Abstract
The methanol leaf extracts of female cultivars of the carob tree [Ceratonia siliqua L. (Fabaceae)] and of hermaphrodite and male trees were investigated for their contents of phenolic compounds, their in vitro antioxidant activity, measured by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging and linoleic acid system assays, and their in vitro tumor growth inhibition on HeLa cells. The different cultivars and trees showed high levels of phenols, and considerable variations in the amount of these compounds. The extracts showed significant radical scavenging activity (RSA), which was not significantly affected by the gender of the tree. From the female cultivars tested, Galhosa exhibited the highest RSA. Gender significantly affected the antioxidant activity of the extracts measured by the linoleic acid system assay, and males and hermaphrodites showed the highest activities. The extracts displayed a remarkable ability to inhibit tumor cell proliferation, and their bioactivity varied with different cultivars or trees tested. Extracts from male and hermaphrodite trees exhibited higher capacity to inhibit the proliferation of HeLa cells than the female cultivars.
Introduction
Free radicals (reactive oxygen species, ROS, and reactive nitrogen species, RNS) are products of normal cellular metabolism, and are extremely reactive and potentially damaging transient chemical species. The delicate balance between beneficial and harmful effects of free radicals is very important to living organisms and is maintained by “redox regulation” defense mechanisms, which include preventive and repair mechanisms, physical defenses, and antioxidant defenses provided by vitamins, carotenoids, sterols, and phenols (CitationValko et al., 2007).
There have been numerous studies on the biological activities of phenols, which are potent antioxidants and free radical scavengers (CitationSugihara et al., 1999). Furthermore, many isolated phenol compounds or extracts rich in these compounds have been reported to possess anticancer, anti-carcinogenic, anti-mutagenic, antibacterial, antiviral, or anti-inflammatory activities (CitationYamagishi et al., 2000; CitationGreenspan et al., 2005; CitationLambert et al., 2005; CitationMoreno et al., 2006; CitationSong et al., 2007). Phenolic compounds may also have antioxidant effects used for managing oxidation stress-related chronic diseases such as diabetes and hypertension (CitationKwon et al., 2008).
The carob tree [Ceratonia siliqua L. (Fabaceae)] is one of the most useful trees of the Mediterranean basin. It is mainly used in the food industry as a source of gum extracted from the seeds (LBG; E410). The pulp is used in infants for the treatment of diarrhea of bacterial and viral origin (CitationLoeb et al., 1989). The leaves and pulps have antioxidant (CitationKumazawa et al., 2002; CitationMakris & Kefalas, 2004, CitationOwen et al., 2003; CitationPapagiannopoulos et al., 2004), antiproliferative (CitationCorsi et al., 2002), and antimicrobial activities (CitationKivçak et al., 2002). Leaf extracts have potential anxiolytic and sedative effects (CitationAvallone et al., 2002).
The chemical composition of pods varies among the different cultivars, and includes a high amount of different types of polyphenolic compounds (CitationAvallone et al., 1997; CitationCorsi et al., 2002; CitationKumazawa et al., 2002; CitationOwen et al., 2003; CitationPapagiannopoulos et al., 2004), which are also present in leaves in higher amounts (CitationCorsi et al., 2002). Some previously isolated constituents include gallic acid, hydrolyzable and condensed tannins, flavonolglycosides, flavonoids (CitationOwen et al., 2003; CitationPapagiannopoulos et al., 2004), and pinitol (CitationBaumgartner et al., 1986).
As far as our literature survey could ascertain, there is no information about the phenolic profile or biological activities of leaf extracts of Portuguese female cultivars of this species; nor are there any comparative studies of the variation in chemical composition between the three genders. In this context, we assessed the amounts of phenol compounds from the methanol leaf extracts of six representative Portuguese female cultivars of the carob tree growing in the Algarve region, and the leaf extracts of two male and two hermaphrodite individuals. The antioxidant activity of the extracts was assessed using two established in vitro methods, and this activity was compared to the activity of pure phenolic compounds. Furthermore, we investigated the antitumor activity of the extracts using HeLa cells, a cell line of human uterine cervix adenocarcinoma, as a model system.
Materials and methods
Collection of plant material and preparation of extracts
Samples from six of the most representative female Portuguese cultivars of the carob tree in terms of fruit productivity, namely Mulata, Galhosa, Aida, Costela/Canela, Gasparinha, and Preta de Lagos, and from two hermaphrodite (H1 and H2) and two male trees (M1 and M2) were sampled during August and September of 2005. The different cultivars and trees are from a cultivar collection from the Ministry of Agriculture, Rural Development and Fisheries (Direcção Regional de Agricultura do Algarve, Delegação de Tavira), and were identified by J. Graça. Fully expanded leaves were randomly collected from the middle third of the branches in all canopy orientations, dried at 40°C for 2 days, crushed and milled in a laboratory-scale hammer mill, and stored in the dark at −20°C until extraction. The extracts were prepared as described by CitationOwen et al. (2003). Samples (10 g) were extracted in a Soxhlet apparatus first with n-hexane (2 × 3 h), to remove lipids, and then with methanol (5 h). The methanol extracts were centrifuged at room temperature (RT) during 10 min at 3000 rpm, the supernatant was collected, and the solvent was removed by rotary evaporation with vacuum. The extracts were resuspended in methanol and kept at −20°C in the dark until analysis. Every extraction was performed at least twice.
Chemicals used for experimentation
Dulbecco’s modified Eagle medium (DMEM), l-glutamine, penicillin, and streptomycin were purchased from Biological Industries; fetal bovine serum (FBS) was from PAA Laboratories and n-hexane was from Labscan. Sigma Chemical Co. supplied DPPH (1,1-diphenyl-2-picrylhydrazyl), chlorogenic acid, (+)-catechin, linoleic acid, chloroform and β-carotene, whereas WST-1 [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium], monosodium salt) was purchased from Roche. Folin–Ciocalteu reagent, (–)-epicatechin, gallic acid, methanol (MeOH), Tween 40, p-dimethylaminocinnamaldehyde (DMACA), aluminum chloride (AlCl3), and sodium carbonate (Na2CO3) were from Fluka Biochemika. Catechol was purchased from Acros organics. All the other chemicals where of analytical grade.
Measurement of total phenol content
The total phenol content of the extracts was determined in samples (2 mg/mL) by the Folin–Ciocalteu colorimetric method (CitationJulkunen-Tiitto, 1985). Briefly, aliquots of the extracts (0.1 mL, 2 mg/mL) were added to 5 mL of distilled water and 0.5 mL of Folin–Ciocalteu reagent and vigorously shaken. After 3 min, 1 mL of a saturated solution of sodium carbonate (Na2CO3) was added, and the volume made up to 10 mL with distilled water. The mixtures were allowed to stand for 60 min at RT for complete reaction, and the total phenols were determined by colorimetry at 720 nm (Shimadzu UV-160A). The amount of total polyphenols was calculated as a gallic acid equivalent from the calibration curve of gallic acid standard solutions, and expressed as gallic acid equivalents (GAE) in milligrams per gram of initial dry plant material.
Determination of total condensed tannins content
The amount of condensed tannins was determined by the p-dimethylaminocinnamaldehyde (DMACA) method, according to CitationArnous et al. (2001). Samples (0.4 mL, 2 mg/mL) were mixed with 2 mL of DMACA solution (0.1% in 1 N HCl in MeOH). The mixture was vortexed, allowed to react at RT for 10 min, and the absorbances were read at 640 nm on a Shimadzu UV-160A spectophotometer. The concentration of condensed tannins was estimated from a calibration curve, constructed by plotting known solutions of (+)-catechin, and results were expressed as catechin equivalents (CE) in milligrams per gram of initial dry plant material.
Determination of total flavonoids
The flavonoid content was estimated in the extracts by the aluminum chloride (AlCl3) method (CitationLamaison & Carnat, 1990). In short, 1 mL of methanol extract (2 mg/mL) was added to 1 mL of 2% methanol AlCl3.6H2O. The absorbance was measured 10 min later at 430 nm (Shimadzu UV-160A). The results were expressed as rutin equivalents (RE) in milligrams per gram of initial dry plant material by comparison with the values obtained from standard rutin treated in the same conditions.
Determination of antioxidant activity
DPPH radical scavenging activity
The effect of extracts on the DPPH radical was estimated using the method of CitationBrand-Williams et al. (1995). Aliquots (0.1 mL) of test sample at concentrations ranging from 400 to 50 μg/mL were reacted with 2.5 mL of methanol DPPH solution (100 μM) for 30 min in the dark, and the decrease in absorbance at 517 nm was measured spectrophotometrically (Shimadzu UV-160A). The DPPH radical scavenging activity (RSA) was calculated by the following equation: RSA (%) = [(control absorbance − sample absorbance)]/(control absorbance) × 100. Results were expressed as IC50 value (μg/mL), which is the amount of sample necessary to decrease by 50% the absorbance of DPPH, and as antiradical efficiency (AE), which is 1000-fold the inverse IC50 value (CitationParejo et al., 2003).
Linoleic acid system
The activity of the extracts was determined according to CitationTepe et al. (2006). A stock solution of β-carotene–linoleic acid mixture was prepared by dissolving β-carotene (0.5 mg) in chloroform (50 mL), and adding linoleic acid (25 μL) and Tween 40 (200 mg). Chloroform was completely evaporated using a vacuum evaporator, and 100 mL of distilled water was added with vigorous shaking. Aliquots (3 mL) of this reaction mixture were dispensed to test tubes, the extracts (0.1 mL) were added, and the emulsion system was incubated for 60 min at 50°C. Oxidation of the emulsion was assessed spectrophotometrically (Shimadzu UV-160A) by measuring absorbance at 470 nm after the 60 min period. The antioxidant activity is expressed as percent inhibition relative to the control after a 60 min incubation using the following equation: AA = 100(DRC – DRS)/DRC, where AA is the antioxidant activity, DRC is the degradation rate of the control [= ln(a/b)/60], DRS is the degradation rate in the presence of the sample [= ln(a/b)/60], a is the initial absorbance at time 0, and b is the absorbance at 60 min. Extracts and polyphenol compounds were evaluated at the final concentrations of 1 mg/mL in the assay mixture. Both the scavenging and the antioxidant activities of the extracts were compared to those of pure phenolic compounds, namely gallic acid, (+)-catechin, chlorogenic acid, (–)- epicatechin, and catechol.
Antitumor activity
In vitro bioassay test
Human cervical adenocarcinoma cells (HeLa line) from the American type culture collection (Rockville, MD) were grown and maintained in Dulbecco’s modified Eagle medium (DMEM) containing 1 g/mL of glucose, and supplemented with 10% (v/v) fetal bovine serum (FBS), l-glutamine (2 mM), penicillin (50 U/mL), and streptomycin (50 μg/mL). The cells were grown in an incubator at 37°C, in a 5.1% CO2 humidified atmosphere.
Tumor growth inhibition was determined by the WST-1 assay (CitationChoudhary et al., 2006; CitationWang et al., 2006). Exponentially growing cells were seeded on 96-well plates at a density of 7 × 103 cells/well. After incubation for 24 h at 37°C, cells were treated with several dilutions of the extracts (400–25 μg/mL), and incubated for 24, 48, and 72 h. Two hours before the end of incubation, 20 μL of WST-1 were added to each well and further incubated for 2 h at 37°C, and the optical density (OD) was measured at 450 nm on a microplate reader (Power Wave XS spectrophotometer). The cytotoxicity of the extracts was expressed as the percentage viability of the cells, half maximal inhibitory concentration (IC50), and maximal degree of inhibition.
Statistical analysis
The experimental results are expressed as mean ± standard deviation (SD). All experiments were conducted in triplicate, and all tests and measurements were repeated at least three times. The data were analyzed using the analysis of variance (ANOVA) method to assess differences with the SPSS statistical package for Windows (release 15.0; SPSS Inc.), and significance between means was tested by Duncan’s new multiple range test (p = 0.05). The correlation between antioxidant and free-radical scavenging activities versus the polyphenol, tannin, and flavonoid contents was determined using the correlation and regression program of Microsoft Excel. IC50 values were calculated by sigmoidal fitting of the data in the GraphPad Prism V 4.0 microcomputer program.
Results
Polyphenol contents
The total content of phenol compounds was not different between extracts from trees of different genders (p > 0.05). Among female trees, the total amount of phenols varied among different cultivars; the levels of these compounds were markedly higher in the female cultivars Galhosa, Aida, and Preta de Lagos than in the cultivars Mulata and Gasparinha ().
Table 1. Contents of total phenol compounds, condensed tannins and total flavonoids in methanol leaf extracts of male, female and hermaphrodite carob trees.
The amount of condensed tannins varied significantly between the different genders and cultivars (p < 0.05), ranging from 1.8 to 23.7 mg CE g−1, with the lowest amount obtained from extracts from the female cultivar Preta de Lagos and the highest amount obtained from the female cultivar Galhosa and hermaphrodite tree H1 (). We found that the leaves of the hermaphrodites were richer in tannins, and the level of these compounds was markedly higher in tree H1. In female cultivars, tannins were present in highest amounts in the cultivar cultivar Galhosa (). While there was no significant difference in total flavonoid contents of extracts from different genders of tree, significant differences were observed between female cultivars (p < 0.05) (). Among the female cultivars, Galhosa and Mulata had the highest and lowest values, respectively, while among hermaphrodites, flavonoids were present in the highest amounts in leaves from tree H2 (). Among males, M2 had the highest content of these compounds ().
Radical scavenging activity (RSA)
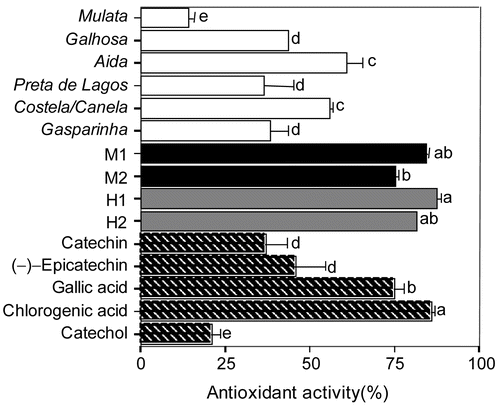
The extracts showed a significant RSA (), which was concentration-dependent (data not shown). There was no gender-based variation in RSA (p > 0.05), but there was a significant variation in RSA between extracts taken from different cultivars and different individual trees (p < 0.001). Among the female cultivars, Galhosa exhibited the highest activity, denoted by the lowest IC50 and highest antiradical efficiency values, while among the male and hermaphrodite trees, M1 and H2 exhibited the highest RSA, respectively (). Furthermore, the RSAs of extracts from the three genders were similar to those displayed by chlorogenic acid and (+)-catechin.
Table 2. DPPH radical scavenging activity of methanol leaf extracts from female, male and hermaphrodite carob trees, and pure polyphenolic compounds.
A correlation analysis was conducted between the RSA and the amount of phenols, tannins, and flavonoids in all of the trees studied, and in the female cultivars (). For all trees studied, the RSA was closely correlated to the total amount of phenols and flavonoids, but was not correlated to the amount of tannins in the extracts. In extracts from female trees, the amount of total phenols and of flavonoids was very closely correlated with the RSA.
Table 3. Correlation coefficients (R) between radical scavenging activity (RSA), antioxidant activity (AA) and contents of total phenols (GAE), tannins (CE) and flavonoids (RE).
Antioxidant activity in the linoleic acid system
Significant gender-based differences in the antioxidant activity of the extracts were observed (p < 0.05), with males and hermaphrodites having the highest activities (). Overall, extracts from males and hermaphrodites are better than or as good at preventing the discoloration of β-carotene as pure phenolic compounds. As for the RSA, a correlation analysis was conducted between the AA and the amount of phenols, tannins, and flavonoids in extracts of all trees studied, and in extracts from the female cultivars (). For extracts from females, high correlation coefficients were found between the AA and the total amount of flavonoids, but not between the AA and the total amount of phenols or tannins. When all samples were considered, there was no correlation between the analyzed parameters.
Figure 1. Antioxidant activity of methanol leaf extracts from female, male, and hermaphrodite carob trees, and of pure polyphenol compounds. Values represent means ± SD of three assessments of the percent inhibition of autoxidation of the linoleic acid/β-carotene emulsion. Extracts and phenol compounds were evaluated at final concentrations of 1 mg/mL. Values followed by different letters are significantly different at p < 0.05 (one-way ANOVA, Duncan’s new multiple range test).
Antitumor activity
Except for the female cultivar Galhosa, whose extracts had no effect on HeLa cell proliferation, carob extracts significantly inhibited HeLa cell proliferation in a dose-dependent manner (p < 0.05) (). The arrest of HeLa cell growth was not time-dependent and was already evident after a treatment lasting 24 h. For the highest concentration tested (400 μg/mL), cell survival was generally below 25% (). There was significant variation in bioactivity between the extracts from different genders of tree. Regardless of the concentration, treatment with extracts from males and hermaphrodites resulted in a significantly higher decrease in cell proliferation than treatment with extracts from females (p < 0.01). Extracts from the hermaphrodite tree H1 produced the highest inhibition capacity (IC50 < 25 μg/mL), while extracts from the female cultivar Galhosa were the least effective at preventing the proliferation of HeLa cells (IC50 > 400 μg/mL) (). The maximal inhibition values were high (generally 100%) (). A correlation analysis was performed between cytotoxic activity and RSA for all the trees studied and for the female cultivars, but no relationship was observed (data not shown).
Figure 2. Effect of treatment with methanol leaf extracts from female (A), hermaphrodite (B), and male (C) carob trees on cell proliferation. Each point represents the mean of three independent experiments.
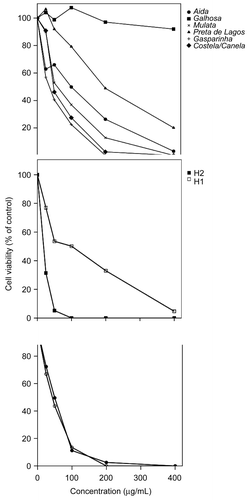
Table 4. Inhibitory concentrations (IC50) and maximal degree of inhibition (Max.) of methanol leaf extracts of female, hermaphrodite and male carob trees on HeLa cells.
Discussion and conclusions
The idea of using leaves of carob, a typical Mediterranean tree, as a source of polyphenols, was raised after the evidence that carob leaves contain a high amount of these compounds (CitationCorsi et al., 2002). Our results support these findings since in this study, except for female cv. Mulata, all the cultivars and trees displayed remarkably high levels of total phenolic content, with GAE values >20 mg/g dry weight (DW) (CitationKähkönen et al., 1999). On the other hand, the amount of total phenols in leaf extracts from the carob tree were higher than those observed in Salvia spinosa L. (Lamiaceae), a plant species that is well known as a rich source of polyphenols (CitationAlali et al., 2007).
Cross-varietal screening tests have repeatedly shown that certain genotypes within a plant species can have widely divergent levels of inherent antioxidants (CitationLila, 2006). In this work, we observed a general variation in the amount of phenolic compounds between extracts from different carob tree genders and different cultivars (). Working with the same species, CitationAvallone et al. (1997) observed different levels of total polyphenols in different organs of female trees, and among trees from different locations. CitationEmmons and Peterson (2001) also found significant differences among cultivars in concentrations of the majority of the phenol compounds measured in Avena sativa L. (Poaceae). Similar results were found by other authors in different plant species (Cetkovic et al., 2004; Maksimović et al., 2005; Gattuso et al., 2007; Li et al., 2007).
In this study, the free radical scavenging activity (RSA) of leaf extracts was assessed against DPPH radicals, and their antioxidant activity (AA) was assessed using the β-carotene–linoleic acid system. The extracts showed a significant RSA (), suggesting that they are capable of scavenging free radicals, and thus, may be able to prevent the initiation of free radical-mediated chain reactions by preventing the abstraction of hydrogen from susceptible polyunsaturated fatty acids. On the other hand the RSA varied between extracts from different genotypes. There was a significant gender-based difference in the AA of the extracts, and overall, extracts from males and hermaphrodites were equally or better able to prevent the discoloration of β-carotene than pure phenolic compounds ().
Extracts with a higher phenol or flavonoid content generally show higher antioxidant activity, and good correlations have been found among these parameters (CitationAlali et al., 2007; CitationLi et al., 2007). Generally, the RSA was closely correlated to the total amount of phenols and flavonoids suggesting that these compounds are major contributors to the RSA of the extracts (). These results agree with previous reports which found that phenols and flavonoids contribute significantly to the RSA of different plant species (CitationParejo et al., 2003; CitationAlali et al., 2007).
Interestingly, despite the fact that the extracts have a higher content of tannins than flavonoids, no significant correlation was found between RSA and the amount of tannins in an extract (), suggesting that flavonoids may be the major contributor to the RSA of the extracts. Flavonoids do indeed show strong antioxidant and radical scavenging activities (CitationPietta, 2000), and the use of flavonoid-containing drugs is associated with a reduced risk for certain chronic diseases, some cardiovascular disorders, and certain types of cancerous processes (CitationKris-Etherton et al., 2002). Their antiradical properties stem from targeting ROS that are implicated in the initiation of lipid peroxidation. Moreover, flavonoids are soluble chain-breaking inhibitors of the peroxidation process. They scavenge intermediate peroxyl and alkoxyl radicals and chelate metal ions, the latter of which are among the major components in the initiation of radical reactions (CitationPietta, 2000).
For female cultivars, high correlation coefficients were found between the AA and the total flavonoid content, but not with total phenol or tannin content (). When we considered all samples together, there was no correlation between the analyzed parameters (). These results are consistent with the findings of other authors in the same species (CitationAlali et al., 2007), and suggest that apart from phenols and flavonoids, other compounds in the extracts may also contribute significantly to the antioxidant activity of carob tree leaf extracts. Moreover, a plant with high antioxidant activity, for which phenolic content versus antioxidant activity falls above the regression line, should be a plant in which novel antioxidants may be found, and the carob tree is such a plant (CitationAlali et al., 2007).
The search for new therapeutic drugs for the treatment of cancer from natural products is based mainly on the cytotoxic properties of the natural samples (CitationGoldin et al., 1981). In this sense, the incubation of HeLa cells with carob leaf extracts resulted in a remarkable concentration-dependent decrease of cell proliferation. Such an effect has been previously reported in several cancer cell cultures, after the application of different plant extracts (CitationWedge et al., 2001; CitationChen et al., 2004; CitationRamos et al., 2005). Cytotoxicity and the inhibition of cell proliferation are also displayed by several anticancer drugs derived from natural materials that are in wide clinical use, such as vincristine and vinblastine from Catharanthus roseus G. Don (Apocynaceae), paclitaxel (Taxol®) and taxotere from species of yew (Taxus), etoposide derived from lignans of Podophyllum spp. (Berberidaceae), and camptothecin analogs, such as topotecan, from Camptotheca acuminate D. (Nyssaceae) (CitationHoughton et al., 2007).
Interestingly, the arrest of HeLa cell growth after treatment was not time-dependent and was already evident after a treatment lasting 24 h. This indicates that the polyphenols in the extracts promptly initiated a series of cellular events leading to the inhibition of cell proliferation and/or the induction of cell death. However, it should be noted that by using the WST-1 assay it is not possible to differentiate between cell growth inhibition and an increase in cell death. In this regard, data from studies focusing on elucidation of the molecular basis of the putative anticancer activity of polyphenols indicate that both the inhibition of cell growth and the induction of cell death play a role in the antitumor activity of polyphenols (CitationWenzel et al., 2000; CitationLazzè et al., 2004).
The bioactivity of the extracts varied between different cultivars and trees tested (). Similarly, the extracts from male and hermaphrodite trees showed a higher antitumor activity, which also corresponds to the AA results. The RSA of the different extracts was directly correlated to the total amount of phenols and flavonoids, but there was no correlation relationship between RSA and antitumor activity (data not shown). These results suggest that the inhibition of tumor cell proliferation in vitro by the methanol leaf extracts of carob cannot be solely explained by the concentration of phenol/flavonoid compounds. Other phytochemicals may play a significant role in the antiproliferative activity.
This is the first time that the carob tree has been investigated for antitumor activity against human cancer cells. Taken together with results for the phenolic profile and antioxidant activities, our results suggest that the methanol leaf extracts possess exploitable antioxidants, and that they could be a source of phenolic compounds with potential antitumor activity. Our findings therefore serve as a prelude to in vitro and in vivo studies that may lead to verification of the efficacy of the antitumor activities of carob leaf extracts against various human cancers, elucidation of the mechanisms of their cytotoxic activity, and identification of the active components.
Declaration of interest: This work was partially supported by CICYT (CTQ2006-03794/BQU), Instituto de Salud Carlos III (CB06_01_0074 and PI060624), the Generalitat de Catalunya (2005SGR 00662), the Institute for Research in Biomedicine, and the Barcelona Science Park. One of the authors (L.C.) thanks the Portuguese Foundation for Science and Technology (FCT) for a post-doctoral grant (grant SFRH/BPD/20736/2004).
References
- Alali F, Tawaha K, El-Elimat T, Syouf M, El-Fayad M, Abulaila K, Nielsen S, Wheaton W, Falkinham J, Oberlies N (2007): Antioxidant activity and total phenolic content of aqueous and methanol extracts of Jordanian plants: an ICBG project. Nat Prod Res 21: 1121–1131.
- Arnous A, Makris D, Kefalas P (2001): Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J Agric Food Chem 49: 5736–5742.
- Avallone R, Plessi M, Baraldi M, Monzani A (1997): Determination of chemical composition of carob (Ceratonia siliqua): protein, fat, carbohydrates, and tannins. J Food Comp Anal 10: 166–172.
- Avallone R, Cosenza F, Farina F, Baraldi C, Baraldi M (2002): Extraction and purification from Ceratonia siliqua of compounds acting on central and peripheral benzodiazepine receptors. Fitoterapia 73: 390–396.
- Baumgartner S, Genner-Ritzmann R, Haas J, Amadò R, Neukom H (1986): Isolation and identification of cyclitols in carob pods (Ceratonia siliqua L.). J Agric Food Chem 34: 827–829.
- Brand-Williams W, Cuvelier ME, Berset C (1995): Use of a free radical method to evaluate antioxidant activity. Lebens-Wiss Technol 28: 25–30.
- Cetkovic G, Djilas S, Canadanovic-Brunet J, Tumbas V (2004): Antioxidant properties of marigold extracts. Food Res Int 37: 643–650.
- Chen M, Chen D, Dou Q (2004): Inhibition of proteasome activity by various fruits and vegetables is associated with cancer cell death. In Vivo 18: 73–80.
- Choudhary M, Jali, A, Israr M, Rahman A (2006). Inhibition of respiratory burst in human neutrophils an lipoxygenase enzyme by compounds from Haloxylon griffithii. Phytother Res 20: 840–843.
- Corsi L, Avallone R, Cosenza F, Farina F, Baraldi C, Baraldi, M (2002): Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 73: 674–684.
- Emmons C, Peterson D (2001): Antioxidant activity and phenolic content of oat as affected by cultivar and location. Crop Sci 41: 1676–1681.
- Gattuso G, Barreca D, Caristi C, Gargiulli C, Leuzzi U (2007): Distribution of flavonoids and furocoumarins in juices from cultivars of Citrus bergamia Risso. J Agric Food Chem 55: 9921–9927.
- Goldin A, Venditt JM, MacDonald JS, Muggia FM, Henney JE, Devita VT (1981): Current results of the screening program at the division of cancer treatment, National Cancer Institute. Eur J Cancer 17: 129–142.
- Greenspan P, Bauer J, Pollock S, Gangemi J, Mayer E, Ghaffar A, Hargrove J, Hartle D (2005): Antiinflammatory properties of the muscadine grape (Vitis rotundifolia). J Agric Food Chem 53: 8481–8484.
- Houghton PJ, Fang R, Techatanawat I, Steventon GJ, Hylands PJ, Lee CC (2007): The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 42: 377–387.
- Julkunen-Tiitto R (1985): Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J Agric Food Chem 33: 213–217.
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M (1999): Antioxidant activity of plant extracts containing phenolic compounds. J Agri Food Chem 47: 3954–3962.
- Kivçak B, Mert T, öztürk H (2002): Antimicrobial and cytotoxic activities of Ceratonia siliqua L. extracts. Turk J Biol 26: 197–200.
- Kris-Etherton P, Hecker K, Bonanome A, Coval S, Binkoski A, Hilpert K, Griel A, Etherton T (2002): Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am J Med 113: 71–88.
- Kumazawa S, Taniguchi M, Susuki Y, Shimura M, Kwon M-S, Nakayama T (2002): Antioxidant activity of polyphenols in carob pods. J Agric Food Chem 50: 373–377.
- Kwon Y-I, Apostolidis E, Shetty A (2008): In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 3 diabetes and hypertension. Bioresource Technol 99: 2981–2988.
- Lamaison L, Carnat A. (1990): Teneurs en acide rosmarinique, en dérivés hydroxycinnamiques totaux et activités antioxydantes chez les Apiacées, les Borraginacées et les Lamiacées médicinales. Pharm Acta Helv 65: 315–320.
- Lambert J, Hong J, Yang G-Y, Liao J, Yang C (2005): Inhibition of carcinogenesis by polyphenols: Evidence from laboratory studies. Am J Clin Nutr 81: 284S–291S.
- Lazzè M, Savio M, Pizzala R, Cazzalini O, Perucca P, Scovassi A, Stivala L, Bianchi L (2004): Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis 25: 1427–1433.
- Li W, Gao Y, Zhao J, Wang Q (2007): Phenolic, flavonoid, and lutein ester content and antioxidant activity of 11 cultivars of Chinese marigold. J Agric Food Chem 255: 8478–8484.
- Lila M (2006): The nature-versus-nurture debate on bioactive phytochemicals: The genome versus terroir. J Sci Food Agric 86: 2510–2515.
- Loeb H, Vandenplas Y, Wursch P, Guesry P (1989): Tannin-rich carob pod for the treatment of acute-onset diarrhea. J Pediatr Gastr Nutr 8: 480–485.
- Maksimović Z, Malenčić D, Kovačević N (2005): Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresource Technol 96: 873–877.
- Makris D, Kefalas P (2004): Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxidants. Food Technol Biotechnol 42: 105–108.
- Moreno S, Scheyer T, Romano C, Vojnov A (2006): Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res 40: 223–231.
- Owen R, Haubner R, Hull W, Erben G, Spiegelhalder B, Bartsch H, Haber B (2003): Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem Toxicol 41: 1727–1738.
- Papagiannopoulos M, Wollseifen H, Mellenthin A, Haber B, Galensa R (2004): Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MS. J Agric Food Chem 52: 3784–3791.
- Parejo I, Viladomata F, Bastida J, Rosas-Romero A, Saavedra G, Murcia M. Jiménez A, Codina C (2003): Investigation of Bolivian plant extracts for their radical scavenging activity and antioxidant activity. Life Sci 73: 1667–1681.
- Pietta PG (2000): Flavonoids as antioxidants. J Nat Prod 63: 1035–1042.
- Ramos S, Alia M, Bravo L, Goya L (2005): Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (HepG2). J Agric Food Chem 53: 1271–1280.
- Song J, Park K, Lee K, Byun Y, Park J, Kim S, Kim J, Seong B (2007): Biological evaluation of anti-influenza viral activity of semi-synthetic catechin derivates. Antiviral Res 76: 178–185.
- Sugihara N, Arakawa T, Ohnishi M, Furuno K (1999): Anti- and pro-oxidative effects of flavonoids on metal induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes located with α-linolenic acid. Free Radic Biol Med 27: 1313–1323.
- Tepe B, Akpulat A, Sokmen M, Daferera D, Yumrutas O, Aydin E, Polissiou M, Sokmen A (2006): Screening of the antioxidative and antimicrobial properties of the essential oils of Pimpinella anisetum and Pimpinella flabellifolia from Turkey. Food Chem 97: 719–724.
- Valko M, Leibfritz D, Moncol J, Cronin M, Mazur M, Telser J (2007): Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84.
- Yamagishi M, Natsume M, Nagaki A, Adachi T, Osakabe N, Takizawa T. Kumon H, Osawa T (2000): Antimutagenic activity of cacao: inhibitory effect of cacao liquor polyphenols on the mutagenic action of heterocyclic amines. J Agric Food Chem 48: 5074–5078.
- Wang Y, Deng T, Lin L, Pan Y, Zheng X (2006): Bioassay-guided isolation of antiatherosclerotic phytochemicals from Artocarpus altilis. Phytother Res 20: 1052–1055.
- Wedge D, Meepagala K, Magee J, Smith S, Huang G, Larcom L (2001): Anticarcinogenic activity of strawberry, blueberry, and raspberry extracts to breast and cervical cancer cells. J Med Food 4: 49–51.
- Wenzel U, Kuntz S, Brendel M, Daniel H (2000): Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res 60: 3823–3831.
