Abstract
Kinetics and inhibition of Na+/K+-ATPase and Mg2+-ATPase activity from rat synaptic plasma membrane (SPM), by separate and simultaneous exposure to transition (Cu2+, Zn2+, Fe2+ and.Co2+) and heavy metals (Hg2+and Pb2+) ions were studied. All investigated metals produced a larger maximum inhibition of Na+/K+-ATPase than Mg2+-ATPase activity. The free concentrations of the key species (inhibitor, MgATP2 − , MeATP2 − ) in the medium assay were calculated and discussed. Simultaneous exposure to the combinations Cu2+/Fe2+ or Hg2+/Pb2+caused additive inhibition, while Cu2+/Zn2+ or Fe2+/Zn2+ inhibited Na+/K+-ATPase activity synergistically (i.e., greater than the sum metal-induced inhibition assayed separately). Simultaneous exposure to Cu2+/Fe2+ or Cu2+/Zn2+ inhibited Mg2+-ATPase activity synergistically, while Hg2+/Pb2+ or Fe2+/Zn2+ induced antagonistic inhibition of this enzyme. Kinetic analysis showed that all investigated metals inhibited Na+/K+-ATPase activity by reducing the maximum velocities (Vmax) rather than the apparent affinity (Km) for substrate MgATP2-, implying the noncompetitive nature of the inhibition. The incomplete inhibition of Mg2+-ATPase activity by Zn2+, Fe2+ and Co2+ as well as kinetic analysis indicated two distinct Mg2+-ATPase subtypes activated in the presence of low and high MgATP2 − concentration. EDTA, L-cysteine and gluthathione (GSH) prevented metal ion-induced inhibition of Na+/K+-ATPase with various potencies. Furthermore, these ligands also reversed Na+/K+-ATPase activity inhibited by transition metals in a concentration-dependent manner, but a recovery effect by any ligand on Hg2+-induced inhibition was not obtained.
Introduction
Na+/K+-ATPase and Mg2+-ATPase are membrane enzymes ubiquitous in animal cells that involve adenosine triphosphate (ATP) as a substrate for their functioning Citation1-3. The ouabain-sensitive Na+/K+-ATPase, or sodium pump, is a membrane bound protein that establishes and maintains the high internal K+ and low internal Na+ concentrations, characteristic of most animal cells. The ouabain-insensitive Mg2+-ATPase is much less well characterized, but apparently consists of at least two forms with different molecular weights [Citation4] and sensitivity to metal ions [Citation5,Citation6].
Hydrolysis of ATP catalysed by these ATPases supplies the energy required for many biochemical processes [Citation7] and depends sensitively on pH, temperature, presence of metal ions, such as Na+, K+ and Mg2+ as well as presence of some modulators. It was reported that highly toxic heavy metals induced inhibition of Na+/K+-ATPase and Mg2+-ATPase activities in a concentration-dependent manner Citation8-11. The metals of the first transition series (Fe, Co, Cu, Zn) are apparently necessary to some physiological processes, but as previously reported, the higher concentrations alter plasma membrane functioning in a variety of tissues, both in vitro and in vivo Citation12-14. Inhibitory effects of Cu2+, Zn2+ and Fe2+ on bovine cerebral cortex Na+/K+-ATPase activity have been obtained and the extent of inhibition seemed to depend on the presence of chelators [Citation15].
Recently, we investigated the in vitro influence of some metal ions (Cu2+, Co2+, Hg2+, Cd2+) on Na+/K+-ATPase and Mg2+-ATPase activity using rat synaptic plasma membranes (SPM) as a model system [Citation5,Citation6,Citation8,Citation9,Citation16]. The present paper continues our study and investigates the effects of some first transition series elements (Cu2+, Zn2+, Fe2+, Co2+) and heavy metals (Hg2+ and Pb2+) on the activity of SPM Na+/K+-ATPase and Mg2+-ATPase. On the one hand, these effects were induced by separate, and on the other, by simultaneous exposures to investigated metals. It has been shown that some of the investigated ions (Cu2+, Zn2+ and Fe2+) form complex entities with ATP. Therefore, our aim was to elucidate whether the inhibition was caused by a decrease in substrate (MgATP2 − ) concentration or was due to interaction with the enzyme. Consequently we followed the influence of the investigated metals on MgATP2 − concentration in the enzyme medium. We also investigated the abilities of − SH containing ligands (gluthathione and l-cysteine) and EDTA to prevent and recover the metal ion – induced inhibition since chelators are able to protect against some toxic effects of metal ions [Citation17]. In addition, extensive kinetic studies were undertaken to determine the nature of the enzyme inhibition by the investigated metals.
Material and methods
Chemicals
All chemicals for the medium assay were commercially available from Sigma (St. Louis, MO, USA) and were of reagent grade. The metal ion salts: mercury (II) chloride, lead (II) chloride, cobalt (II) chloride, zinc (II) chloride, cooper (II) chloride, iron (II) chloride, NaCl, KCl, stannous chloride, ammonium molybdate, were from Merck (Darmstadt, Germany). All solutions were prepared using de-ionized water.
Animals
Adult male Wistar rats three months, 330–400 g, were housed under standard laboratory conditions in the animal house of the Vinča Institute of Nuclear Sciences (Beograd, Serbia & Montenegro). Animals were kept under controlled illumination (lights on: 5:00 a.m.–5:00 p.m.) and temperature (23 ± 2°C), and had free access to food and water. The “Guiding Principles for the Care and Use of Animals” based upon Helsinki Declaration (1964) and “Protocol of the “Vinča” Institute on care and treatment of laboratory animals” were strictly followed.
Synaptic plasma membrane (SPM) preparation
SPM were isolated from the whole rat brain. After decapitation with a guillotine (Harvard Apparatus), brains were rapidly excised and pooled (6/pool) for immediate preparation of synaptic plasma membranes. The SPM were isolated according to the method of Cohen et al. [Citation18], as modified by Towle and Sze [Citation19]. The mitochondrial contamination and protein content were determined according to the standard procedure [Citation16]. SPM were stored at − 70oC until used.
ATPase assay
The standard assay medium for investigation of ATPases activity contained (in mM) 50 Tris–HCl, pH 7.4; 100 NaCl; 20 KCl; 5 MgCl2; 2 ATP; 25 μg SPM proteins. After preincubation for 10 min at 37°C in the absence (control) or in the presence of investigated metals, the reaction was initiated by addition of ATP and stopped after 10 min by adding 22 μl ice cold HClO4 and immediate cooling on ice. The released inorganic orthophosphate (Pi) liberated from the hydrolysis of ATP was determined by a modified spectrophotometric method [Citation6]. The spectrophotometric measurements were performed on a Beckman 5260 UV VIS spectrophotometer. The activity obtained in the presence of Mg2+ alone was attributed to Mg2+-ATPase activity. Na+/K+ -ATPase activity was calculated by subtracting the Mg2+-ATPase activity from the total ATPase activity in the presence of Na+, K+ and Mg2+ ions. The results are expressed as mean % enzyme activity compared to the corresponding control value ± S.E.M. of at least three independent experiments done in triplicate.
Since some of the investigated metal ions form complexes with ATP, their presence required the monitoring of the concentration of MgATP2 − in the reaction mixture which is the actual substrate for both ATPases. The following reactions in the medium assay were taken into consideration for calculation the concentration of MgATP2 − , MeATP2 − and free, uncomplexed metal ion concentrations:
Free metal ion, MeATP2– and MgATP2– concentrations were calculated according to the well-known Storer and Cornish-Bowden method [Citation20] taking into account all the equilibrium reactions involving Mg2+, investigated metal ions and ATP. The stability and equilibrium constants were taken from references [Citation21,Citation22].
The effect of EDTA and − SH containing ligands (l-cysteine and glutathione) on the prevention of the metal ion induced inhibition was measured under the same conditions as described above, with the ligand added to the medium assay before the exposure to metal ions. The recovery of inhibited enzyme activity by investigated ligand was examined by adding ligand in the experimental tube, after 10 min of preincubation in the presence of metal ion at a concentration which induced complete enzyme inhibition. The control tubes contained the corresponding concentration of chelators without metal ions.
Kinetic analysis
Kinetic analysis was carried out according to a slightly modified method of Phylips [Citation23], by following the initial velocity of the enzymatic reaction in the presence of inhibitor (concentration near IC50) and rising concentrations of ATP (0.25–5.5 mM), while maintaining the concentrations of other ions (Mg2+, Na+, K+) constant. The data were analyzed by the softwere package developed [Citation6] and the results were recalculated using EZ FIT [Citation24].
Results
Effects of metal ions on SPM ATPase activities and substrate concentrations
The separate influence of metal ions on Na+/K+- ATPase and Mg2+- ATPase activity was investigated in the concentration range 1 × 10–8–1 × 10–2 M. The increasing concentrations of metal ions induced inhibition of enzymatic activity in a concentration—dependent manner. Sigmoidal inhibition curves for both enzymes were obtained. The half-maximum inhibitory concentrations (IC50) of the investigated ions for both enzymes were determined by Hill analysis of the experimental results and are summarized in . In addition, since it is generally believed that the ionic form of metal ions is responsible for protein interactions, corresponding IC50 values of the “uncomplexed”,”free” form of metals that form complexes with ATP, were calculated and are presented in . It is clearly apparent that Na+/K+ -ATPase is more sensitive to all the investigated metals than Mg2+- ATPase. It is interesting to note that transition metals (except Cu2+) do not inhibit Mg2+-ATPase completely, even when present in concentrations above 1 × 10–3 M. The inhibition of Mg2+-ATPase activity asymptotically approaches 57% for Zn2+, 80% for Fe2+ and 78% for Co2+ in contrast to 100% for Na+/K+-ATPase (results not shown).
Table I. Experimental and recalculated “free” IC50 values (μM).
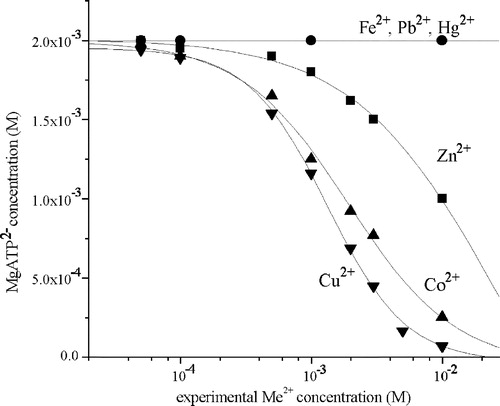
The dependence of MgATP2– concentration on metal concentration in the assay medium assay at pH 7.4 is presented in . The results show that at a concentration level below 2 × 10–4 M, metal ions do not have a significant effect on substrate concentration in the enzyme mixture since under the experimental conditions, the concentration of MgATP2– was in excess. By increasing metal ion concentration above 2 × 10–4 M, MgATP2– concentration significantly decreased.
Inhibition of SPM ATPases by simultaneous exposure to metal ions
The effect of simultaneous exposure to combinations of metal ions on the activity of Na+/K+-ATPase and Mg2+-ATPase was studied by using the mixtures of Cu/Fe, Cu/Zn, Fe/Zn, and Pb/Hg. The concentrations of metals were 5 × 10–7–2 × 10–5 M, i.e. limited to producing the inhibition in the concentration range lower than the IC50 values. The inhibition obtained by exposure to several combinations was compared to the inhibition in the presence of each single metal. The mean values of experimentally determined and mathematically added values are presented in . The mathematically determined means were calculated as the sum of metal ion-induced inhibitions measured separately. Analysis of variance (One way ANOVA) was used to compare the mathematical sum of inhibitions caused by separate exposure to metal ions with inhibitions induced by exposure to two metals in combination (simultaneously). When a significant F value (P < 0.05) was obtained, the post hoc test Bonferoni was used to determine the differences.
Table II. Inhibition of Na+/K+-ATPase and Mg2+-ATPase activity by simultaneous exposure to metal ions in mixtures.
A synergistic effect was defined as a statistically significant (P < 0.05) difference between inhibitions caused by simultaneous exposure and mathematically calculated mean values of enzyme inhibition for a pair of metals, where the former is larger than the latter. An antagonistic effect was defined as above, except that the inhibition caused by simultaneous exposure is less than the mathematically calculated mean value.
Cu2+ and Fe2+ together caused an additive inhibition, while simultaneous exposure to Cu2+ and Zn2+, or Fe2+ and Zn2+ induced synergistic inhibition of SPM Na+/K+- ATPase activity at all investigated concentrations. Simultaneous exposure of Mg2+-ATPase to Cu2+ and Fe2+ or Cu2+ and Zn2+ ions caused synergistic inhibition, while Fe2+ and Zn2+ together inhibited the enzyme antagonistically. Exposure to the Pb2+/Hg2+ combination induced an additive inhibition of Na+/K+- ATPase activity as well as antagonistic inhibition of Mg2+-ATPase activity at all concentrations examined.
Effects of chelators on the prevention and recovery of inhibited Na+/K+-ATPase activity
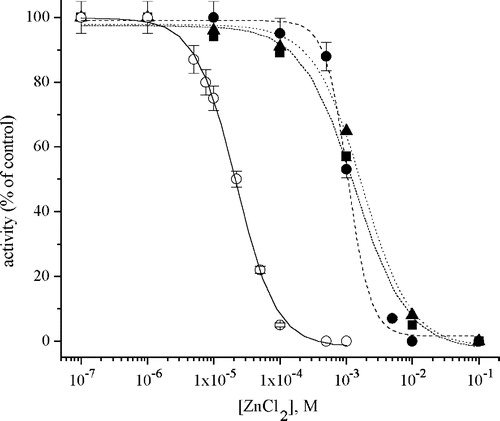
The inhibition of Na+/K+-ATPase induced by metal ions was investigated in the presence of 1 mM EDTA, 10 mM l-cysteine and 10 mM GSH. The Zn2+-induced inhibition curves of Na+/K+-ATPase activity in the presence and absence of chelators are shown in , and similar results were obtained for the other metals of the first transition series. It is obvious that the presence of EDTA in the reaction mixture at a 1 mM concentration prevents enzyme inhibition at a metal concentration below 1 mM, since 10 mM l-cysteine and 10 mM GSH induce the same effect. Moreover, in the presence of 1 mM EDTA, or 10 mM sulphur-containing chelators, the effect on the enzyme activity of these investigated metals in the reaction mixture at a total concentration below 1 × 10–4 M was negligible.
Figure 2 Inhibition of Na+/K+-ATPase by Zn2+ in the absence (○) and presence of: 10 mM l-Cysteine (▪); 10 mM glutathione (▴) and 1 mM EDTA (•).
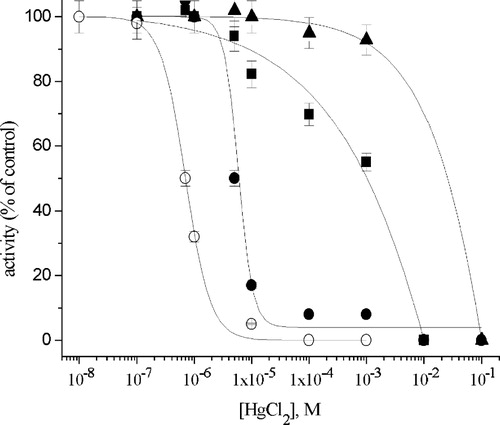
The Hg2+-induced inhibition curves of Na+/K+-ATPase activity in the presence and absence of chelators are shown in . It could be noted that the most protective effect on Na+/K+-ATPase activity was achieved in the presence of 10 mM GSH in the reaction mixture, while the ability of EDTA to prevent the Hg2+-induced inhibition was the least. In the presence of 10 mM GSH, the IC50 value of HgCl2 was almost five orders of magnitude higher than the IC50 value in the absence of any chelator, while the presence of 10 mM l-cysteine caused a four orders of magnitude increase in the IC50 value for HgCl2.
Figure 3 Inhibition of Na+/K+-ATPase by Hg2+ in the absence (○) and presence of: 1 mM EDTA (•); 10 mM l-cysteine (▪) and 10 mM glutathione (▴).
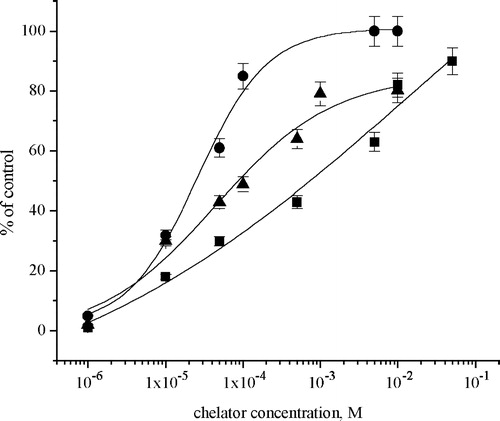
Since it was demonstrated that EDTA, l-cysteine and GSH prevent the metal ion-induced inhibition of Na+/K+-ATPase, the recovery of the inhibited enzyme activities was investigated by varying the chelator concentration from 1 × 10–6–1 × 10–1 M. The effect of chelators was investigated in the presence 1 × 10–4 M of metal ions, given the fact that at this concentration, investigated metals induced complete inhibition of Na+/K+-ATPase. The results presented in show that the chelators had a dose-dependent recovery effect on Na+/K+-ATPase activity exposed to all metal ions that belong to the first transition series. On the contrary, recovery of Hg2+-induced inhibition was not achieved, even with a chelator's concentrations above 0.01 M.
Kinetic analysis
In order to evaluate the nature of metal ion-induced Na+/K+-ATPase and Mg2+-ATPase inhibition, kinetic analysis in the presence and absence of inhibitors was performed. The influence of metal ions (concentrations near IC50 values) on catalytic activity was investigated by following the initial velocities vs. the concentration of ATP (from 0.25–5.50 mM). Dependence of the reaction rate vs. MgATP2 − concentration for Na+/ K+-ATPase in the presence and absence of metal ions exibited typical Michaelis–Menten kinetics in all cases. The kinetic parameters (Km, Vmax) were estimated from Eadie–Hofstee transformation of the data and are summarized in . It is obvious that all metal ions that belong to the first transition series inhibited enzyme activity noncompetitively by decreasing its Vmax value, without changing significantly its apparent affinity for ATP. It is worthy to note that the saturating levels of MgATP2– were always present in the assay medium assay at an IC50 of metal ions.
Table III. Kinetic analysis of Na+/K+-ATPase and Mg2+-ATPase activity in the absence (control) and presence of metal ions.
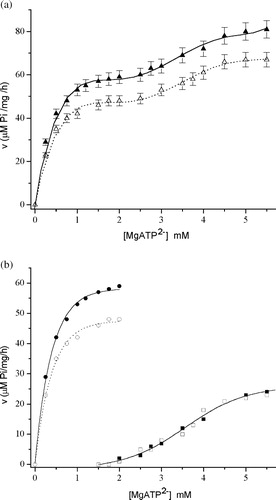
Mg2+-ATPase activity exhibited biphasic dependence on increasing MgATP2– concentration in the absence and presence of all investigated metal ions. The Mg2+-ATPase activity as a function of MgATP2– concentration, in the presence and absence of ZnCl2 is presented in (a). The obtained functions are not purely of the Michaelis–Menten type, and could be approximated by two overlapping curves separated by a plateau. The results indicate that the enzyme activity at each ATP concentration represent the sum of the activity of two distinct Mg2+-ATPase subtypes i.e. the plot of the total activity represents the line for “two enzymes acting on one substrate”. The first one, active at a substrate concentration below 1 mM, is denoted as “high affinity”, while the second, active at MgATP2– concentration above 2 mM, is denoted as “low affinity” Mg2+-ATPase. Analysis of the data by a PC software package [Citation20] revealed the Michaelis–Menten enzyme kinetics at low and sigmoidal kinetics at high ATP concentrations. The theoretical curves for “high” and “low affinity” Mg2+-ATPase subtypes in the presence and absence of Zn2+ are presented in (b). The kinetic parameters for “high affinity” subtype, in the presence and absence of metal ions, were calculated by the Eadie–Hofstee transformation and are summarized in . These results suggest that Zn2+, Fe2+, Co2+and Cu2+ are noncompetitive inhibitors of this enzyme, because they significantly decrease its Vmax value without changing significantly its apparent affinity for ATP. The activity of the “low affinity” subtype was reconstructed by subtracting the calculated “high affinity” values at each ATP concentration from the experimentally measured total activity. From the obtained results, initial velocity was recalculated. The iterative procedure was repeated until the best fit of the experimental results was achieved. The kinetic constants of the “low affinity” Mg2+-ATPase were calculated by Hill analysis and are presented in . It is notable that the effects of Zn2+, Fe2+and Co2+ on the kinetic parameters of the “low affinity” Mg2+-ATPase was negligible.
Figure 5 (a) The dependence of Mg2+-ATPase activity on MgATP2– in the absence (▴) and in the presence (Δ) of 15 μM ZnCl2. Symbols represent experimental points. (b) The Mg2+-ATPase theoretical kinetic curves (Mg2+-ATPase activity vs MgATP2 − concentration) of: “high affinity “Mg2+-ATPase subtype (○) in the absence of ZnCl2 (•) and in the presence of ZnCl2 (○); and “low affinity” Mg2+-ATPase subtype (squares) in the absence of ZnCl2 (▪) and in the presence of ZnCl2 (▪ or □).
Discussion
Results of our study showed that the examined metal ions, as previously reported [Citation5,Citation6,Citation9,Citation25], inhibited rat brain Na+/K+-ATPase and Mg2+-ATPase activities and had various potencies (). It is reasonable to assume that this potency depends on the nature of the metal ion as well as the nature of the enzyme. Obtained IC50 values of Zn2+ and Fe2+ for Na+/K+-ATPase are of the same order of magnitude as the IC50 values reported for bovine cerebral cortex Na+/K+-ATPase [Citation15]. Experimental IC50 values () of all investigated ions that form inactive MeATP2– complex are below 2 × 10–4 M for both enzymes, while the concentration of substrate, MgATP2–, remains practically unchanged ().These results indicate that inhibition of the enzyme activities was due to the metal ion binding on the enzyme binding sites rather than the influence on the formation of an inactive MeATP2– complex.
Furthermore, incomplete inhibition of Mg2+-ATPase by Zn2+, Fe2+ and Co2+ is in accordance with kinetic analysis that confirms the existence of at least two Mg2+-ATPase subtypes. One of them, denoted as “low affinity” Mg2+-ATPase, being insensitive towards the inhibition induced by Zn2+, Fe2+ and Co2+ and their presence, had no effect on kinetic parameters. Incomplete inhibition of Mg2+-ATPase activity supports the results of earlier studies of rat brain ATPases which suggested that there are two different Mg-ATPases differentiated by their sensitivity to metal ions [Citation25] and ehacrinic acid [Citation4]. Two distinct Mg2+-ATPase activities differentiated by their sensitivity to Ca2+, La3+ and F-, were isolated from Triton-X-100 solubilized human erythrocyte membranes [Citation26]. On the contrary, as previously reported [Citation6,Citation9], Cu2+ and heavy metals (Pb2+, Cd2+ and Hg2+) inhibit Mg2+-ATPase activity completely, while significantly changing the kinetic parameters for both Mg2+-ATPases. These results are also in accordance with synergistic inhibition caused by simultaneous exposure to Cu2+ and Zn2+ or Cu2+ and Fe2+ suggesting different modes of interaction of Cu2+ and Zn2+ (as well as Cu2+ and Fe2+) with Mg2+-ATPases.
The additive inhibition of SPM Na+/ K+-ATPase activity obtained by simultaneous exposure to Cu2+ and Fe2+ (as well as Hg2+ and Pb2+) suggests that both metal ions are competing for the same inhibitor binding sites and there is an excess of inhibitor binding sites within the concentration ranges examined. Competition between Hg2+ and Pb2+ is in agreement with the chemical similarities of these ions [Citation21,Citation22], as well as with the previously reported fact that both ions are noncompetitive inhibitors of the investigated enzyme [Citation9]. The results that Cu2+ in combination with Zn2+, as well as Fe2+ in combination with Zn2+, inhibit Na+/K+-ATPase activity synergistically, but additivelly with each other (Cu2+/Fe2+), suggest that Zn2+ binds at different binding sites, which are linked to different but associated mechanisms of inhibition. The Zn2+-induced inhibition may include, as suggested earlier [Citation15], Zn2+ binding to the Mg2+-binding site, which directly affects the phosphorylation step in the enzyme cycle [Citation15], while Cu2+- and Fe2+-induced inhibition involve interactions with other functional groups on the enzyme. However, Zn2+ binding to the Mg2+-binding site could not be responsible for Mg2+-ATPase inhibition, since this enzyme appears not to belong to the P-type ATPases although synergistic inhibition obtained by simultaneous exposure to Cu2+ and Zn2+ (as well as Cu2+ and Fe2+) suggest that these pairs of metals bind to different types of binding site. The antagonistic inhibition of Mg2+-ATPase activity with Fe2+ and Zn2+ at all concentrations examined suggest that the two metals are competing for a limited number of binding sites or the binding of one metal decreases the binding of second through a conformational change in the enzyme.
Our study clearly showed that the presence of 1 mM EDTA, 10 mM l-cysteine and 10 mM GSH prevents enzyme inhibition induced by Zn2+, Fe2+, Co2+ and Cu2+. These results are in agreement with the strong chelating effects of the investigated ligands which significantly decrease the ionic form of metals able to interact with enzymes. Strong protective effects of − SH containing-ligands on Hg2+-induced inhibition are a sufficient basis for a plausible assumption that Hg2+ is a potent reagent for thiol groups. Previous studies have shown that Hg2+ inhibition is due to its binding to sulphydryl groups of Na+/K+-ATPase [Citation27,Citation28], which means that the lowest protective effect of EDTA can be explained by supposing that Hg2+ binds more efficiently to the − SH groups of enzyme in comparison to this ligand.
Moreover, the recovery of transition metal-induced inhibition of Na+/ K+ -ATPase is probably due to competition between the functional groups of protein and ligands for complex formation with these metal ions. These results are consistent with the kinetic analysis suggesting that these ions are reversible, noncompetitive inhibitors of this enzyme. In spite of the fact that the kinetic analysis showed that Hg2+—induced inhibition was reversible [Citation9], the attempt to reactivate the enzyme clearly failed. Hence, we may assume that the Hg binding affinity for the enzyme far exceeds its affinity towards either of the investigated chelators.
To summarize, our results confirm that the inhibitory effects of metal ions may be attributed to their ionic forms and that the induced inhibition is likely due to direct interaction between metal ions and enzymes instead of between ions and ATP. Kinetic analysis showed that the nature of Na+/ K+—ATPase inhibition by the investigated metals is non-competitive. The incomplete inhibition by Zn2+, Fe2+ and Co2+, as well as kinetic analysis indicated two distinct SPM Mg2+-ATPase subtypes with different sensitivity to metal ions. The inhibitory effects can be prevented by addition of strong metal—ion chelators, such as EDTA, l-cysteine and GSH. Finally, the effect of these chelators on the recovery of transition metal-induced enzyme inhibition is dose-dependent, but recovery of the Hg2+-induced inhibition was not achieved, even when the chelators were present at concentrations above 0.01 M.
Acknowledgements
This study was supported by the Ministry of Science, Technology and Development of the Republic of Serbia, Project No 1991.
Notes
*Hg2+, Fe2+ and Pb2+ don't form complexes with ATP.
References
- Vasilets LA, Schwarz W. Biochim Biophys Acta 1993; 1154: 201–222
- Rodrigez de Lores Aranaiz G, Pena C. Neurochem Int 1995; 27: 319–327
- Post RL, Merrit CR, Kinsolving CR, Allright CD. J Biol Chem 1960; 253: 1796–1802
- Tanaka T, Inagake C, Kunugy Y, Takaori S. Jpn J Pharmacol 1987; 45: 205–211
- Vujisić LJ, Krstić D, Vučetić J. J Serb Chem Soc 2000; 65: 507–515
- Vasić V, Jovanović D, Krstić D, Nikezić G, Horvat A, Vujisić Lj, Nedeljković N. Toxicol Lett 1999; 110: 95–104
- Voet D, Voet J. Transport through membranes. Biochemistry2nd ed. John Willey and Sons, New York 1995; 524
- Nikezić G, Horvat A, Todorović S, Vasić V, Vujisić LJ. Book of abstracts. 1997; 325, 11th BBBD Balkan Biochemical Biophysical Days, Thessaloniki, Greece
- Vasić V, Jovanović D, Horvat A, Momić T, Nikezić G. Anal Biochem 2002; 300: 113–120
- Diamont GL, Zalups RK. Toxicol Pathol 1998; 26: 92–103
- Preininger C, Wolfbeis OS. Biosens Bioelectron 1996; 11: 981–990
- Dykastra M, Intveld G, Vandenburg GJ, Muller M, Kuipers F, Vonk R. J Clin Investig 1995; 95: 412–416
- Dunning JC, Ma Y, Marquis Re. Appl Environ Microbiol 1998; 64: 27–33
- Prakash NJ, Fontana J, Henkin RI. Life Sci 1973; 12: 249
- Hexum TD. Biochem Pharmacol 1974; 23: 3441–3447
- Nikezić G, Horvat A, Nedeljković N, Todorović S, Nikolić V, Kanazir D, Vujisić Lj, Kopečni M. Gen Physiol Biophys 1998; 17: 15–23
- Peyser YM, Ben-Hur M, Weher MM, Muhlard A. Biochemistry 1996; 35: 4409–4416
- Cohen RS, Blomberg F, Berzins K, Siekevitz P. J Cell Biol 1977; 74: 181–203
- Towle AC, Sze PY. J Steroid Biochem 1983; 18: 135–143
- Storer AC, Cornish-Bowden A. Biochem J 1976; 159: 1–5
- Högfeldt E. Stability constants, Part A: Inorganic ligands. Pergamon, Oxford 1983
- Silen LG, Martell AE. Stability constants of metal ligand complexes. Special Publication No 25, The Chemical Society, London 1971
- Phyllips TD, Hayes AW, Ho IK, Desiah D. J Biol Chem 1978; 253: 3487–3493
- Perrella FW. Anal Biochem 1988; 174: 437–447
- Cafagna MA, Ponsler GD, Muhobeac BB. Chem Biol Inteact 1996; 100: 53–65
- Merran EA, Michael BM, Basil DR. Arch Biochem Biophys 1994; 312: 272–277
- Chandra SV, Murthy RC, Husian T, Bansal SK. Acta Pharmacol Toxicol 1984; 54: 210–218
- Ahamadsahib KI, Ramamurthi R, Desaiah DJ. Biochem Toxicol 1987; 2: 169–176