed that the ascorbic acid, being the standard, had the highest value of 8268.43 mM Fe++ mg−1, followed by D. benthamianus ethanol extract which had a value of 4068.06 mM Fe++ mg−1, while the values of the other extracts were very low.
Figure 1. IC50 values of D. benthamianus and Z. zanthoxyloides extracts and standard ascorbic acid. DE, D. benthamianus ethanol extract; DA, D. benthamianus aqueous extract; ZE, Z. zanthoxyloides ethanol extract; ZA, Z. zanthoxyloides aqueous extract. DPPH solution was mixed with an equal volume of the extract and the absorbance was measured at 520 nm. Results are averages ± SD for ≥ 3 determinations.
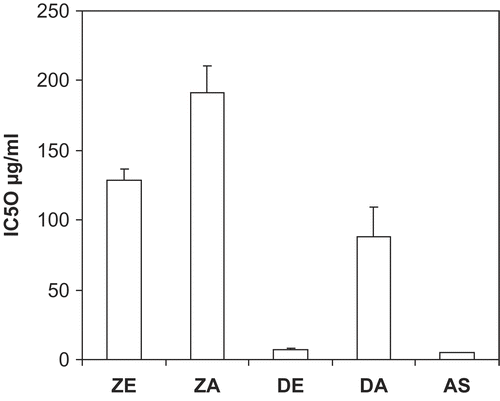
Figure 2. Ascorbic acid equivalent antioxidant capacity values of D. benthamianus and Z. zanthoxyloides extracts. Antioxidant properties of the extracts were expressed as ascorbic acid equivalent antioxidant capacity, calculated from at least three different concentrations of extract tested in the assay giving a linear response. DE, D. benthamianus ethanol extract; DA, D. benthamianus aqueous extract; ZE, Z. zanthoxyloides ethanol extract; ZA, Z. zanthoxyloides aqueous extract. Results are averages ± SD for ≥ 3 determinations.
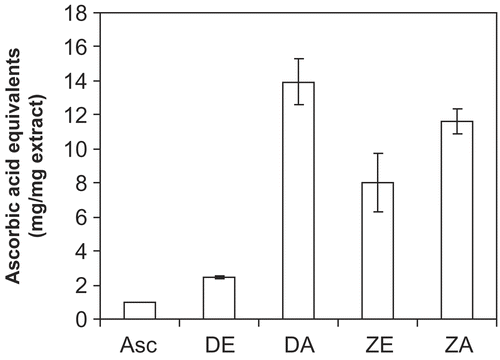
Figure 3. Total antioxidant capacity determined as FRAP (Ferric reducing/antioxidant power) of D. benthamianus, Z. zanthoxyloides extracts and standard ascorbic acid. DE, D. benthamianus ethanol extract; DA, D. benthamianus aqueous extract; ZE, Z. zanthoxyloides ethanol extract; ZA, Z. zanthoxyloides aqueous extract. Results are averages ± SD for ≥ 3 determinations.
Discussion
None of the tested microbial strains was sensitive to the aqueous extracts of the two plants studied. This may be due to the fact that the active agents that are needed to inhibit these organisms may be present in low concentrations. These active substances may be soluble in the organic extract thus making the organisms susceptible to the ethanol extracts (CitationNair & Chanda, 2006). As the traditional use of chewing sticks involves an aqueous environment, one begins to wonder how effective they are when they are being used for dental hygiene. It is likely that during the process of chewing, the active ingredients present in the plant materials may react with the saliva in the mouth through enzymatic processes thus making it active against the pathogens present. The poor antimicrobial activity of these aqueous extracts is similar to the work of CitationAgbelusi et al. (2007) in which most of the aqueous extracts from chewing sticks screened were not active against the pathogens tested. Although most of the tested microbial strains used in this study were not strictly oral pathogens, they are known to play a notable role in oral hygiene, potentially causing dental diseases, periapical lesions, periodontal abscesses and possible gingivitis (CitationVan Vuuren & Viljoen, 2006).
The tested strains of E. coli were not sensitive to any of the extracts. E. coli is resistant to many antibiotics which are effective against Gram-positive organisms. Gram-negative organisms tend to have a higher intrinsic resistance to most antimicrobial agents (CitationNdukwe et al., 2005). However, in this study P. aeruginosa was susceptible to the ethanol extract of D. benthamianus. P. aeruginosa is known to be naturally resistant to antibacterial agents (CitationKonning et al., 2004). In addition, D. benthamianus ethanol extract was highly active against most of the tested strains. The extract totally inhibited the methicillin-resistant strains of S. aureus and S. epidermidis. Methicillin-resistant S. aureus (MRSA) is an important human pathogen and represents a growing public health burden due to the emergence and spread of epidemic strains, particularly within the hospital environment (CitationWannet et al., 2004). Reports have shown that MRSA strains account for 10% to 40% of S. aureus isolated from some European hospitals (CitationVoss et al., 1994). Reports have shown that oropharyngeal carriage of MRSA can be difficult to eradicate (CitationSmith et al., 2003). Oral carriage of MRSA may serve as a reservoir for colonization of other body sites, or cross-infection to other patients or healthcare workers (CitationMartin & Hardy, 1991). In this study, it is noteworthy that D. benthamianus extract was able to inhibit MRSA and methicillin-resistant Staphylococcus epidermidis (MRSE). In their work, CitationSmith et al. (2003) reported that oral staphylococcal isolates appeared less susceptible to some oral products because of a likely adaptation to daily exposure to such products.
Most of the Gram-positive organisms used in this work were more susceptible to the ethanol extracts of the plants. It has been suggested that the higher resistance of Gram-negative organisms to plant extracts may be due to the thick murein layer in their outer membrane which prevents the entrance of inhibitor substances into the cell (CitationDurmaz et al., 2006). In this study, the results suggest that the ethanol extracts of the two Nigerian plants may have active substances that were able to inhibit the microbial tested strains in varying proportions, thus making their usage in oral and dental hygiene feasible. The antibiotic susceptibility test carried out in the laboratory from which the organisms were collected showed that some of the extracts were able to inhibit the growth of the clinical isolates which some notable antibiotics were unable to achieve. This may be due to the fact that these organisms were not yet exposed to these extracts, thus having no resistance against them. This may be a promising development in order to produce potent antimicrobial agents.
Nigerian Zanthoxylum species have been known to have antibacterial properties because of some of the metabolites they possess (CitationAdesina, 2005). Likewise, the ethanol extract of Z. zanthoxyloides used in this work showed some antibacterial activity against E. faecalis, B. cereus and three strains of S. aureus. The extract also showed strong inhibitory activity against the yeast C. albicans; this is remarkable because the organism causes oral thrush. Those who suffer from oral Candida often lose a lot of weight because of a sore throat, which prevents them from eating (CitationSanne, 2001). The advent of the human immunodeficiency virus infection and the increasing prevalence of compromised individuals in the community due to modern therapeutic advances have resulted in a resurgence of opportunistic infections, including oral candidosis, which is by far the most common oral fungal infection in man (CitationSoysa et al., 2007). Oral candidiasis caused by C. albicans is the most frequently reported oral infection in HIV-infected patients; this may be due to the fact that they are immunocompromised and the presence of this fungal infection may serve as a marker for serious opportunistic infections (CitationLynch, 1997). The oral manifestations of HIV disease are the first indication of HIV infection or are markers for progression to AIDS. An increased presence of Candida in the oral cavity of patients with diabetes has also been recognized (CitationSamaranayake, 1990). In their work, CitationGuggenheimer et al. (2000) discovered that oral soft tissue diseases attributable to candidiasis were found to be five times more likely in people with diabetes than in control subjects without diabetes. Because most infections in the oral cavity are associated with disseminated disease, early diagnosis and prompt treatment is important for these life threatening infections (CitationLynch, 1997).
The antimicrobial results showed that the ethanol extract of D. benthamianus inhibited more of the tested strains than Z. zanthoxyloides. These results support the traditional use of Z. zanthoxyloides ethanol extract in toothache to ease pain (CitationAdesina, 2005) and D. benthamianus extract in dental hygiene (CitationAderinokun et al., 1999) and other ailments, notably caused by the agents they inhibited. Further research needs to be done to determine the efficacy of chewing sticks when used in the traditional way. Thus, the ethanol extracts of the plants studied may be potential sources of potent antimicrobial agents in the treatment of oral infections and diseases. Several researchers have reported the inhibitory action of chewing sticks against pathogens (CitationFadulu, 1975; CitationSote & Wilson, 1995; CitationTaiwo et al., 1999; CitationNdukwe et al., 2005; CitationAgbelusi et al., 2007). This also supports the efficacy of the ethanol extracts of the chewing sticks used in the present study. Medicinal plants with MIC values below 8 mg mL−1 have been reported as being effective for antimicrobial therapy (CitationFabry et al., 1998). Toothbrush stick extracts have been reported to have MIC ranges between 0.25 and 8 mg mL−1 for most pathogens, though there are some exceptions. In this study moderate sensitivity of the extracts was observed at MIC ≤ 2.64 mg mL−1 for the ethanol D. benthamianus extract on P. aeruginosa, E. faecalis, and B. cereus, while S. aureus and S. epidermidis had MIC value ≤ 0.44 mg mL−1. However, Z. zanthoxyloides ethanol extract was less effective, but noteworthy was the MIC value ≤ 2.52 mg mL−1against C. albicans and 0.28 mg/mL against some S. aureus strains.
In recent years, on account of adverse effects of synthetic drugs, attempts have been made to establish the potential of phytochemicals for the prevention and treatment of periodontal diseases. Several plant species are known to have helped in the cure and treatment of periodontal diseases, particularly in alleviation of toothaches. Herbal oral hygiene products have an advantage of combining positive effects such as anti-inflammatory, antimicrobial and healing (CitationAdamkova et al., 2004). Broad-spectrum antibiotics used in the treatment of a wide range of disease conditions have also been attributed as a predisposing factor of oral candidosis (CitationSoysa et al., 2007). Consequently, there is a need to source and use naturally occurring antioxidants in dental hygiene rather than synthetic ones. The antioxidant activity results of the chewing stick extracts revealed that the ethanol extracts seem to have better activity than the aqueous extracts. D. benthamianus ethanol extract had the best antioxidant activity and high reducing power. Generally, herbal medicines do not produce significant side effects, perhaps because the active ingredients are combined with other compounds in the herb and administered in different dosages. In their work CitationBattino et al. (2002) described that several mouth rinses possess antioxidant activity which may be due to the presence of some active components. They concluded that this finding may be useful in the treatment of periodontal diseases and should be considered when preparing new mouth rinse formulations. In addition, several forms of periodontal diseases are often associated with activated phagocytosing leukocytes and contemporary free radical production. Host antioxidant defenses could benefit from mouth rinses used as adjuncts to counteract plaque-associated bacteria (CitationBattino et al., 2002, Citation2005). In the present study, it was discovered that ethanol extracts, especially that of D. benthamianus, exhibited significant antioxidant activity.
The present study suggests that utilization of active ingredients in Nigerian chewing sticks, when producing herbal oral products, may be a reliable way of producing effective, cheap and affordable products to alleviate and possibly reduce periodontal diseases, thus preventing dental plaque and gingivitis.
Acknowledgements
This research was supported by the Nelson Mandela Metropolitan University Research and Development Fund and the National Research Foundation (FA2006021600006).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adamkova H, Vicar J, Palasova J, Ulrichova J, Simanek V (2004): Macleya chordate and Prunella vulgaris in oral hygiene products – their efficacy in control of gingivitis. Biomed Pap 148: 103–105.
- Aderinokun GA, Lawoyin JO, Onyeaso CO (1999): Effect of two Nigerian chewing sticks in gingival health and oral hygiene. Odonto-Stomatol Trop 87: 13–17.
- Adesina SK (2005): The Nigerian Zanthoxylum: Chemical and biological values. Afri J Trad Compl Med 2: 282–301.
- Agbelusi GA, Odukoya OA, Otegbeye AF (2007): In vitro screening of chewing sticks extracts and sap on oral pathogens: Immune compromised infections. Biotech 6: 97–100.
- Andrews JM (2001): Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48, S5–16.
- Battino M, Ferreiro MS, Armeni T, Politi A, Bompadre S, Massoli A, Bullon P (2005): In vitro antioxidant activities of antioxidant-enriched toothpastes. Free Rad Res 39: 243–350.
- Battino M, Ferreiro MS, Fattorini D, Bullon P (2002): In vitro antioxidant activities of mouth rinses and their components. J Clin Periodontol 29: 462–467.
- Benzie IFF, Strain JJ (1996): The ferric reducing ability of plasma (FRAP) as measurement of “antioxidant power”: The FRAP assay. Anal Biochem 239: 70–76.
- Durmaz H, Sagun E, Tarakci Z, Ozgokce F (2006): Antibacterial activities of Allium vineale, Chaerophyllum macropodum and Prangos ferulacea. Afri J Biotech 5: 1795–1798.
- Fabry W, Okemo PO, Ansorg R (1998): Antibacterial activity of East African medicinal plants. J Ethnopharmacol 60: 79–84.
- Fadulu SO (1975): Antimicrobial properties of the buffer actions of chewing sticks used in Nigeria. Planta Med 27: 123–126.
- Guggenheimer J, Myers D, Weyant R (2000): Insulin-dependent diabetes mellitus and oral soft tissue pathologies. Oral Surg Oral Med Oral Pathol 89: 570–576.
- Konning GH, Agyare C, Ennison B (2004): Antimicrobial activity of some medicinal plants from Ghana. Fitoterapia 75: 65–67.
- Lynch DP (1997): Oral manifestations of HIV disease: An update. Sem Cutaneous Med Surg 16: 257–264.
- Martin MV, Hardy P (1991): Two cases of oral infection by methicillin-resistant Staphylococcus aureus. Br Dent J 170: 63–64.
- Nair R, Chanda S (2006): Activity of some medicinal plants against certain pathogenic bacterial strains. Ind J Pharmacol 38: 142–144.
- Ndukwe KC, Lamikanra A, Okeke IN (2004): Antibacterial activity in plants used as chewing sticks in Africa. Drugs Fut 29: 1221–1233.
- Ndukwe KC, Okeke IN, Lamikanra A, Adesina SK, Aboderin O (2005): Antimicrobial activity of aqueous extracts of selected chewing sticks. J Contemp Dent Pract 6: 86–94.
- Prieto P, Pineda M, Aguilar MM (1999): Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 269: 337–341.
- Samaranayake LP (1990): Host factors and oral candidosis. In: Samaranyake LP, MacFarlane TW, eds., Oral Candidosis. London, Wright, pp. 66–103.
- Sanne I (2001) Treating HIV/AIDS. Archimed 43: 32–34.
- Smith AJ, Morrison D, Robertson D (2003): Efficacy of oral hygienic products against MRSA and MSSA isolates. J Antimicrob Chemother 52: 738–739.
- Sote EO, Wilson M (1995): In vitro antibacterial effect of Nigerian tooth cleaning sticks on periondontopathic bacteria. Afri Dent J 9: 15–19.
- Soysa NS, Samaranayake LP, Ellepola ANP (2008): Antimicrobials as a contributory factor in oral candidosis – A brief overview. Oral Dis 14:138–143.
- Taiwo O, Xu H, Lee S (1999): Antibacterial activities of extracts from Nigeria chewing sticks. Phytotherapy Res 13: 675–679.
- Tang SY, Whiteman M, Peng ZF, Jenner A, Yong EL, Halliwell B (2004): Characterization of antioxidant and antiglycation properties and isolation of active ingredients from traditional Chinese medicines. Free Rad Biol Med 36: 1575–1587.
- Van Vuuren SF, Viljoen AM (2006): In vitro antimicrobial activity of toothbrush sticks used in Ethiopia. South Afri J Bot 73: 646–648.
- Voss A, Milatovic D, Wallrauch-Schwarz C, Rosdahl VT, Braveny I (1994): Methicillin resistance Staphylococcus aureus in Europe. Eur J Clin Microbiol Infect Dis 13: 50–55.
- Wannet WJB, Spalburg E, Heck, , MEOC Pluister GN, Willems RJL, de Neeling AJ (2004): Widespread dissemination in the Netherlands of the epidemic Berlin methicillin-resistant Staphylococcus aureus clone with low-level resistance to oxacillin. J Clin Microbiol 42: 3077–3082.
