Abstract
Medical care for transgender people is multi-faceted and attention to individual reproductive aspirations and planning are an essential, yet often overlooked aspect of care. Given the impact of hormonal therapy and other gender affirmation procedures on reproductive function, extensive counselling and consideration of fertility preservation is recommended prior to their commencement. This review article explores the reproductive aspirations of transgender women and considers the current disparity between stated desires regarding utilisation of fertility preservation services. Current fertility preservation options and prospective treatments currently showing promise in the research arena are explored.
Introduction
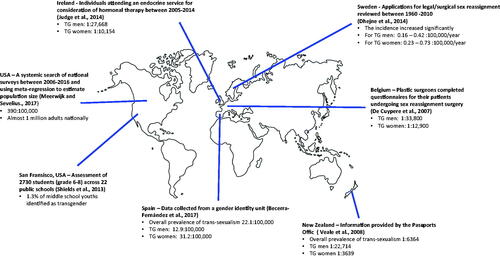
Gender dysphoria is defined as a feeling of discomfort or distress arising from a disjunction between a person’s felt gender and their gender assigned at birth (American Psychiatric Association, Citation2013). While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) reports the prevalence of gender dysphoria to be 5–14 per 1000 of those assigned male at birth, and 2–3 per 1000 of those assigned female, these figures are based upon those who seek specialist care, and as such, exclude those who do not present to services (American Psychiatric Association, Citation2013; Karasic & Drescher, Citation2006). An extensive literature search and meta-regression model analysis indicate that the transgender population of the USA is approximately one million (Meerwijk & Sevelius, Citation2017). While there are no validated figures for the UK, it is estimated that 300,000 people exhibit some degree of gender dysphoria, which may be defined as behaviour or gender expression that does not match masculine or feminine gender norms (Reed et al., Citation2009). As such, the reported true prevalence of gender dysphoria among children, adolescents and adults is more likely between 0.5% and 2% (Crissman et al., Citation2017; Goodman et al., Citation2019). Prevalence rates vary internationally, which is often attributed to local, social, and cultural demographics that can affect perceptions and acceptability, as summarised in (Becerra-Fernández et al., Citation2017; De Cuypere et al., Citation2007; Dhejne et al., Citation2014; Judge et al., Citation2014; Meerwijk & Sevelius, Citation2017; Shields et al., Citation2013; Veale, Citation2008).
Whilst founding a family is regarded as a fundamental aspect of individual and social life, trans and gender variant individuals remain a cohort of individuals for whom the right to reproduce and genetically parent remains ill-defined, and one that is breached more often than observed (Lauterpacht, Citation1948; McGuinness & Alghrani, Citation2008). A recent controversial High Court case ruled that children are highly unlikely to be able to consent to puberty blockers (EPATH, Citation2020). The Bell v. Tavistock case has brought the topic of trans reproduction and fertility preservation to the forefront of discussions (Beattie, Citation2022). This, alongside the increasing willingness of The European Court of Human Rights to scrutinise laws in European states which mandate transgender sterilisation requirements and the increasing ways in which novel assisted reproductive technologies may facilitate fertility preservation and genetic reproduction post transition, it is both timely and imperative that discussion and debate around fertility preservation and restoration takes place (Alghrani, Citation2018; Dunne, Citation2017). The aim of this review is to comprehensively summarise the reproductive aspirations of transgender women, overview the fertility preservation options currently available, and introduce the prospect of reproductive realignment with uterine transplantation.
Management of gender dysphoria
Treatment of gender dysphoria aims to achieve congruence, to allow those who experience it to feel comfortable within their bodies, thereby improving psychological wellbeing (Coleman et al., Citation2012). Not all individuals who experience gender dysphoria will seek treatment. Of those that do, many require only partial treatment and/or social transition to achieve congruence, whereas others find comfort only after hormonal and/or surgical intervention, with the intention of changing genitalia and sexual characteristics (Coleman, Citation2017). Bottom surgeries and hormonal interventions aimed at physical realignment of a transgender person’s body with their gender identity may result in infertility, forcing those with reproductive aspirations to make difficult choices between alleviating their dysphoria and fulfilling their reproductive goals. Treatment is therefore multi-faceted and dynamic, necessitating individualisation according to circumstance and values, including consideration of future reproductive aspirations. Although the number of transgender people presenting to specialised services is increasing, they remain at high-risk of poor medical outcomes. This has been attributed to a multitude of reasons including a knowledge gap in management and long-term outcomes (Olson-Kennedy et al., Citation2016), lack of access to care, financial barriers, discrimination, and socioeconomic barriers (Safer et al., Citation2016). Moreover, this is compounded further by a lack of training and subsequent shortage of professionals with sufficient expertise, knowledge, confidence of transgender medicine (Sanchez et al., Citation2009; Unger, Citation2015; Vance et al., Citation2015). Qualitative studies demonstrate that most transgender youths, adolescents, adults (88%) and their parents (93%) prefer fertility preservation counselling to be provided by gender clinic physicians and one third would prefer mental health specialists or fertility specialists to provide counselling (Quain et al., Citation2021). It is therefore important that healthcare professionals across all disciplines are trained in the provision of care.
For those at pre or peripubertal ages (i.e., those who have not yet reached Tanner Stage V), the aim of hormonal therapy is twofold: (i) to suppress puberty; and (ii) for gender affirmation. Gonadotrophin releasing hormone (GnRH) analogues can be administered to delay puberty and provide time to determine a comprehensive and individualised treatment plan (Coleman, Citation2017; Marshall & Tanner, Citation1970). Gender-affirming oestrogen based hormonal therapy has been shown to improve symptoms of dysphoria, psychological wellbeing, sexual function, and overall quality of life (Costa & Colizzi, Citation2016; Murad et al., Citation2010). However, long-term oestrogen administration may impair spermatogenesis and deplete Leydig cells in testicular tissue (Jindarak et al., Citation2018; Lübbert et al., Citation1992; Matoso et al., Citation2018; Schulze, Citation1988; Thiagaraj et al., Citation1987; Venizelos & Paradinas, Citation1988). Although the use of low dose ethinyl oestradiol does not impair sperm motility, impairment has been demonstrated at higher doses (Lübbert et al., Citation1992). Long-term oestrogen use is also associated with histological changes to testicular tissue, including germ cell hypoplasia, hypoplasia, or absence of Leydig cells and epididymal hyperplasia (Matoso et al., Citation2018). The negative impact of hormonal therapy on reproductive function has not been consistently shown and data remains conflicting (Schneider et al., Citation2017). Comparison studies assessing the difference in sperm parameters in transgender women with current, previous, or no exposure to gender-affirming hormonal treatments have shown that those undergoing current gender-affirming hormone treatment had poorer sperm parameters (Adeleye et al., Citation2019). However, sperm parameters in those with previous use were comparable to those with no use, suggesting potential reversibility (Adeleye et al., Citation2019). Moreover, data from transgender women on long-term hormonal therapy prior to orchidectomy identified the majority (80%) still had germ cells present, with function maintained in 40% (Jiang et al., Citation2019). Although the duration of hormonal therapy did not impact preservation of germ cells, commencing therapy at a younger age appeared to be associated with the presence of fewer germ cells.
Traditionally, little attention has focussed on the reproductive aspirations of transgender people: some European countries even mandated sterilisation as part of the legal gender confirmation process (Dunne, Citation2017). However, The European Court of Human Rights has declared such legal mandates to be a breach of the individual's Article 8 right to a private and family life. In recent years, there has been a greater emphasis on the importance of fertility preservation counselling among transgender populations (Nahata et al., Citation2016). The World Professional Association for Transgender Health and the Endocrine Society now recommends comprehensive fertility counselling prior to commencing treatment in both adolescents and adults (Coleman, Citation2017; Deutsch & Feldman, Citation2013; Hembree et al., Citation2017). This has been reiterated by the American Society for Reproductive Medicine Ethics Committee, who emphasises fertility preservation counselling plays a role in avoiding the unethical exclusion of transgender individuals from assisted reproductive services (Ethics Committee of the American Society for Reproductive Medicine, Citation2015).
Reproductive aspirations
Reproductive and sexual health is an important consideration for transgender individuals including contraception, prevention of sexually transmitted infections, family planning and fertility treatment (Mehringer & Dowshen, Citation2019). Despite this, it is often overlooked both clinically and academically. This is epitomised by a recent review which identified <1% of published literature related to transgender medicine addressed the topic of reproduction, and where reproduction was discussed, the focus was primarily on ethical concerns rather than clinical outcomes (Wanta & Unger, Citation2017).
summarises the fertility aspirations among transgender populations reported in 9 published studies. Desire for parenthood among transgender women appears to match those held by cisgender women, with many stating they would consider fertility preservation if given the opportunity (Neblett & Hipp, Citation2019). Despite international recommendations from professional bodies, detailed fertility counselling is not universally undertaken (Coleman, Citation2017; Hembree et al., Citation2017; Shumer et al., Citation2016). Indeed, while surveys of healthcare workers from medical/psychiatric backgrounds, demonstrate high levels of knowledge regarding fertility preservation in transgender populations, this is not reflected in practice (Chen et al., Citation2019), and is therefore similar to the early literature which described how men with cancer did not always receive adequate opportunity to bank sperm prior to their treatment (Pacey & Eiser, Citation2011). A recent online survey of 156 individuals identified that while 26-44% of transgender or gender non-conforming youths expressed an interest in future biological parenthood, only 21% discussed fertility implications with clinicians, and just 14% were informed of the deleterious impact of hormonal therapy on their reproductive potential (Chen et al., Citation2018).
Table 1. Fertility aspirations and influencing factors in transgender women.
Appropriate counselling can lead to a high uptake of fertility preservation
Although some clinics report that 82% of transgender women receiving adequate counselling pursue fertility preservation, high rates have not been consistently reported and uptake can range from 7% to 82%. Fertility preservation counselling does not always equate to high uptake rates. In one clinic, most transgender youths declined preservation despite receiving fertility discussions, indicating that patient specific factors will affect an individual’s choice (Wakefield et al., Citation2019). As summarised in , fertility preservation uptake may remain low despite adequate fertility counselling, highlighting a potential mismatch between desire and uptake.
Table 2. Fertility preservation uptake rates and prohibiting factors in transgender women.
A number of barriers may explain the disparity between desires and uptake of fertility preservation, including financial considerations, a desire to not delay transition and a perception of contradiction or incompatibility with female identity (Auer et al., Citation2018; Chen et al., Citation2017; Jones, Reiter, et al., Citation2016; Millar et al., Citation2015; Persky et al., Citation2020; Wierckx et al., Citation2012). A survey-based questionnaire amongst transgender adults demonstrated that 90% did not perceive the loss of fertility important enough to delay transition, although 50% felt having biological offspring was important and 51% would have seriously considered fertility preservation had they received counselling (Pfäfflin et al., Citation2002). In a survey of 13 transgender adults, although many expressed an interest in parenthood, it was the mechanisms in place to achieve this, which differ from how women assigned female at birth reproduce and contradicted their beliefs towards parenthood and pregnancy (von Doussa et al., Citation2015). Other data has identified that transgender women may be deterred by the invasive nature of fertility preservation (Chen et al., Citation2017; Van Voorhis, Citation2007), or amid concerns future offspring may be similarly affected (Riggs & Bartholomaeus, Citation2018), owing to the potential genetic component to gender dysphoria.
Public perception may also dissuade transgender women from pursuing parenthood. In-depth interviews have raised concerns amongst transgender women that judgments will be made by friends and family for pursuing parenthood, leading to the breakdown of relationships (von Doussa et al., Citation2015). Public perceptions extend beyond stigma felt by friends and family. Over one fifth of transgender individuals report daily discrimination, harassment, and victimisation. Moreover, there have been reports of transgender people feeling victimised by healthcare providers (Kattari & Hasche, Citation2016). In insurance-based medical systems transgender individuals are also less likely than the general population to have health insurance coverage, adding a further barrier in the form of health discrimination (Dickey et al., Citation2016; Nahata et al., Citation2017). Legal barriers have also been described (Tornello & Bos, Citation2017). Reassuringly, public perception may be becoming more inclusive: a recent online survey of 986 US residents demonstrated that most respondents (76%) were supportive of transgender people having biological children (Goldman et al., Citation2017). Whilst some countries such as Sweden offer fertility preservation services to transgender individuals under the publicly funded healthcare system, this is not the case in other countries (Rodriguez-Wallberg et al., Citation2021).
Having biologically related children has been shown to be an important consideration for some transitioning individuals (Tornello & Bos, Citation2017). The decision to undergo fertility preservation seems closely aligned to the importance of having genetically related offspring; 71% of transgender adults who underwent fertility preservation rated having genetic relatedness as important (Riggs & Bartholomaeus, Citation2018). However, not all transgender women express desire for biologically related offspring (Chiniara et al., Citation2019; Strang et al., Citation2018).
Fertility preservation rates are higher among transgender women, compared to transgender men (Alpern et al., Citation2022). Concerns regarding the fertility preservation process itself, fear of gender dysphoria caused by hormonal treatment and concerns regarding the attitude of medical staff were more likely to dissuade transgender men from fertility preservation when compared with transgender women (Alpern et al., Citation2022). Higher fertility preservation rates among transgender women than transgender men have also been demonstrated by others; interestingly, the opposite trend was seen in transgender adolescents (Amir et al., Citation2020). Being older and not having had gender-affirming hormonal treatment was associated with higher rates of fertility preservation among transgender women (Amir et al., Citation2020).
Age at presentation to services will also impact reported fertility aspirations. One study among adult transgender women with a median age of 41 years, concluded that with every year of advancing age, the desire to have children drops by 5.3% (Auer et al., Citation2018). Transgender women typically present at a later age than men, impacting both their reproductive desires and options (Couch et al., Citation2007). Results from an Israeli clinic showed that presentation at an earlier age increased the likelihood of undergoing fertility preservation (Segev-Becker et al., Citation2020). Contrastingly, other studies have identified that younger transgender girls are less likely to utilise fertility preservation (Brik et al., Citation2019). The influence of age is therefore not universal, whereas cultural and social aspects have been shown to be highly influential (Segev-Becker et al., Citation2020). Additionally, ethnicity may also play a role with suggestion that Caucasian transgender girls are less likely to pursue fertility preservation (Brik et al., Citation2019).
In those who already have children prior to the onset of treatment, future desire for children may be reduced, as is the case among the general population (Stöbel-Richter et al., Citation2005). However, results from a transgender centre in Germany demonstrated that having children prior to gender-affirming treatment did not necessarily impact future desire for further offspring (Auer et al., Citation2018). As the average age of transition decreases, a smaller proportion will have children prior to transition, thereby amplifying the need to consider future reproductive aspirations. This is exemplified by a recent study assessing reproductive aspirations among transgender women where almost three quarters of the cohort were aged 16–30. Although <10% had children prior to transition, 95% held the desire for children in the future (Jones, Rajamanoharan, et al., Citation2020).
Fertility preservation
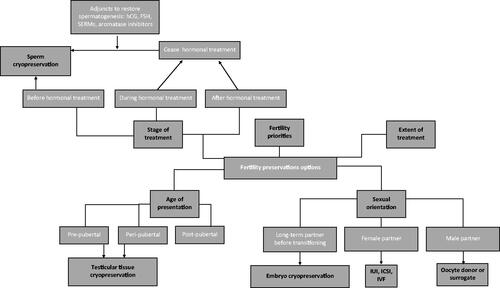
Transgender women are now presented with viable options regarding the preservation of their reproductive potential, as summarised in . Fertility preservation can take place prior to transition, utilising sperm cryopreservation, with subsequent in-vitro fertilisation (IVF) or intra uterine insemination (IUI) with a female partner or surrogate using donor oocytes. However, sperm cryopreservation is not possible in those wishing to undergo treatment prior to the onset of sexual maturity, owing to the absence of mature sperm. In this population, electroejaculation and testicular biopsies can be carried out in early puberty before virilisation (Parikh et al., Citation2021; Peri et al., Citation2021). Each option presents advantages and disadvantages, which vary depending on individual circumstance. To avoid compromising future reproductive aspirations and risk involuntary childlessness, it is essential extensive reproductive counselling takes place prior to hormonal or surgical therapy (Martinez, Citation2017). The key to optimising patient-centred care and shared decision-making is the provision of accurate, supportive, and empowering counselling. It should explore all available options to ameliorate the adverse, and potentially irreversible impact of hormonal and surgical treatment on fertility. It should also be individualised, respecting differences in priorities and preferences among patients who may legitimately privilege speedy transition over fertility preservation and/or be dissuaded by the experimental or invasive nature of certain options (Chen et al., Citation2017, Citation2018; Nahata et al., Citation2017; Pang et al., Citation2020).
Figure 2. Available methods of fertility preservation for transgender women and influencing factors.
Sperm cryopreservation
Sperm cryopreservation is an established and effective method of preserving male fertility. A systematic review and meta-analysis have shown that pregnancy and fertilisation rates with cryopreserved sperm are now comparable to rates with fresh sperm (Ohlander et al., Citation2014). In post-pubertal individuals, management options depend on whether hormonal therapy has commenced. Prior to hormonal therapy, or where evidence of spermatogenesis remains after commencement, sperm cryopreservation can be offered. In cases where spermatogenesis is not restored following the cessation of hormonal therapy, gonadotrophins, selective oestrogen receptor modulators (SERMs) and aromatase inhibitors can be used to facilitate the return of spermatogenesis, although evidence surrounding the usage of such agents remains limited (McBride & Coward, Citation2016; Wenker et al., Citation2015). Indeed, most data evaluating pharmacological restoration of spermatogenesis concern cisgender males, with a lack of data amongst transgender women. In men with hypogonadotrophic hypogonadism, administration of human chorionic gonadotrophin (hCG) has been shown to induce spermatogenesis (Vicari et al., Citation1992). Additionally, recombinant follicle stimulating hormone (FSH), SERMS (such as clomiphene citrate) and aromatase inhibitors (such as anastrazole and letrozole), can be used in adjunct to hCG to restore spermatogenesis in men following use of anabolic androgenic steroid use or exogenous testosterone use. In transgender women, successful restoration of testosterone levels and increased sperm motility have been reported following treatment with FSH and clomiphene citrate (Alford et al., Citation2020). A case of recovered spermatogenesis in an individual who had initiated treatment of gender dysphoria with leuprolide acetate has been reported, suggesting that sperm cryopreservation is an option for those who have commenced gender-affirming therapy (Barnard et al., Citation2019).
Sperm cryopreservation is not without its limitations. Sperm is usually acquired via masturbation, which can be problematic for transgender women (De Roo et al., Citation2016). For those with ejaculatory dysfunction, ejaculation can be achieved by neurostimulatory methods including penile vibratory stimulation and electroejaculation (McBride & Lipshultz, Citation2018). Hormonal therapy may also impair erectile and ejaculatory function, further challenging the acquisition of sperm through masturbation (Hembree et al., Citation2017). Other methods of acquiring sperm are by microsurgical testicular sperm extraction or by microsurgical epidydimal sperm aspiration (Schneider et al., Citation2017).
An additional point of consideration involves the cost of sperm cryopreservation. The average cost for sperm cryopreservation of one vial is $745 USD for the first year and $343 USD for each year thereafter (Gilbert et al., Citation2018). If sperm cryopreservation is performed earlier in childhood, fees will undoubtedly mount. Such costs may therefore limit the feasibility of sperm cryopreservation for some individuals.
Reproductive medicine clinics report an increase in fertility preservation referrals for transgender patients, particularly for semen banking. In one Swedish clinic, fertility preservation referrals increased by 60% on average per year and the proportion who went on to undergo sperm cryopreservation increased from 56% in 2013 to 91% in 2018 (Rodriguez-Wallberg et al., Citation2021). With increasing demand, an improved understanding of reproductive aspirations and options is required.
Embryo cryopreservation
Embryo cryopreservation may also be suitable for transgender women with female partners, or those that choose to use donor oocytes. Although this offers the benefit of having genetically related embryos stored with an existing partner, there are disadvantages, such as having to undergo IVF prior to transitioning, which can be invasive, time consuming and expensive. The additional possibility of subsequent relationship breakdown, which may render the stored embryos unusable or at least lead to disputes over their use, must also be considered. This risk may be amplified by the dynamic nature of sexual orientation following transition. For example, a study of 232 transgender women showed that while 54% were attracted to women before gender reassignment surgery, this reduced to just 25% post-operatively and while 9% were attracted to men pre-operatively, this increased to 34% following gender reassignment surgery (Lawrence, Citation2005).
Testicular tissue cryopreservation
For transgender women who transition prior to puberty and are therefore unable to undergo sperm cryopreservation, an alternative is testicular tissue cryopreservation. While still an experimental procedure from which no children have yet been produced, clinical trials are offering testicular tissue cryopreservation to pre-pubertal children who may have their reproductive potential compromised.
Testicular tissue can be obtained surgically and will contain spermatogonial stem cells which have the potential to either self-replicate or to differentiate into mature sperm. This was first theorised in 1994, when spermatogenesis was demonstrated after testicular tissue was harvested from a mouse and re-implanted into recipient mouse seminiferous tubules (Brinster & Zimmermann, Citation1994). A subsequent study successfully extracted and cultured immature mouse testicular tissue, enabling spermatogenesis to occur in vitro, following which intracytoplasmic sperm injection (ICSI) was used to reproduce offspring (Sato et al., Citation2011). However, human spermatogenesis is more complex, and in vitro human spermatogenesis requires a specific micro-environment, which has yet to be determined (Komeya et al., Citation2018). At present, human studies are focussing on optimising the process to achieve feasibility, including culture environments, cryoprotectants and preservation protocols (Baert et al., Citation2013). However, challenges extend beyond refining the technique, notably including issues regarding obtaining adequately informed and appropriate consent from children. This issue is epitomised by a questionnaire of pre-pubertal boys diagnosed with cancer which demonstrated that just 33.3% of those aged <12 were able to comprehend information given to them about fertility preservation (Wyns et al., Citation2015). This may be further complicated in the case of transgender patients due to increased potential for psychological co-morbidities and dysphoria surrounding fertility preservation itself (Chen & Simons, Citation2018). Nevertheless, at present, testicular tissue cryopreservation remains experimental and has only been applied to the human model in oncology cases.
Assisted reproductive technology
When fertility treatment is subsequently embarked upon, the cryopreserved sperm could be used for in-vitro fertilisation (IVF) or intra uterine insemination (IUI) with a female partner or surrogate using donor oocytes. Pregnancy rates from IUI are strongly influenced by the concentration and quality of sperm, requiring five to ten million total motile sperm (Dickey et al., Citation2001). A recent French series demonstrated no major impairment of semen parameters in transgender women who had not started gender affirming hormonal therapy when compared with sperm donors (Sermondade et al., Citation2021). However, analysis of sperm parameters in transgender women has revealed that, even before the onset of hormonal therapy, sperm quality both prior to cryopreservation and following warming, are poor (Hamada et al., Citation2015). This is confirmed by data showing that prior to gender-affirming treatment, semen parameters are lower in transgender women compared to the general population, even when body mass index, alcohol intake, cannabis use, and use of gender-affirming hormones are accounted for (de Nie et al., Citation2020). Other studies have similarly found transgender women to have poorer sperm parameters when compared to cisgender men, irrespective of gender-affirming hormonal treatment use (Li et al., Citation2018; Marsh et al., Citation2019; Rodriguez-Wallberg et al., Citation2021). When compared with fertile cisgender men, semen parameters including sperm concentration, total sperm per ejaculate, total motile sperm, volume, and sperm morphology were significantly lower in transgender women prior to the initiation of gender-affirming treatment (Marsh et al., Citation2019). Behaviours such as wearing tight underwear, keeping the genitals tight against the body, bringing the testicles into the inguinal canal and a low frequency of masturbation may all influence sperm function and quality (de Nie et al., Citation2020; Mieusset et al., Citation1987; Povey et al., Citation2012; Tiemessen et al., Citation1996). Lifestyle and medical factors that may influence semen parameters in transgender women should not be over-looked. For example, transgender women exhibit higher levels of stress, anxiety and depression when compared with cisgender men (Marsh et al., Citation2019). Stress, depression, and anxiety have been identified to negatively impact semen parameters (Li et al., Citation2011; Nordkap et al., Citation2016). Moreover, use of selective serotonin reuptake inhibitors for the treatment of depression is also associated with poorer semen quality (Drobnis & Nangia, Citation2017; Nørr et al., Citation2016). High rates of cigarette, alcohol and recreational drugs use has been reported among transgender groups, which may impact semen parameters (Li et al., Citation2011; Newcomb et al., Citation2020). When semen quality was analysed in a group of transgender women prior to the use of gender-affirming treatment, poor semen factors were not correlated with medical and lifestyle factors such as smoking, alcohol, depression, anxiety, antidepressant use or cannabis use, concluding that the cause for poorer semen parameters in transgender women without use of gender-affirming treatment is unclear and likely to be multi-factorial (Amir et al., Citation2022).
Subsequently, in order to acquire substantial samples for successful IUI, multiple ejaculatory samples are often required. Even among groups of transgender women with high uptakes of sperm cryopreservation, subsequent pregnancy rates remain low. In one group of transgender women cryopreserved sperm was only utilised by one in nine transgender women (Jones, Reiter, et al., Citation2016). In those with suboptimal sperm quality, IVF with intracytoplasmic sperm injection (ICSI) should be considered. Whereas reproductive outcomes in transgender women remain sparse, case reports have shown successful IVF outcomes, but highlighted healthcare providers need to expand their clinical experience owing to additional complexities in this population (Broughton & Omurtag, Citation2017). Larger studies exploring the IVF outcomes of transgender women are lacking, exposing a research priority to further improve access to reproductive services.
Reproductive realignment—Uterine transplantation
Uterine transplantation (UTx) is a surgical intervention that restores reproductive potential in women with absolute uterine infertility which allows them the opportunity to gestate and give birth to their own children. More than 70 cases of uterine transplantation in cisgender women have now been undertaken worldwide, and 23 livebirths have been reported (Jones et al., Citation2021). Following the rapid development of UTx and demonstration of its feasibility in cisgender women, speculation has escalated regarding the possibility of undertaking UTx in transgender women (Jones, Saso, et al., Citation2019; Lefkowitz et al., Citation2013; Murphy, Citation2015). Numerous psychosocial, ethical, and legal concerns, as well as significant anatomical and physiological challenges, must however, be considered and/or overcome prior to establishing the feasibility of UTx in this population (Alghrani, Citation2016a; Jones, Williams, et al., Citation2019).
Demand for uterine transplantation in transgender women
Uterine transplantation (UTx) and the ability to gestate may be welcome by trans women as a way of expressing and consolidating a maternal identity, namely a parental identity that aligns with gender identity (Kyweluk et al., Citation2018). Initial evidence suggested that less than a quarter (23.3%) of transgender women would consider UTx (Auer et al., Citation2018). However, a more recent questionnaire study exploring the perceptions of 182 transgender women demonstrated that 95% of respondents felt the risks associated with UTx were outweighed by the benefits (Jones, Rajamanoharan, et al., Citation2020), and highlighted the desire to experience physiological processes unique to cisgender women, such as menstruation and gestation. Moreover, three quarters of the cohort felt discrimination through alternative ways of acquiring parenthood, such as adoption and surrogacy. This desire to gestate has previously been identified to supersede the importance of having a biological child in some cases (Pfäfflin et al., Citation2002).
Surgical and hormonal considerations
Anatomical differences add additional surgical complexity to UTx in transgender women. Three key surgical challenges have previously been highlighted, including the vascular anastomoses, the neovaginal anastomosis and ligamentous support. Whereas minor modifications to the surgical technique may overcome the issues related to the major pelvic vessels and ligamentous insertion, the neovaginal anastomosis requires more consideration. The structure of the vaginal microbiome is associated with various clinical and reproductive implications that are vital in the process of UTx (Jones, Saso, et al., Citation2020). Skin lined neovaginas in transgender women have been demonstrated to be colonised with bacteria traditionally identified in skin, intestine, or bacterial vaginosis (Weyers et al., Citation2009), and be lactobacilli deplete in more than 95% of transgender women (Weyers et al., Citation2009). As such, the absence of physiologically functioning vaginal mucosa could interfere with successful UTx in transgender women (Jones, Williams, et al., Citation2019), and consideration of the method used for neovagina creation is now warranted.
The impact of exogenous hormonal therapy must also be considered. Following UTx, sequential hormone replacement therapy (HRT) would be preferable over the usually used continuous HRT regimen, as graft function can be determined by the presence of withdrawal bleeding (Jones, Williams, et al., Citation2019). In addition, withdrawal bleeding could facilitate the alleviation of gender dysphoria by mirroring menstruation (Brantelid et al., Citation2014; Jones, Williams, et al., Citation2019; Jones, Rajamanoharan, et al., Citation2020). Moreover, if anti-androgens such as finasteride or spironolactone are being taken, these should also be stopped in advance of fertility treatment, owing to their potential teratogenic effects.
Ethical considerations
Given the need to ensure justice and equality in access to medical treatment, and acknowledgement that the potential benefits transgender women may seek from UTx reflect those of women as a group more widely, there is a prima facie case for offering UTx to transgender women. However, some important ethical and practical concerns remain that may cast doubt on its appropriateness for this group.
These have been widely discussed by reproductive ethics scholars and there thus exists a wealth of literature exploring the different ethical and policy questions raised. These explore a diverse range of topics including the extent to which UTx is motivated by and contributes towards harmful social and cultural attitudes which valorise genetic and gestational parenthood (Lotz, Citation2016; Wilkinson & Williams, Citation2016). Other important considerations include: (i) informed consent from UTx recipients and donors; (ii) the use of living donors for quality of life-enhancing interventions; (iii) priority setting and funding for UTx and (iv) the extent to which the benefits of UTx, such as experiencing gestation and childbirth, can truly outweigh the potential harms risked to both recipient and the future child (Alghrani, Citation2016b; Catsanos et al., Citation2013; Wilkinson & Williams, Citation2016; Williams, Citation2016).
Welfare of the woman
Physicians aiming to provide UTx to transgender women must carefully consider whether or not, as in UTx in women assigned female at birth, the physical and psychological harms are likely to be outweighed by the benefits provided. While the benefits transgender women seek from UTx will generally be like those of other women with absolute uterine factor infertility (AUFI), the risks are potentially significantly greater. Thus, it may be the case that UTx in transgender women will simply prove too risky to justify. If so, then the position of transgender women in this respect would be like other groups excluded on the grounds of risk. This includes women with serious systemic diseases which is currently an exclusion criterion for UTx (Jones, Saso, et al., Citation2016). In cisgender women, the uterus is removed following the completion of their family, allowing the cessation of immunosuppressive medications. However, if UTx is undertaken to help alleviate gender dysphoria in addition to facilitate the achievement of reproductive aspirations, transgender women may desire permanent UTx, requiring lifelong immunosuppression. A recent perceptions study of transgender women regarding UTx highlighted that more than a third of transgender women (39%) were undecided or may refuse a hysterectomy following completion of their family (Jones, Rajamanoharan, et al., Citation2020). As the risks associated with immunosuppression, such as cancer and infection, are cumulative, this would increase the associated risk of the process significantly.
Welfare of the child
As with any new assisted reproductive technology, physicians considering performing UTx in transgender women are ethically obliged to consider the physical and psychological welfare of children who may be born as a result. In countries such as the UK, they are also legally mandated by the Human Fertilisation and Embryology Act (Citation2008) to consider the welfare of any resulting child prior to offering fertility treatment. Thus, if it can be shown that children born to transgender women through UTx are likely to suffer ‘serious harm’ this would cast doubt on the appropriateness of the procedure. In the context of undertaking UTx in women assigned female at birth, concerns have been raised regarding the physical risk exposed to the foetus because of gestation inside a donated uterus (Daar & Klipstein, Citation2016). These may be increased by unknown factors following UTx in transgender women. However, the risks posed to the developing foetus are unlikely to be greater than those in women with chronic medical conditions such as renal disease, diabetes, autoimmune diseases, and hypertensive disorders. Thus, provided adequate steps are taken to ensure a safe gestational environment for the developing foetus, UTx in transgender women is unlikely to prove any less ethically acceptable than other high-risk pregnancies.
Concerns may, however, be raised about potential psychological harm to children born following UTx. With regard to previous trials of UTx it has been noted that children may, like the first IVF babies, experience unwanted media attention, confusion or indeed abuse from others regarding their origin and perceived differences (Williams, Citation2016). These concerns may be exacerbated by the possible harms children of transgender parents may experience in terms of their self-perception, identity development, family relationships and social relationships. Yet, whilst such concerns warrant consideration there is a distinct absence of meaningful evidence that children born to transgender parents are injured in systematic or significant ways because of their parent’s gender identity (Murphy, Citation2012). Available studies on child outcomes suggest transgender parents are no less likely to exhibit supportive, stable, and loving parenting than cisgender parents (Green, Citation1998). Moreover, children with transgender parents are equally likely meet developmental milestones, and no more likely to exhibit distress and confusion regarding their gender or sexual identities (Stotzer et al., Citation2014). They are also unlikely to be harmed by knowledge of their parent’s gender identity, provided it is disclosed early in childhood (Chiland et al., Citation2013). Instead, it is more likely that psychological distress reported by such children may be secondary to harassment or discriminatory behaviour outside of the family unit. Such harms, as with racism and sexism, are best prevented through education of those who harass or discriminate, and not through preventing the births of those who are discriminated against or harassed. Moreover, given that children who are younger at the time of their parent’s transitioning demonstrate better adaptation and maintain healthier relationships with their parents, it is likely outcomes would improve further if they were born into their post-transition family unit (White & Ettner, Citation2007). As such there is also no compelling reason to refrain from trialling UTx in transgender women on the basis of child welfare.
Legal implications
Whereas legislation varies internationally, there remains dispute regarding whether transgender women would legally be permitted in England to undergo embryo transfer after UTx (Hammond-Browning., Citation2019; Jones, Alghrani, et al., Citation2019). Section 3(2) of the Human Fertilisation and Embryology Act (Citation2008), states ‘no person shall place in a woman a live embryo other than a permitted embryo’, and explains in section 3ZA that the terms “woman” and “man” include respectively a girl and a boy (from birth)” and that a permitted embryo refers to an unaltered embryo “created by the fertilisation of a permitted egg by permitted sperm.” Given these explanations, some have suggested that women need to have been assigned female from birth to undergo embryo transfer (Hammond-Browning, Citation2019). However, an alternative interpretation is that the reference to “a girl and boy (from birth)” is intended to refer to girls who have not yet become ‘women’ by definition with the aim of permitting the use, in fertility treatment, of cryopreserved oocytes and ovarian tissue obtained from individuals rendered infertile prior to the age of 16 (Jones, Alghrani, et al., Citation2019).
Even if the legislation did make the transfer of embryos illegal in transgender women, this would conflict with the Gender Recognition Act (Citation2004), which states a person’s gender becomes, for all purposes, the acquired gender, once a full gender recognition certificate has been issued. A transgender woman seeking UTx may also challenge the aforementioned provisions on the grounds that it breaches her Article 8 and 14 rights under the European Convention of Human Rights, in the absence of any persuasive reason or legitimate aim which justifies the interference. This is supported by the ESHRE Task Force which highlighted the denial of assisted reproduction clashes with human rights (De Wert et al., Citation2014). As such, if UTx proves feasible in transgender women, it is essential that governing regulations evolve concomitantly to meet the demands and reproductive aspirations of modern-day families.
Conclusion
One of the principal aims of the clinical management of gender dysphoria is the reduction of any disjunction between the person’s felt or experienced gender and their physiological characteristics and capacities. Taking transgender women’s reproductive aspirations seriously is therefore an important aspect of care and failing to do so could further harm. Further studies exploring the attitudes of transgender women to fertility are required. With a deeper understanding of their perspectives, barriers to accessing services can be more readily be overcome and services more effectively designed. For experimental techniques, such as testicular tissue cryopreservation and uterine transplantation, further scientific progress is required. In the meantime, the reproductive desires of transgender women should be approached with considerations of equality and wellbeing at the forefront of clinicians’ minds. Fertility preservation counselling should be part of routine care and transgender women’s reproductive plans should be considered and discussed in detail before any fertility-compromising treatment commences.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adeleye, A. J., Reid, G., Kao, C. N., Mok-Lin, E., & Smith, J. F. (2019). Semen parameters among transgender women with a history of hormonal treatment. Urology, 124, 136–141. https://doi.org/10.1016/j.urology.2018.10.005
- Alford, A. V., Theisen, K. M., Kim, N., Bodie, J. A., & Pariser, J. J. (2020). Successful ejaculatory sperm cryopreservation after cessation of long-term estrogen therapy in a transgender female. Urology, 136, e48–e50. https://doi.org/10.1016/j.urology.2019.08.021
- Alghrani, A. (2016a). Uterus transplantation: Does procreative liberty encompass a right to gestate? Journal of Law and the Biosciences, 3(3), 636–641. https://doi.org/10.1093/jlb/lsw048
- Alghrani, A. (2016b). Yes, uterus transplants should be publicly funded! Journal of Medical Ethics, 42(9), 566–567. https://doi.org/10.1136/medethics-2015-103231
- Alghrani, A. (2018). Regulating assisted reproductive technologies: New horizons. Cambridge University Press.
- Alpern, S., Yaish, I., Wagner-Kolasko, G., Greenman, Y., Sofer, Y., Paltiel Lifshitz, D., Groutz, A., Azem, F., & Amir, H. (2022). Why fertility preservation rates of transgender men are much lower than those of transgender women. Reproductive Biomedicine Online, 44(5), 943–950. https://doi.org/10.1016/j.rbmo.2022.01.003
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). APA. https://psychiatry.org/psychiatrists/practice/dsm
- Amir, H., Perl, L., Barda, S., Lantsberg, D., Becker, A. S., Israeli, G., Azem, F., & Oren, A. (2022). Adolescent transgender females present impaired semen quality that is suitable for intracytoplasmic sperm injection even before initiating gender-affirming hormone treatment. Reproductive Sciences (Thousand Oaks, Calif.), 29(1), 260–269. https://doi.org/10.1007/s43032-021-00561-y
- Amir, H., Yaish, I., Oren, A., Groutz, A., Greenman, Y., & Azem, F. (2020). Fertility preservation rates among transgender women compared with transgender men receiving comprehensive fertility counselling. Reproductive Biomedicine Online, 41(3), 546–554. https://doi.org/10.1016/j.rbmo.2020.05.003
- Auer, M. K., Fuss, J., Nieder, T. O., Briken, P., Biedermann, S. V., Stalla, G. K., Beckmann, M. W., & Hildebrandt, T. (2018). Desire to have children among transgender people in Germany: A cross-sectional multi-center study. The Journal of Sexual Medicine, 15(5), 757–767. https://doi.org/10.1016/j.jsxm.2018.03.083
- Baert, Y., Van Saen, D., Haentjens, P., In’t Veld, P., Tournaye, H., & Goossens, E. (2013). What is the best cryopreservation protocol for human testicular tissue banking? Human Reproduction (Oxford, England), 28(7), 1816–1826. https://doi.org/10.1093/humrep/det100
- Barnard, E. P., Dhar, C. P., Rothenberg, S. S., Menke, M. N., Witchel, S. F., Montano, G. T., Orwig, K. E., & Valli-Pulaski, H. (2019). Fertility preservation outcomes in adolescent and young adult feminizing transgender patients. Pediatrics, 144(3), e20183943. https://doi.org/10.1542/peds.2018-3943
- Beattie, C. (2022). High court should not restrict access to puberty blockers for minors. Journal of Medical Ethics, 48(1), 71–76. https://doi.org/10.1136/medethics-2020-107055
- Becerra-Fernández, A., Rodríguez-Molina, J. M., Asenjo-Araque, N., Lucio-Pérez, M. J., Cuchí-Alfaro, M., García-Camba, E., Pérez-López, G., Menacho-Román, M., Berrocal-Sertucha, M. C., Ly-Pen, D., & Aguilar-Vilas, M. V. (2017). Prevalence, incidence, and sex ratio of transsexualism in the autonomous region of Madrid (Spain) according to healthcare demand. Archives of Sexual Behavior, 46(5), 1307–1312. https://doi.org/10.1007/s10508-017-0955-z
- Brantelid, I. E., Nilvér, H., & Alehagen, S. (2014). Menstruation during a lifespan: A qualitative study of women’s experiences. Health Care for Women International, 35(6), 600–616. https://doi.org/10.1080/07399332.2013.868465
- Brik, T., Vrouenraets, L. J., Schagen, S. E., Meissner, A., de Vries, M. C., & Hannema, S. E. (2019). Use of fertility preservation among a cohort of transgirls in the Netherlands. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 64(5), 589–593. https://doi.org/10.1016/j.jadohealth.2018.11.008
- Brinster, R. L., & Zimmermann, J. W. (1994). Spermatogenesis following male germ-cell transplantation. Proceedings of the National Academy of Sciences of the United States of America, 91(24), 11298–11302. https://doi.org/10.1073/pnas.91.24.11298
- Broughton, D., & Omurtag, K. (2017). Care of the transgender or gender-nonconforming patient undergoing in vitro fertilization. International Journal of Transgenderism, 18(4), 372–375. https://doi.org/10.1080/15532739.2017.1352554
- Catsanos, R., Rogers, W., & Lotz, M. (2013). The ethics of uterus transplantation. Bioethics, 27(2), 65–73. https://doi.org/10.1111/j.1467-8519.2011.01897.x
- Chen, D., Kolbuck, V. D., Sutter, M. E., Tishelman, A. C., Quinn, G. P., & Nahata, L. (2019). Knowledge, practice behaviors, and perceived barriers to fertility care among providers of transgender healthcare. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 64(2), 226–234. https://doi.org/10.1016/j.jadohealth.2018.08.025
- Chen, D., Matson, M., Macapagal, K., Johnson, E. K., Rosoklija, I., Finlayson, C., Fisher, C. B., & Mustanski, B. (2018). Attitudes toward fertility and reproductive health among transgender and gender-nonconforming adolescents. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 63(1), 62–68. https://doi.org/10.1016/j.jadohealth.2017.11.306
- Chen, D., & Simons, L. (2018). Ethical considerations in fertility preservation for transgender youth: A case illustration. Clinical Practice in Pediatric Psychology, 6(1), 93–100. https://doi.org/10.1037/cpp0000230
- Chen, D., Simons, L., Johnson, E. K., Lockart, B. A., & Finlayson, C. (2017). Fertility preservation for transgender adolescents. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 61(1), 120–123. https://doi.org/10.1016/j.jadohealth.2017.01.022
- Chiland, C., Clouet, A. M., Golse, B., Guinot, M., & Wolf, J. P. (2013). A new type of family: Transmen as fathers thanks to donor sperm insemination. A 12-year follow-up exploratory study of their children. Neuropsychiatrie de l'Enfance et de l'Adolescence, 61(6), 365–370. https://doi.org/10.1016/j.neurenf.2013.07.001
- Chiniara, L. N., Viner, C., Palmert, M., & Bonifacio, H. (2019). Perspectives on fertility preservation and parenthood among transgender youth and their parents. Archives of Disease in Childhood, 104(8), 739–744. https://doi.org/10.1136/archdischild-2018-316080
- Coleman, E. (2017). Standards of care for the health of transsexual, transgender, and gender-nonconforming people: An introduction. In Principles of gender-specific medicine (pp. 69–75). Elsevier. https://doi.org/10.1080/15532739.2011.700873
- Coleman, E., Bockting, W., Botzer, M., Cohen-Kettenis, P., DeCuypere, G., Feldman, J., Fraser, L., Green, J., Knudson, G., Meyer, W. J., Monstrey, S., Adler, R. K., Brown, G. R., Devor, A. H., Ehrbar, R., Ettner, R., Eyler, E., Garofalo, R., Karasic, D. H., … Zucker, K. (2012). Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. International Journal of Transgenderism, 13(4), 165–232. https://doi.org/10.1080/15532739.2011.700873
- Costa, R., & Colizzi, M. (2016). The effect of cross-sex hormonal treatment on gender dysphoria individuals’ mental health: A systematic review. Neuropsychiatric Disease and Treatment, 12, 1953–1966. https://doi.org/10.2147/NDT.S95310
- Couch, M., Pitts, M., Patel, S., Mulcare, H., Croy, S., & Mitchell, A. (2007). TranZnation - a report on the health and wellbeing of transgender people in Australia and New Zealand. Australian Research Centre in Sex, Health & Society, La Trobe University. https://doi.org/10.4225/50/557E5CFB09E8E
- Crissman, H. P., Berger, M. B., Graham, L. F., & Dalton, V. K. (2017). Transgender demographics: A household probability sample of US adults, 2014. American Journal of Public Health, 107(2), 213–215. https://doi.org/10.2105/AJPH.2016.303571
- Daar, J., & Klipstein, S. (2016). Refocusing the ethical choices in womb transplantation. Journal of Law and the Biosciences, 3(2), 383–388. https://doi.org/10.1093/jlb/lsw031
- De Cuypere, G., Van Hemelrijck, M., Michel, A., Carael, B., Heylens, G., Rubens, R., Hoebeke, P., & Monstrey, S. (2007). Prevalence and demography of transsexualism in Belgium. European Psychiatry: The Journal of the Association of European Psychiatrists, 22(3), 137–141. https://doi.org/10.1016/j.eurpsy.2006.10.002
- de Nie, I., Meißner, A., Kostelijk, E., Soufan, A., Voorn-de Warem, I., den Heijer, M., Huirne, J., & van Mello, N. (2020). Impaired semen quality in trans women: Prevalence and determinants. Human Reproduction (Oxford, England), 35(7), 1529–1536. https://doi.org/10.1093/humrep/deaa133
- De Roo, C., Tilleman, K., T'Sjoen, G., & De Sutter, P. (2016). Fertility options in transgender people. International Review of Psychiatry (Abingdon, England), 28(1), 112–119. https://doi.org/10.3109/09540261.2015.1084275
- De Wert, G., Dondorp, W., Shenfield, F., Barri, P., Devroey, P., Diedrich, K., Tarlatzis, B., Provoost, V., & Pennings, G. (2014). ESHRE Task Force on Ethics and Law 23: Medically assisted reproduction in singles, lesbian and gay couples, and transsexual people. Human Reproduction (Oxford, England), 29(9), 1859–1865. https://doi.org/10.1093/humrep/deu183
- Deutsch, M. B., & Feldman, J. L. (2013). Updated recommendations from the world professional association for transgender health standards of care. American Family Physician, 87(2), 89–93. https://www.aafp.org/pubs/afp/issues/2013/0115/p89.html
- Dhejne, C., Öberg, K., Arver, S., & Landén, M. (2014). An analysis of all applications for sex reassignment surgery in Sweden, 1960–2010: Prevalence, incidence, and regrets. Archives of Sexual Behavior, 43(8), 1535–1545. https://doi.org/10.1007/s10508-014-0300-8
- Dickey, L. M., Budge, S. L., Katz-Wise, S. L., & Garza, M. V. (2016). Health disparities in the transgender community: Exploring differences in insurance coverage. Psychology of Sexual Orientation and Gender Diversity, 3(3), 275–282. https://doi.org/10.1037/sgd0000169
- Dickey, R. P., Pyrzak, R., Taylor, S. N., Rye, P. H., Lu, P. Y., & Sartor, B. M. (2001). Decision to do IUI or IVF–sperm count? Fertility and Sterility, 76(5), 1086–1087. https://doi.org/10.1016/S0015-0282(01)02530-4
- Drobnis, E. Z., & Nangia, A. K. (2017). Psychotropics and male reproduction. Advances in Experimental Medicine and Biology, 1034, 63–101. https://doi.org/10.1007/978-3-319-69535-8_8
- Dunne, P. (2017). Transgender sterilisation requirements in Europe. Medical Law Review, 25(4), 554–581. https://doi.org/10.1093/medlaw/fwx028
- Ethics Committee of the American Society for Reproductive Medicine. (2015). Access to fertility services by transgender persons: An Ethics Committee opinion. Fertility and Sterility, 104(5), 1111–1115. https://doi.org/10.1016/j.fertnstert.2015.08.021
- EPATH (2020). WPATH, EPATH, USPATH, AsiaPATH, CPATH, AusPATH, PATHA Response to Bell v. Tavistock judgment statement regarding medical affirming treatment including puberty blockers for transgender adolescents. https://epath.eu/joint-statement-regarding-medical-affirming-treatment-including-puberty-blockers-for-transgender-adolescents/
- Gender Recognition Act (2004). https://www.legislation.gov.uk/ukpga/2004/7/contents
- Gilbert, K., Nangia, A. K., Dupree, J. M., Smith, J. F., & Mehta, A. (2018). Fertility preservation for men with testicular cancer: Is sperm cryopreservation cost effective in the era of assisted reproductive technology? Urologic Oncology, 36(3), 92.e1–92–e9. https://doi.org/10.1016/j.urolonc.2017.11.002
- Goldman, R. H., Kaser, D. J., Missmer, S. A., Farland, L. V., Ashby, R. K., Ginsburg ., & E. S., Scout. (2017). Fertility treatment for the transgender community: A public opinion study. Journal of Assisted Reproduction and Genetics, 34(11), 1457–1467. https://doi.org/10.1007/s10815-017-1035-y
- Goodman, M., Adams, N., Corneil, T., Kreukels, B., Motmans, J., & Coleman, E. (2019). Size and distribution of transgender and gender nonconforming populations: A narrative review. Endocrinology and Metabolism Clinics of North America, 48(2), 303–321. https://doi.org/10.1016/j.ecl.2019.01.001
- Green, R. (1998). Transsexuals’ children. International Journal of Transgenderism, 2(4), 1–6. http://web.archive.org/web/20070506211157/http://www.symposion.com/ijt/ijtc0601.htm
- Hamada, A., Kingsberg, S., Wierckx, K., T’Sjoen, G., De Sutter, P., Knudson, G., & Agarwal, A. (2015). Semen characteristics of transwomen referred for sperm banking before sex transition: a case series. Andrologia, 47(7), 832–838. https://doi.org/10.1111/and.12330
- Hammond-Browning, N. (2019). Uterine transplantation in transgender women: Medical, legal and ethical considerations. BJOG : An International Journal of Obstetrics and Gynaecology, 126(2), 157. https://doi.org/10.1111/1471-0528.15482
- Hembree, W. C., Cohen-Kettenis, P. T., Gooren, L., Hannema, S. E., Meyer, W. J., Murad, M. H., Rosenthal, S. M., Safer, J. D., Tangpricha, V., & T’Sjoen, G. G. (2017). Endocrine treatment of gender-dysphoric/gender-incongruent persons: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism, 102(11), 3869–3903. https://doi.org/10.1210/jc.2017-01658
- Human Fertilisation and Embryology Act (2008). https://www.legislation.gov.uk/ukpga/2008/22/contents
- Jiang, D. D., Swenson, E., Mason, M., Turner, K. R., Dugi, D. D., Hedges, J. C., & Hecht, S. L. (2019). Effects of estrogen on spermatogenesis in transgender women. Urology, 132, 117–122. https://doi.org/10.1016/j.urology.2019.06.034
- Jindarak, S., Nilprapha, K., Atikankul, T., Angspatt, A., Pungrasmi, P., Iamphongsai, S., Promniyom, P., Suwajo, P., Selvaggi, G., & Tiewtranon, P. (2018). Spermatogenesis abnormalities following hormonal therapy in transwomen. BioMed Research International, 2018, 7919481. https://doi.org/10.1155/2018/7919481
- Jones, B. P., Alghrani, A., & Smith, J. R. (2019). Re: Uterine transplantation in transgender women: medical, legal and ethical considerations. BJOG: An International Journal of Obstetrics & Gynaecology, 126(4), 545–545. https://doi.org/10.1111/1471-0528.15558
- Jones, B. P., Kasaven, L., Vali, S., Saso, S., Jalmbrant, M., Bracewell-Milnes, T., Thum, M. Y., Quiroga, I., Friend, P., Diaz-Garcia, C., Ghaem-Maghami, S., Yazbek, J., Lees, C., Testa, G., Johannesson, L., Jones, B., & Smith, J. R. (2021). Uterine transplantation: Review of livebirths and reproductive implications. Transplantation, 105(8), 1695–1707. https://doi.org/10.1097/TP.0000000000003578
- Jones, B., Rajamanoharan, A., Vali, S., Williams, N. J., Saso, S., Thum, M.-Y., Ghaem-Maghami, S., Quiroga, I., Diaz-Garcia, C., Thomas, P., Wilkinson, S., Yazbek, J., & Smith, J. R. (2020). Uterine transplantation in male to female transgender women; A cross-sectional survey assessing perceptions, acceptability and demand. The Lancet. Available at SSRN https://ssrn.com/abstract=3526304 or https://doi.org/10.2139/ssrn.3526304
- Jones, B. P., Saso, S., Bracewell-Milnes, T., Thum, M. Y., Nicopoullos, J., Diaz-Garcia, C., Friend, P., Ghaem-Maghami, S., Testa, G., Johannesson, L., Quiroga, I., Yazbek, J., & Smith, J. R. (2019). Human uterine transplantation: A review of outcomes from the first 45 cases. BJOG : An International Journal of Obstetrics and Gynaecology, 126(11), 1310–1319. https://doi.org/10.1111/1471-0528.15863
- Jones, B. P., Saso, S., L’Heveder, A., Bracewell-Milnes, T., Thum, M. Y., Diaz-Garcia, C., MacIntyre, D. A., Quiroga, I., Ghaem-Maghami, S., Testa, G., Johannesson, L., Bennett, P. R., Yazbek, J., & Smith, J. R. (2020). The vaginal microbiome in uterine transplantation. BJOG : An International Journal of Obstetrics and Gynaecology, 127(2), 230–238. https://doi.org/10.1111/1471-0528.15881
- Jones, B. P., Saso, S., Yazbek, J., & Smith, J. R. (2016). Uterine transplantation: Past, present and future. BJOG : An International Journal of Obstetrics and Gynaecology, 123(9), 1434–1438. https://doi.org/10.1111/1471-0528.13963
- Jones, B. P., Williams, N. J., Saso, S., Thum, M. Y., Quiroga, I., Yazbek, J., Wilkinson, S., Ghaem‐Maghami, S., Thomas, P., & Smith, J. R. (2019). Uterine transplantation in transgender women. BJOG : An International Journal of Obstetrics and Gynaecology, 126(2), 152–156. https://doi.org/10.1111/1471-0528.15438
- Jones, C. A., Reiter, L., & Greenblatt, E. (2016). Fertility preservation in transgender patients. International Journal of Transgenderism, 17(2), 76–82. https://doi.org/10.1080/15532739.2016.1153992
- Judge, C., O’Donovan, C., Callaghan, G., Gaoatswe, G., & O’Shea, D. (2014). Gender dysphoria–prevalence and co-morbidities in an Irish adult population. Frontiers in Endocrinology, 5, 87. https://doi.org/10.3389/fendo.2014.00087
- Karasic, D., & Drescher, J. (2006). Sexual and gender diagnoses of the diagnostic and statistical manual (DSM) a reevaluation. Journal of Psychology & Human Sexuality, 17(3-4), 1–5. https://doi.org/10.1300/J056v17n03_01
- Kattari, S. K., & Hasche, L. (2016). Differences across age groups in transgender and gender non-conforming people’s experiences of health care discrimination, harassment, and victimization. Journal of Aging and Health, 28(2), 285–306. https://doi.org/10.1177/0898264315590228
- Komeya, M., Sato, T., & Ogawa, T. (2018). In vitro spermatogenesis: A century‐long research journey, still half way around. Reproductive Medicine and Biology, 17(4), 407–420. https://doi.org/10.1002/rmb2.12225
- Kyweluk, M. A., Sajwani, A., & Chen, D. (2018). Freezing for the future: Transgender youth respond to medical fertility preservation. International Journal of Transgenderism, 19(4), 401–416. https://doi.org/10.1080/15532739.2018.1505575
- Lauterpacht, H. (1948). The universal declaration of human rights. British Yearbook of International Law, 25, 354–381.
- Lawrence, A. A. (2005). Sexuality before and after male-to-female sex reassignment surgery. Archives of Sexual Behavior, 34(2), 147–166. https://doi.org/10.1007/s10508-005-1793-y
- Lefkowitz, A., Edwards, M., & Balayla, J. (2013). Ethical considerations in the era of the uterine transplant: An update of the Montreal Criteria for the Ethical Feasibility of Uterine Transplantation. Fertility and Sterility, 100(4), 924–926. https://doi.org/10.1016/j.fertnstert.2013.05.026
- Li, K., Rodriguez, D., Gabrielsen, J. S., Centola, G. M., & Tanrikut, C. (2018). Sperm cryopreservation of transgender individuals: Trends and findings in the past decade. Andrology, 6(6), 860–864. https://doi.org/10.1111/andr.12527
- Li, Y., Lin, H., Li, Y., & Cao, J. (2011). Association between socio-psycho-behavioral factors and male semen quality: Systematic review and meta-analyses. Fertility and Sterility, 95(1), 116–123. https://doi.org/10.1016/j.fertnstert.2010.06.031
- Lotz, M. (2016). Commentary on Nicola Williams and Stephen Wilkinson: ‘Should uterus transplants be publicly funded?’ Journal of Medical Ethics, 42(9), 570–571. https://doi.org/10.1136/medethics-2015-103230
- Lübbert, H., Leo-Roßberg, I., & Hammerstein, J. (1992). Effects of ethinyl estradiol on semen quality and various hormonal parameters in a eugonadal male. Fertility and Sterility, 58(3), 603–608. https://doi.org/10.1016/S0015-0282(16)55271-6
- Marsh, C., McCracken, M., Gray, M., Nangia, A., Gay, J., & Roby, K. F. (2019). Low total motile sperm in transgender women seeking hormone therapy. Journal of Assisted Reproduction and Genetics, 36(8), 1639–1648. https://doi.org/10.1007/s10815-019-01504-y
- Marshall, W. A., & Tanner, J. M. (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45(239), 13–23. https://doi.org/10.1136/adc.45.239.13
- Martinez, F. (2017). Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: Indications, results and future perspectives. Fertility and Sterility, 108(3), 407–415.e11. https://doi.org/10.1016/j.fertnstert.2017.05.024
- Matoso, A., Khandakar, B., Yuan, S., Wu, T., Wang, L. J., Lombardo, K. A., Mangray, S., Mannan, A., & Yakirevich, E. (2018). Spectrum of findings in orchiectomy specimens of persons undergoing gender confirmation surgery. Human Pathology, 76, 91–99. https://doi.org/10.1016/j.humpath.2018.03.007
- McBride, J. A., & Coward, R. M. (2016). Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use. Asian Journal of Andrology, 18(3), 373–380. https://doi.org/10.4103/1008-682X.173938
- McBride, J. A., & Lipshultz, L. I. (2018). Male fertility preservation. Current Urology Reports, 19(7), 49. https://doi.org/10.1007/s11934-018-0803-2
- McGuinness, S., & Alghrani, A. (2008). Gender and parenthood: The case for realignment. Medical Law Review, 16(2), 261–283. https://doi.org/10.1093/medlaw/fwn010
- Meerwijk, E. L., & Sevelius, J. M. (2017). Transgender population size in the United States: A meta-regression of population-based probability samples. American Journal of Public Health, 107(2), e1–e8. https://doi.org/10.2105/AJPH.2016.303578
- Mehringer, J., & Dowshen, N. L. (2019). Sexual and reproductive health considerations among transgender and gender-expansive youth. Current Problems in Pediatric and Adolescent Health Care, 49(9), 100684. https://doi.org/10.1016/j.cppeds.2019.100684
- Mieusset, R., Bujan, L., Mansat, A., Pontonnier, F., & Grandjean, H. (1987). Hyperthermia and human spermatogenesis: Enhancement of the inhibitory effect obtained by ‘artificial cryptorchidism’. International Journal of Andrology, 10(4), 571–580. https://doi.org/10.1111/j.1365-2605.1987.tb00356.x
- Millar, A., Kim, B. H., Livne-Segev, D., Fung, R., Jarvi, K., & Millar, A. C. (2015). Attitudes, knowledge and beliefs regarding fertility preservation among people of transgendered experience: preliminary results. Canadian Journal of Diabetes, 39(6), 536. https://doi.org/10.1016/j.jcjd.2015.09.040
- Murad, M. H., Elamin, M. B., Garcia, M. Z., Mullan, R. J., Murad, A., Erwin, P. J., & Montori, V. M. (2010). Hormonal therapy and sex reassignment: A systematic review and meta‐analysis of quality of life and psychosocial outcomes. Clinical Endocrinology, 72(2), 214–231. https://doi.org/10.1111/j.1365-2265.2009.03625.x
- Murphy, T. F. (2012). The ethics of fertility preservation in transgender body modifications. Journal of Bioethical Inquiry, 9(3), 311–316. https://doi.org/10.1007/s11673-012-9378-7
- Murphy, T. F. (2015). Assisted gestation and transgender women. Bioethics, 29(6), 389–397. https://doi.org/10.1111/bioe.12132
- Nahata, L., Quinn, G. P., Caltabellotta, N. M., & Tishelman, A. C. (2017). Mental health concerns and insurance denials among transgender adolescents. LGBT Health, 4(3), 188–193. https://doi.org/10.1089/lgbt.2016.0151
- Nahata, L., Quinn, G. P., & Tishelman, A. (2016). A call for fertility and sexual function counseling in pediatrics. Pediatrics, 137(6), e20160180. https://doi.org/10.1542/peds.2016-0180
- Nahata, L., Tishelman, A. C., Caltabellotta, N. M., & Quinn, G. P. (2017). Low fertility preservation utilization among transgender youth. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 61(1), 40–44. https://doi.org/10.1016/j.jadohealth.2016.12.012
- Neblett, M. F., & Hipp, H. S. (2019). Fertility considerations in transgender persons. Endocrinology and Metabolism Clinics of North America, 48(2), 391–402. https://doi.org/10.1016/j.ecl.2019.02.003
- Newcomb, M. E., Hill, R., Buehler, K., Ryan, D. T., Whitton, S. W., & Mustanski, B. (2020). High burden of mental health problems, substance use, violence, and related psychosocial factors in transgender, non-binary, and gender diverse youth and young adults. Archives of Sexual Behavior, 49(2), 645–659. https://doi.org/10.1007/s10508-019-01533-9
- Nordkap, L., Jensen, T. K., Hansen, Å. M., Lassen, T. H., Bang, A. K., Joensen, U. N., Blomberg Jensen, M., Skakkebaek, N. E., & Jørgensen, N. (2016). Psychological stress and testicular function: A cross-sectional study of 1,215 Danish men. Fertility and Sterility, 105(1), 174–187.e872. https://doi.org/10.1016/j.fertnstert.2015.09.016
- Nørr, L., Bennedsen, B., Fedder, J., & Larsen, E. R. (2016). Use of selective serotonin reuptake inhibitors reduces fertility in men. Andrology, 4(3), 389–394. https://doi.org/10.1111/andr.12184
- Ohlander, S., Hotaling, J., Kirshenbaum, E., Niederberger, C., & Eisenberg, M. L. (2014). Impact of fresh versus cryopreserved testicular sperm upon intracytoplasmic sperm injection pregnancy outcomes in men with azoospermia due to spermatogenic dysfunction: A meta-analysis. Fertility and Sterility, 101(2), 344–349. https://doi.org/10.1016/j.fertnstert.2013.10.012
- Olson-Kennedy, J., Cohen-Kettenis, P. T., Kreukels, B. P., Meyer-Bahlburg, H. F., Garofalo, R., Meyer, W., & Rosenthal, S. M. (2016). Research priorities for gender nonconforming/transgender youth: Gender identity development and biopsychosocial outcomes. Current Opinion in Endocrinology, Diabetes, and Obesity, 23(2), 172–179. https://doi.org/10.1097/MED.0000000000000236
- Pacey, A. A., & Eiser, C. (2011). Banking sperm is only the first of many decisions for men: What healthcare professionals and men need to know. Human Fertility (Cambridge, England), 14(4), 208–217. https://doi.org/10.3109/14647273.2011.634480
- Pang, K. C., Peri, A., Chung, H. E., Telfer, M., Elder, C. V., Grover, S., & Jayasinghe, Y. (2020). Rates of fertility preservation use among transgender adolescents. JAMA Pediatrics, 174(9), 890–891. https://doi.org/10.1001/jamapediatrics.2020.0264
- Parikh, N., Chattha, A., Gargollo, P., & Granberg, C. (2021). Fertility preservation: A tale of two testicles. Urology, 153, 298–300. https://doi.org/10.1016/j.urology.2020.11.011
- Peri, A., Ahler, A., Gook, D., O’Connell, M. A., Bourne, H., Nightingale, M., Telfer, M., Jayasinghe, Y., & Pang, K. C. (2021). Predicting successful sperm retrieval in transfeminine adolescents after testicular biopsy. Journal of Assisted Reproduction and Genetics, 38(10), 2735–2743. https://doi.org/10.1007/s10815-021-02293-z
- Persky, R. W., Gruschow, S. M., Sinaii, N., Carlson, C., Ginsberg, J. P., & Dowshen, N. L. (2020). Attitudes toward fertility preservation among transgender youth and their parents. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 67(4), 583–589. https://doi.org/10.1016/j.jadohealth.2020.02.027
- Pfäfflin, F., Bockting, W. O., Coleman, E., Ekins, R., King, D., Gray, N. N., & Pellett, E. (2002). The desire to have children and the preservation of fertility in transsexual women: A survey. International Journal of Transgenderism, 6(3), 97–103.
- Povey, A. C., Clyma, J. A., McNamee, R., Moore, H. D., Baillie, H., Pacey, A. A., & Cherry, N. M. (2012). Modifiable and non-modifiable risk factors for poor semen quality: a case-referent study. Human Reproduction (Oxford, England), 27(9), 2799–2806. https://doi.org/10.1093/humrep/des183
- Quain, K. M., Kyweluk, M. A., Sajwani, A., Gruschow, S., Finlayson, C., Gordon, E. J., Johnson, E. K., Persky, R., Dowshen, N., & Chen, D. (2021). Timing and delivery of fertility preservation information to transgender adolescents, young adults, and their parents. The Journal of Adolescent Health : official Publication of the Society for Adolescent Medicine, 68(3), 619–622. https://doi.org/10.1016/j.jadohealth.2020.06.044
- Reed, B., Rhodes, S., Schofield, P., & Wylie, K. (2009). Gender variance in the UK: Prevalence, incidence, growth and geographic distribution (Technical Report). GIRES – the Gender Identity Research and Education Society. Retrieved June, 8, 2011, from http://www.gires.org.uk/assets/Medpro-Assets/GenderVarianceUK-report.pdf
- Riggs, D. W., & Bartholomaeus, C. (2018). Fertility preservation decision making amongst Australian transgender and non-binary adults. Reproductive Health, 15(1), 181. https://doi.org/10.1186/s12978-018-0627-z
- Rodriguez-Wallberg, K. A., Häljestig, J., Arver, S., Johansson, A., & Lundberg, F. E. (2021). Sperm quality in transgender women before or after gender affirming hormone therapy-a prospective cohort study. Andrology, 9(6), 1773–1780. https://doi.org/10.1111/andr.12999
- Safer, J. D., Coleman, E., Feldman, J., Garofalo, R., Hembree, W., Radix, A., & Sevelius, J. (2016). Barriers to healthcare for transgender individuals. Current Opinion in Endocrinology, Diabetes, and Obesity, 23(2), 168–171. https://doi.org/10.1097/MED.0000000000000227
- Sanchez, N. F., Sanchez, J. P., & Danoff, A. (2009). Health care utilization, barriers to care, and hormone usage among male-to-female transgender persons in New York City. American Journal of Public Health, 99(4), 713–719. https://doi.org/10.2105/AJPH.2007.132035
- Sato, T., Katagiri, K., Gohbara, A., Inoue, K., Ogonuki, N., Ogura, A., Kubota, Y., & Ogawa, T. (2011). In vitro production of functional sperm in cultured neonatal mouse testes. Nature, 471(7339), 504–507. https://doi.org/10.1038/nature09850
- Schneider, F., Kliesch, S., Schlatt, S., & Neuhaus, N. (2017). Andrology of male‐to‐female transsexuals: Influence of cross‐sex hormone therapy on testicular function. Andrology, 5(5), 873–880. https://doi.org/10.1111/andr.12405
- Schulze, C. (1988). Response of the human testis to long-term estrogen treatment: Morphology of Sertoli cells, Leydig cells and spermatogonial stem cells. Cell and Tissue Research, 251(1), 31–43. https://doi.org/10.1007/BF00215444
- Segev-Becker, A., Israeli, G., Elkon-Tamir, E., Perl, L., Sekler, O., Amir, H., Interator, H., Dayan, S. C., Chorna, E., Weintrob, N., & Oren, A. (2020). Children and adolescents with gender dysphoria in israel: Increasing referral and fertility preservation rates. Endocrine Practice : Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists, 26(4), 423–428. https://doi.org/10.4158/EP-2019-0418
- Sermondade, N., Benaloun, E., Berthaut, I., Moreau, E., Prades, M., Béranger, A., Chabbert-Buffet, N., Johnson, N., Lévy, R., & Dupont, C. (2021). Reproductive functions and fertility preservation in transgender women: A French case series. Reproductive Biomedicine Online, 43(2), 339–345. https://doi.org/10.1016/j.rbmo.2021.04.016
- Shields, J. P., Cohen, R., Glassman, J. R., Whitaker, K., Franks, H., & Bertolini, I. (2013). Estimating population size and demographic characteristics of lesbian, gay, bisexual, and transgender youth in middle school. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 52(2), 248–250. https://doi.org/10.1016/j.jadohealth.2012.06.016
- Shumer, D. E., Nokoff, N. J., & Spack, N. P. (2016). Advances in the care of transgender children and adolescents. Advances in Pediatrics, 63(1), 79–102. https://doi.org/10.1016/j.yapd.2016.04.018
- Stöbel-Richter, Y., Beutel, M. E., Finck, C., & Brähler, E. (2005). The ‘wish to have a child’, childlessness and infertility in Germany. Human Reproduction (Oxford, England), 20(10), 2850–2857. https://doi.org/10.1093/humrep/dei121
- Stotzer, R. L., Herman, J. L., & Hasenbush, A. (2014). Transgender parenting: A review of existing research. The Williams Institute. https://escholarship.org/uc/item/3rp0v7qv
- Strang, J. F., Jarin, J., Call, D., Clark, B., Wallace, G. L., Anthony, L. G., Kenworthy, L., & Gomez-Lobo, V. (2018). Transgender youth fertility attitudes questionnaire: Measure development in nonautistic and autistic transgender youth and their parents. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 62(2), 128–135. https://doi.org/10.1016/j.jadohealth.2017.07.022
- Thiagaraj, D., Gunasegaram, R., Loganath, A., Peh, K., Kottegoda, S., & Ratnam, S. (1987). Histopathology of the testes from male transsexuals on oestrogen therapy. Annals of the Academy of Medicine, Singapore, 16(2), 347–348.
- Tiemessen, C. H., Evers, J. L., & Bots, R. S. (1996). Tight-fitting underwear and sperm quality. Lancet (London, England), 347(9018), 1844–1845. https://doi.org/10.1016/S0140-6736(96)91670-0
- Tornello, S. L., & Bos, H. (2017). Parenting intentions among transgender individuals. LGBT Health, 4(2), 115–120. https://doi.org/10.1089/lgbt.2016.0153
- Unger, C. A. (2015). Care of the transgender patient: A survey of gynecologists’ current knowledge and practice. Journal of Women’s Health (2002), 24(2), 114–118. https://doi.org/10.1089/jwh.2014.4918
- Van Voorhis, B. J. (2007). Clinical practice. In vitro fertilization. The New England Journal of Medicine, 356(4), 379–386. https://doi.org/10.1056/NEJMcp065743
- Vance, S. R., Jr., Halpern-Felsher, B. L., & Rosenthal, S. M. (2015). Health care providers’ comfort with and barriers to care of transgender youth. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 56(2), 251–253. https://doi.org/10.1016/j.jadohealth.2014.11.002
- Veale, J. F. (2008). Prevalence of transsexualism among New Zealand passport holders. The Australian and New Zealand Journal of Psychiatry, 42(10), 887–889. https://doi.org/10.1080/00048670802345490
- Venizelos, I., & Paradinas, F. (1988). Testicular atrophy after oestrogen therapy. Histopathology, 12(4), 451–454. https://doi.org/10.1111/j.1365-2559.1988.tb01961.x
- Vicari, E., Mongioi, A., Calogero, A., Moncada, M., Sidoti, G., Polosa, P., & D’Agata, R. (1992). Therapy with human chorionic gonadotrophin alone induces spermatogenesis in men with isolated hypogonadotrophic hypogonadism‐long‐term follow‐up. International Journal of Andrology, 15(4), 320–329. https://doi.org/10.1111/j.1365-2605.1992.tb01131.x
- von Doussa, H., Power, J., & Riggs, D. (2015). Imagining parenthood: The possibilities and experiences of parenthood among transgender people. Culture, Health & Sexuality, 17(9), 1119–1131. https://doi.org/10.1080/13691058.2015.1042919
- Wakefield, B. W., Boguszewski, K. E., Cheney, D., & Taylor, J. F. (2019). Patterns of fertility discussions and referrals for youth at an interdisciplinary gender clinic. LGBT Health, 6(8), 417–421. https://doi.org/10.1089/lgbt.2019.0076
- Wanta, J. W., & Unger, C. A. (2017). Review of the transgender literature: Where do we go from here? Transgender Health, 2(1), 119–128. https://doi.org/10.1089/trgh.2017.0004
- Wenker, E. P., Dupree, J. M., Langille, G. M., Kovac, J., Ramasamy, R., Lamb, D., Mills, J. N., & Lipshultz, L. I. (2015). The use of HCG‐based combination therapy for recovery of spermatogenesis after testosterone use. The Journal of Sexual Medicine, 12(6), 1334–1337. https://doi.org/10.1111/jsm.12890
- Weyers, S., Verstraelen, H., Gerris, J., Monstrey, S., Santiago, G., Saerens, B., De Backer, E., Claeys, G., Vaneechoutte, M., & Verhelst, R. (2009). Microflora of the penile skin-lined neovagina of transsexual women. BMC Microbiology, 9, 102. https://doi.org/10.1186/1471-2180-9-102
- White, T., & Ettner, R. (2007). Adaptation and adjustment in children of transsexual parents. European Child & Adolescent Psychiatry, 16(4), 215–221. https://doi.org/10.1007/s00787-006-0591-y
- Wierckx, K., Stuyver, I., Weyers, S., Hamada, A., Agarwal, A., De Sutter, P., & T'Sjoen, G. (2012). Sperm freezing in transsexual women. Archives of Sexual Behavior, 41(5), 1069–1071. https://doi.org/10.1007/s10508-012-0012-x
- Wilkinson, S., & Williams, N. J. (2016). Should uterus transplants be publicly funded? Journal of Medical Ethics, 42(9), 559–565. https://doi.org/10.1136/medethics-2015-102999
- Williams, N. (2016). Should deceased donation be morally preferred in uterine transplantation trials? Bioethics, 30(6), 415–424. https://doi.org/10.1111/bioe.12247
- Wyns, C., Collienne, C., Shenfield, F., Robert, A., Laurent, P., Roegiers, L., & Brichard, B. (2015). Fertility preservation in the male pediatric population: Factors influencing the decision of parents and children. Human Reproduction (Oxford, England), 30(9), 2022–2030. https://doi.org/10.1093/humrep/dev161