Abstract
Nine potential AChE reactivators were synthesized using a modification of currently known synthetic pathways. Their potency to reactivate AChE inhibited by insecticide chlorpyrifos was tested in vitro. 2,2′-Bis(hydroxyiminomethyl)-1,1′-(1,4-phenylenedimethyl)-bispyridinium dibromide seems to be the most potent AChE reactivator. The reactivation potency of these compounds depends on structural factors such as length of the linking chain between both pyridinium rings and position of the oxime moiety on the pyridinium ring.
1 Introduction
Highly toxic organophosphorus compounds (organophosphates, OP), for example the nerve agents (e.g. sarin, soman, tabun and VX) or insecticides (e.g. chlorpyrifos, parathion and paraoxon), inactivate the enzyme acetylcholinesterase (AChE, EC 3.1.1.7) by phosphorylation or phosphonylation of its active site Citation1-3. The inhibition of AChE depends on the chemical structure of the inhibitors whereas reactivation of inhibited AChE depends not only on the inhibitors used but also on the chemical structure of the reactivator [Citation4]. Pralidoxime (1; 2-PAM, 2-hydroxyiminomethyl-1-methylpyridinium chloride) is a commonly used monoquaternary AChE reactivator for the treatment of toxic effects of OP [Citation5,Citation6]. More extended bisquaternary compounds, such as trimedoxime (2; TMB-4, 1,3-bis(4-hydroxyiminomethylpyridinium)propane dibromide), obidoxime (3; Toxogonine®, 1,3-bis(4-hydroxyiminomethylpyridinium)-2-oxapropane dibromide), H-oxime HI-6 (4; 1-(2-hydroxyiminomethylpyridinium)-3-(4-carbamoylpyridinium)-2-oxapropane dichloride) and methoxime (5, bis(4-hydroxyiminomethylpyridinium)methane dichloride) are representatives of these aldoximes Citation7-11.
Due to the high variability of AChE inhibitors, there is no single AChE reactivator having the ability to sufficiently reactivate inhibited enzyme regardless of the chemical structure of the inhibitor. Therefore, many laboratories throughout the world have decided to synthesize new reactivators of OP-inhibited AChE, in order to reactivate OP-inhibited AChE regardless of the chemical structure of the organophosphorus inhibitor.
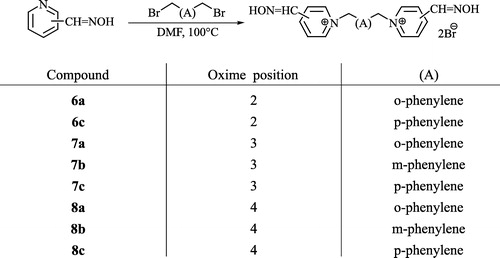
Our research was focused on finding more rigid symmetrical bisquaternary structures than commonly used reactivators have (2, 3). In order to fix the structure of the compounds, we decided to use a xylene linker connecting two pyridinium rings bearing carbaldoxime moieties in various positions (Scheme ). Afterwards, we tested their ability to reactivate AChE inhibited by chlorpyrifos (Scheme ), which is commonly used as an insecticide for agricultural purposes.
2 Materials and methods
2.1 Chemistry
Solvents (acetone, DMF, ethanol) and reagents were purchased from Fluka and Sigma-Aldrich (Czech Republic) and used without further purification. Reactions were monitored by TLC using DC-Alufolien Cellulose F (Merck, Germany) and mobile phase BuOH-CH3COOH-H2O (5: 1: 2). For detection a solution of Dragendorff reagent (solution containing 10 ml CH3COOH, 50 ml H2O and 5 ml of basic solution prepared by mixing of two fractions – fraction I.: 850 mg Bi(NO3)3, 40 ml H2O, 10 ml CH3COOH; fraction II.: 8 g KI, 20 ml H2O) was used. Melting points were measured on a micro heating stage PHMK 05 (VEB Kombinat Nagema, Radebeul, Germany) and are uncorrected.
NMR spectra were generally recorded on a Varian Gemini 300 (1H 300 MHz, 13C 75 MHz, Palo Alto CA, USA). In all cases, the chemical shift values for 1H spectra are reported in ppm (δ) relative to residual CHD2SO2CD3 (δ 2.50) and shift values for 13C spectra are reported in ppm (δ) relative to the solvent peak dimethylsulfoxide – d6 δ 39.43. Signals are quoted as s (singlet), bs (broad singlet), d (doublet), t (triplet) and m (multiplet).
Mass spectra were recorded using a combination of high performance liquid chromatography and mass spectrometry. HP1100 HPLC system was obtained from Agilent Technologies (Waldbronn, Germany). It consisted of vacuum degasser G1322A, quaternary pump G1311A, autosampler G1313A and quadrupole mass spectrometer MSD1456 VL equipped with electrospray ionization source. Nitrogen for the mass spectrometer was supplied by a Whatman 75–720 nitrogen generator. Data were collected in positive ion mode with an ESI probe voltage of 4000 V. The pressure of the nebulizer gas was set up to 35 psig. Drying gas temperature was operated at 335°C and flow at 13 l/min.
2.1.1 Preparation of quaternary salts
Two synthetic pathways were used for preparation of the bisquaternary aldoximes:
A) A solution of the hydroxyiminomethylpyridine (1.0 g, 8.2 mmol) and dibromoxylene (0.97 g, 3.7 mmol) in DMF (10 mL) was stirred at 100°C. The reaction mixture was cooled to room temperature and mixted with acetone (30 mL); the crystalline crude product was collected by filtration, washed with acetone (2 × 20 mL) and recrystalized from acetonitrile.
B) A solution of the hydroxyiminomethylpyridine (1.0 g, 8.2 mmol) and dibromoxylene (0.97 g, 3.7 mmol) in acetonitrile (10 mL) was stirred at 80°C. The reaction mixture was cooled to room temperature and mixted with acetone (30 ml); the crystalline crude product was collected by filtration, washed with acetone (2 × 20 mL) and recrystalized from ethanol.
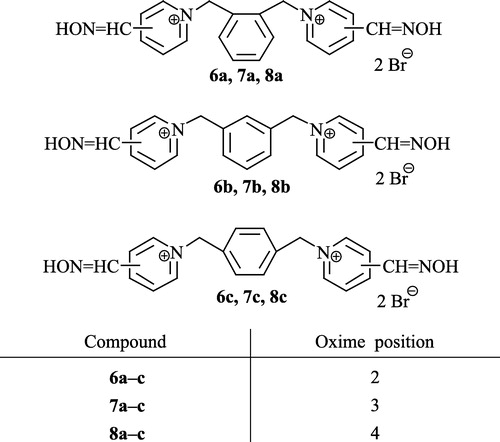
2.1.1.1 2,2′-bis(hydroxyiminomethyl)-1,1′-(1,2-phenylenedimethyl)-bispyridinium dibromide (6a)
Prepared by method A. The reaction was stopped after 8.5 h. Yield 0.93 g (50%), TLC Rf 0.15, m.p. decomp. 170°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.07 (d, 2H, J = 6.32 Hz), 8.80 − 8.58 (m, 6H), 8.32 − 8.23 (m, 2H), 7.38 − 7.30 (m, 2H), 6.57 − 6.49 (m, 2H), 6.39 (s, 4H), 3.56 (bs, 2H). 13C NMR (75 MHz, DMSO d6): δ (ppm) 147.99, 146.21, 145.87, 141.16, 131.41, 129.09, 128.24, 125.76, 125.40, 57.68. Analysis: calculated 47.27% C, 3.97% H, 11.02% N; found 47.51% C, 4.13% H, 10.68% N. ESI-MS: m/z 347.1 [M2+ − H+]+ (calculated for [C20H20N4O22+ − H+]+347.16).
2.1.1.2 2,2′-bis(hydroxyiminomethyl)-1,1′-(1,3-phenylenedimethyl)-bispyridinium dibromide (6b)
Prepared by method B. The reaction was stopped after 5 h. Yield 0.39g (21%), TLC Rf 0.15, m.p. decomp. 199–201°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.20 (d, 2H, J = 6.0 Hz), 8.80 − 8.57 (m, 4H), 8.43 (d, 2H, J = 7.7 Hz), 8.29 − 8.13 (m, 2H), 7.55 − 7.40 (m, 1H), 7.28 (d, 2H, J = 7.7 Hz), 7.17 (s, 1H), 6.11 (s, 4H), 3.95 (bs, 2H). 13C NMR (75 MHz, DMSO d6): δ (ppm) 147.10, 146.32, 145.96, 141.21, 134.89, 130.04, 127.86, 127.76, 126.03, 59.76. Analysis: calculated 47.27% C, 3.97% H, 11.02% N; found 47.00% C, 4.10% H, 10.99% N. ESI-MS: m/z 347.1 [M2+ − H+]+ (calculated for [C20H20N4O22+ − H+]+347.16).
2.1.1.3 2,2′-bis(hydroxyiminomethyl)-1,1′-(1,4-phenylenedimethyl)-bispyridinium dibromide (6c)
Prepared by method A. The reaction was stopped after 7 h. Yield 0.79 g (42%), TLC Rf 0.15, m.p. decomp. 240°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.26 (d, 2H, J = 6.3 Hz), 8.78 − 8.59 (m, 4H), 8.47 − 8,39 (m, 2H), 8.25 − 8.15 (m, 2H), 7.31 (s, 4H), 6.14 (s, 4H), 3.53 (bs, 2H). 13C NMR (75 MHz, DMSO d6): δ (ppm) 147.10, 146.46, 145.98, 141.30, 134.52, 127.97, 127.90, 126.04, 59.68. Analysis: calculated 47.27% C, 3.97% H, 11.02% N; found 47.16% C, 3.99% H, 11.44% N. ESI-MS: m/z 347.1 [M2+ − H+]+ (calculated for [C20H20N4O22+ − H+]+347.16).
2.1.1.4 3,3′-bis(hydroxyiminomethyl)-1,1′-(1,2-phenylenedimethyl)-bispyridinium dibromide (7a)
Prepared by method A. The reaction was stopped after 1.5 h. Yield 1.75 g (93%), TLC Rf 0.15, m.p. 223–226°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.33 (s, 2H), 9.13 (d, 2H, J = 5.8 Hz), 8.82 (d, 2H, J = 8.0 Hz), 8.40 (s, 2H), 8.28 − 8.20 (m, 2H), 7.57 − 7.49 (m, 2H), 7.35 − 7.27 (m, 2H), 6.23 (s, 4H), 3.39 (s, 2H). 13C NMR (75 MHz, DMSO d6): δ (ppm) 144.73, 143.22, 142.64, 142.19, 133.74, 132.52, 130.12, 129.78, 128.53. Analysis: calculated 47.27% C, 3.97% H, 11.02% N; found 47.52% C, 3.85% H, 11.09% N. ESI-MS: m/z 347.1 [M2+ − H+]+ (calculated for [C20H20N4O22+ − H+]+347.16).
2.1.1.5 3,3′-bis(hydroxyiminomethyl)-1,1′-(1,3-phenylenedimethyl)-bispyridinium dibromide (7b)
Prepared by method A. The reaction was stopped after 1.5 h. Yield 1.77 g (94%), TLC Rf 0.15, m.p. 238241°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.50 (s, 2H), 9.25 (d, 2H, J = 5.8 Hz), 8.78 (d, 2H, J = 8.0 Hz), 8.40 (s, 2H), 8.25 − 8.17 (m, 2H), 7.85 (s, 1H), 7.61 (d, 2H, J = 7.4 Hz), 7.57 − 7.49 (m, 1H), 5.96 (s, 4H), 3.38 (s, 2H). 13C NMR (75 MHz, DMSO d6): δ (ppm) 144.53, 143.17, 142.53, 141.90, 134.86, 133.69, 129.98, 129.69, 129.48, 128.46, 62.81. Analysis: calculated 47.27% C, 3.97% H, 11.02% N; found 47.36% C, 3.84% H, 11.08% N. ESI-MS: m/z 347.0 [M2+ − H+]+ (calculated for [C20H20N4O22+ − H+]+347.16).
2.1.1.6 3,3′-bis(hydroxyiminomethyl)-1,1′-(1,4-phenylendimethyl)-bispyridinium dibromide (7c)
Prepared by method A. The reaction was stopped after 2 h. Yield 1.46 g (78%), TLC Rf 0.15, m.p. decomp. 162°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.47 (s, 2H), 9.23 (d, 2H, J = 6.3 Hz), 8.75 (d, 2H, J = 8.0 Hz), 8.36 (s, 2H), 8.24 − 8.15 (m, 2H), 7.67 (s, 4H), 5.95 (s, 4H), 3.38 (s, 2H). 13C NMR (75 MHz, DMSO d6): δ (ppm) 144.42, 143.21, 142.37, 142.01, 135.14, 133.74, 129.63, 128.50, 62.71; Analysis: calculated 47.27% C, 3.97% H, 11.02% N; found 47.26% C, 3.91% H, 10.92% N. ESI-MS: m/z 347.1 [M2+ − H+]+ (calculated for [C20H20N4O22+ − H+]+347.16).
2.1.1.7 4,4′-bis(hydroxyiminomethyl)-1,1′-(1,2-phenylenedimethyl)-bispyridinium dibromide (8a)
Prepared by method A. The reaction was stopped after 2.5 h. Yield 1.23 g (66%), TLC Rf 0.15, m.p. 243 − 244°C, reported 247–248°C, 238.3–239.1°C Citation12Citation14-15. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.10 (d, 4H, J = 6.0 Hz), 8.49 (s, 2H), 8.30 (d, 4H, J = 5.8 Hz), 7.55 − 7.47 (m, 2H), 7.30 − 7.21 (m, 2H), 6.16 (s, 4H), 3.36 (s, 2H). 13C NMR (75 MHz, DMSO d6): δ (ppm) 149.28, 145.63, 145.33, 133.01, 130.30, 129.83, 124.66, 59.90. Analysis: calculated 47.27% C, 3.97% H, 11.02% N; found 47.18% C, 3.82% H, 11.16% N. ESI-MS: m/z 347.1 [M2+ − H+]+ (calculated for [C20H20N4O22+ − H+]+347.16).
2.1.1.8 4,4′-bis(hydroxyiminomethyl)-1,1′-(1,3-phenylenedimethyl)-bispyridinium dibromide (8b)
Prepared by method A. The reaction was stopped after 1.5 h. Yield 1.36 g (73%), TLC Rf 0.15, m.p. 226–228°C, reported 225–226°C [Citation12]. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.21 (d, 4H, J = 6.6 Hz), 8.45 (s, 2H), 8.28 (d, 4H, J = 6.6 Hz), 7.78 (s, 1H), 7.61 − 7.47 (m, 3H), 5.89 (s, 4H), 3.37 (s, 2H). 13C NMR (75 MHz, DMSO d6): δ (ppm) 148.75, 145.12, 145.05, 135.12, 130.01, 129.52, 129.24, 124.33, 62.13. Analysis: calculated 47.27% C, 3.97% H, 11.02% N; found 46.32% C, 3.97% H, 10.71% N. ESI-MS: m/z 347.1 [M2+ − H+]+ (calculated for [C20H20N4O22+ − H+]+347.16).
2.1.1.9 4,4′-bis(hydroxyiminomethyl)-1,1′-(1,4-phenylenedimethyl)-bispyridinium dibromide (8c)
Prepared by method A. The reaction was stopped after 5 h. Yield 1.61 g (86%), TLC Rf 0.15, m.p. decomp. 285°C, reported decomp. 286°C, 290.5–291.5°C Citation12Citation14-15. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.20 (d, 4H, J = 6.6 Hz), 8.42 (s, 2H), 8.25 (d, 4H, J = 6.6 Hz), 7.63 (s, 4H), 5.88 (s, 4H), 3.50 (bs, 2H). 13C NMR (75 MHz, DMSO d6): δ (ppm) 148.75, 145.03, 135.30, 129.55, 124.36, 61.98. Analysis: calculated 47.27% C, 3.97% H, 11.02% N; found 47.24% C, 4.10% H, 10.90% N. ESI-MS: m/z 347.1 [M2+ − H+]+ (calculated for [C20H20N4O22+ − H+]+347.16).
3 Biochemistry
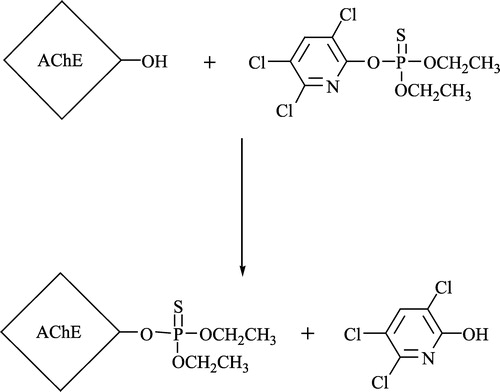
In vitro testing of the synthesized oximes involved a standard collection of experimental procedures. The 10% rat brain homogenate was used as a source of AChE. The brain homogenate (0.5 ml) was mixed with 29 μL of an isopropanol solution of chlorpyrifos (O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl)-phosphorothioate, analytical standard 99.2% from Sigma-Aldrich to achieved 95% inhibition of AChE) and incubated at 25°C for 30 min. A solution of sodium chloride (2.5 mL 3 M) was added to the mixture and filled to a volume of 23 mL with distilled water. Finally, 2 mL of a solution of acetylcholine iodide (0.02 M) was added. The enzyme activity was measured at pH 8.0 and 25°C on an autotitrator RTS 822 (Radiometer, Denmark). Activities of intact AChE (a0) and inhibited AChE (ai) were deduced from the consumption of NaOH solution (0.01 M) with time. After the incubation with chlorpyrifos-inhibited AChE (30 min), the reactivator was added to the solution and the mixture was incubated for 10 min. Activity of the reactivated AChE (ar) was also computed from the consumption of NaOH solution with time.
The percentage of reactivation (%) was calculated from the measured data according to:
Pralidoxime, methoxime, HI-6, obidoxime and trimedoxime of HPLC purity previously synthesized in our laboratory were used as references.The whole method is described in detail in the work of Kuca and Kassa [Citation17].
4 Results and discussion
Previously published reaction conditions but differing in the temperature (100°C) used were applied to the syntheses of the required compounds (Scheme ) [Citation11]. The yields of four former known compounds (6c, 8a–c) are comparable with published data Citation12-15.
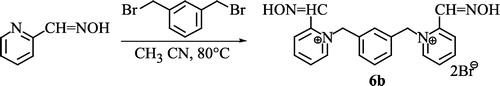
Unfortunately, this system was not applicable in the case of 2,2′-bis(hydroxyiminomethyl)-1,1′-(1,3-phenylenedimethyl)-bispyridinium dibromide (6b), where other conditions were used (Scheme ) [Citation16].
Scheme 4 Synthesis of 2,2′-bis(hydroxyiminomethyl)-1,1′-(1,3-phenylenedimethyl)-bispyridinium dibromide.
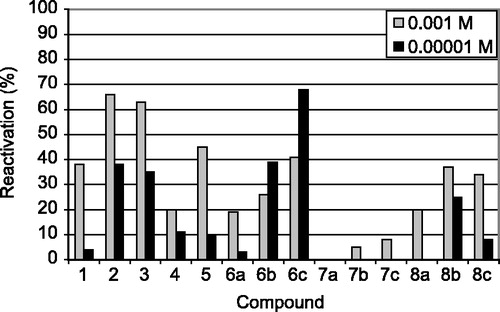
Reactivation potencies of all the synthesized oximes and reference compounds are shown in and .
Table I. Reactivation potencies (%)a of tested oximes. Time of inhibition by chlorpyrifos –30 min; time of reactivation by AChE reactivators –10 min; pH 8; temperature 25°C.
The most potent reactivators of chlorpyrifos-inhibited AChE seem to be the reference compounds obidoxime (3) and TMB-4 (2) at concentrations of 10− 3 M. However, new synthesized oximes (6a–c, 8a–c) showed satisfactory reactivation results, too. Unfortunately, concentration 10− 3 M is not aplicable for further in vivo testing Citation17-21. The concentration 10− 5 M is more suitable from the point of view of the reactivator's toxic effect on the patient [Citation17]. This means that the oxime 6c surpasses all other in vitro tested compounds (68% reactivation) and is the most promising from these compounds tested in vitro. Oximes 6b, 8b, obidoxime (3) and trimedoxime (2) have also satisfactory reactivation ability at 10− 5 M concentration.
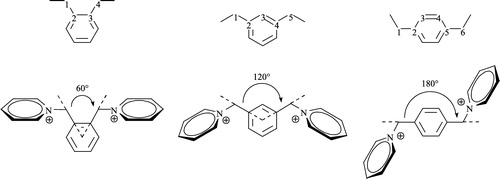
The reactivation potency of the measured compounds depends on the structure of the OP inhibitor Citation17-18. Consequently, we can recommend structural factors appropriate for reactivation of chlorpyrifos-inhibited AChE [Citation17]. The oxime functional group breaks down the bond OP inhibitor-enzyme and is essential for the activity of the reactivator Citation21-23. Our results confirm that the position of the hydroxyiminomethyl group influences the reactivation potency. While the substances bearing the oxime group in position-3 are practically ineffective (7a–c), substances bearing the oxime group in position 2 or 4 show promising results (6a–c, 8a–c) [Citation22]. In comparison (6a–c, 8a–b), the length of the linking chain also influences the reactivation potency [Citation4]. In our case, the length of the linker varies from four to six atoms between the pyridinium rings (Scheme ). The most promising compound (6c) has a six atom-linker in contrast to the active reference substances trimedoxime (2) and obidoxime (3), which have only a three atom-bridge. Than important role is also played by plays also the “rigidity” of the linking chain. The only bonds accessible for free rotation in these molecules are the methylene junctions in contrast to free rotation in the molecules with a propane (2) or 2-oxapropane linking chain (3, 4). These short junctions cause the non-coplanarity of each pyridinium ring to the phenylene ring. Therefore, there could be determined angles between bonds outgoing from the phenylene ring (Scheme ). From this point of view, the most promising are compounds with longer angles meaning a greater distance between the pyridinium rings (6b–c, 8b–c).
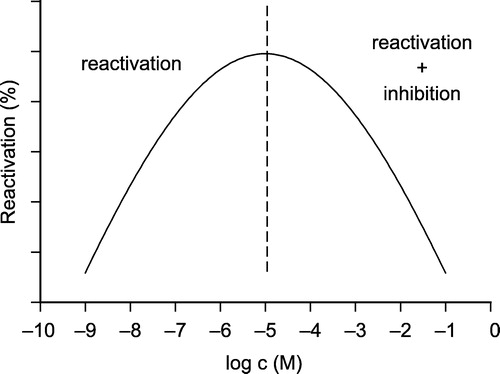
The higher reactivation activity of some compounds (6b–c) at the lower concentration is caused by their inhibition of intact AChE. The measurements were made for two concentrations and the whole concentration scale is bell-shaped (Scheme ) [Citation17]. The reactivation process is characterized in the increasing limb and the decreasing limb shows both reactivation and inhibition of liberated intact AChE by the reactivator itself. Every reactivator varies in the optimal concentration for reactivation and in the case of 6b–c the optimum lies at lower concetrations.
5 Conclusion
Nine potential AChE reactivators have been synthesized using a modification of currently known synthetic pathways and also in vitro tested their potency to reactivate AChE inhibited by the insecticide chlorpyrifos. 2,2′-Bis(hydroxyiminomethyl)-1,1′-(1,4-phenylenedimethyl)-bispyridinium dibromide (6c) seems to be the most potent AChE reactivator. The reactivation potency of these compounds depends on structural factors such as length of the linking chain between both pyridinium rings and position of the functional oxime group on the pyridinium ring.
Acknowledgements
The authors express their appreciation to Mrs. M. Hrabinova for her technical assistance. The study was supported by a grant from the Ministry of Defense No. ONVLAJEP 20031 and by a grant from the Grant Agency of Charles University No. 302/2005/B-CH/FaF.
References
- Bajgar J. Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 2004; 38: 151–216
- Marrs TC. Organophosphate poisoning. Pharmacol Therapeut 1993; 58: 51–66
- Ellin RI, Wills JH. Oximes antagonistic to inhibitors of cholinesterase I. J Pharm Sci 1964; 53: 995–1007
- Kuca K, Patocka J, Cabal J. Reactivation of organophosphate inhibited acetylcholinesterase activity by α,ω-bis-(4-hydroxyiminomethylpyridinium)alkanes in vitro. J Appl Biomed 2003; 1: 207–211
- Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol 2002; 40: 803–816
- Kuca K, Bielavsky J, Cabal J, Bielavska M. Synthesis of potential reactivator of acetylcholinesterase - 1-(4-hydroxyiminomethylpyridinium)-3-(carbamoylpyridinium)-propane dibromide. Tetrahedron Lett 2003; 44: 3123–3125
- Poziomek EJ, Hackley BE, Steinberg GM. Pyridinium aldoximes. J Org Chem 1958; 23: 714–717
- Krejcova G, Kassa J. Neuroprotective efficacy of pharmacological pretreatment and antidotal treatment in tabun-poisoned rats. Toxicology 2003; 185: 129–139
- Rousseaux CG, Gua AK. Pharmacology of HI-6, an H-series oxime. Can. J Physiol Pharmacol 1989; 67: 1183–1189
- Kassa J, Cabal J, Bajgar J, Szinicz L. The choice: HI-6, pralidoxime or obidoxime against nerve agents?. ASA Newslett 1997, 97–4: 16–18
- Kuca K, Cabal J, Patocka J, Kassa J. Synthesis of bisquaternary symmetric - χ,δ(-bis(2-hydroxyimino-methylpyridinium) alkane dibromides and their reactivation of cyclosarin-inhibited acetylcholinesterase. Lett Org Chem 2004; 1: 84–86
- Luettringhaus A, Hagedorn I. Quaternary hydroxyiminomethylpyridinium salts. The dischloride of bis-(4-hydroxyiminomethyl-1-pyridinium-methyl)-ether (LUEH6), a new reactivator of acetylcholinesterase inhibited by organic phosphoric acid esters. Arzneimittel-Forschung 1964; 14: 1–5
- Hagedorn I, Luettringhaus A. Mono-salts of bisquaternary pyridinium compounds. Deutsch. Pat. Appl. 2003; 1965: 102
- Benschop HP, De Jong LPA, Kepner LA. 1-Substituted hydroxyiminomethylpyridinium salts for compositions with an activity against organophosphate poisonings. Eur Pat Appl 1981, EP 0023378
- De Jong LPA, Benschop HP, Van den Berg GR, Wolring GZ, De Korte DC. Reactivation of tabun-inhibited acetylcholinesterase by 1-(hetero)arylmethyl-pyridinium-oximes. Eur J Med Chem 1981; 16: 257–262
- Yang GY, Yoon JH, Seong CM, Park NS, Jung YS. Synthesis of bis-pyridinium oxime antidotes using bis(methylsulfonoxymethyl)ether for organophosphate nerve agents. Bull Korean Chem Soc 2003; 24: 1368–1370
- Kuca K, Kassa J. A comparison of the ability of new bispyridinium oxime 1-(4-hydroxyiminomethylpyridinium)-4-(4-carbamoylpyridinium)butane dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase by in vivo methods. J Enz Inhib Med Chem 2003; 18: 529–535
- Kuca K, Patocka J. Reactivation of cyclosarin-inhibited rat brain acetylcholinesterase by pyridinium-oximes. J Enz Inhib Med Chem 2004; 19: 39–43
- Sevelova L, Kuca K, Krejcova G, Vachek J. The therapeutic efficacy of oxime treatment in cyclosarin-poisoned mice pretreated with a combination of pyridostigmine benactyzine and trihexyphenidyl. J Appl Biomed 2004; 2: 163–167
- Cabal J, Kuca K, Kassa J. Specification of the structure of oximes able to reactivate tabun-inhibited acetylcholinesterase. Pharmacol Toxicol 2004; 95: 81–86
- Kuca K, Picha J, Cabal J, Liska F. Synthesis of three monopyridinium oximes and evaluation of their potency to reactivate acetylcholinesterase inhibited by nerve agents. J Appl Biomed 2004; 2: 51–56
- Heath DF. Organophosphorus poisons: Anticholinesterases and related compounds. Pergamon Press, Oxford 1961; 403
- Cabal J, Hampl F, Liska F, Patocka J, Riedl F, Sevcikova K. Hydrates of quaternary ammonium aldehydes as potential reactivators of sarin-inhibited acetylcholinesterase. Coll Czech Chem Commun 1998; 63: 1021–1030