Abstract
In vitro effects of ketamine and bupivacaine drugs on bovine lactoperoxidase (LPO; E.C. 1.11.1.7) enzyme activity were investigated. Lactoperoxidase was purified with Amberlite CG 50 resin, CM Sephadex C-50 ion-exchange chromatography, and Sephadex G-100 gel filtration chromatography from skimmed bovine milk. Rz(A412/A280) value for the purified LPO was found to be 0.8. Inhibition or activation effects of the drugs on LPO enzyme were determined using 2,21-azino-bis (3-ethylbenzthiazoline-6 sulfonic acid) diammonium salt (ABTS) as a chromogenic substrate at pH = 6.0. The I50 values of ketamine and bupivacaine were 0.29 mM and 0.155 mM, respectively and the Ki constants for ketamine and bupivacaine were 0.019 ± 0.031 and 0.015 ± 0.021 mM, respectively; they were non-competitive inhibitors.
Introduction
Lactoperoxidase (LPO) (donor: hydrogen peroxide oxidoreductase E.C.1.11.1.7) has been reported in bovine milk [Citation1]. Peroxidase activity is present guinea pig, cow, sheep, pig, I lama mouse and human milk Citation2-3. This enzyme is one of the prominent enzymes in milk and has the ability to catalyze the oxidation of halides and pseudohalides, such as thiocynate, by hydrogen peroxide to form potent oxidant and bactericidal agents [Citation4]. LPO is an oxidoreductase secreted into milk and plays an important role in protecting the lactating mammary gland and the intestinal tract of newborn infants against pathogenic microorganism [Citation5]. The enzyme catalyzes the oxidation of endogenous thiocyanate (SCN− ) to the antibacterial hypothiocyanate (OSCN− ) [Citation6] and is a redox enzyme with an antibacterial property found in several biological fluids, e.g. milk, tears, saliva [Citation7]. The peroxidase activity in bovine milk is attributable to LPO secreted from the mammary gland; other biological effects of this protein including antitumor activity have been reported. The enzyme has a single polypeptide chain containing 612 amino acid residues, a heme prosthetic group, and four or five carbohydrate chains which occupy approximately 10% of the total mass; its molecular weight is about 85 kDaltons [Citation8,Citation9].
Anesthetics are widely used in dentistry and in the therapy of chronic pain. Bupivacaine is perhaps the most commonly used local anesthetic and plays an important role in the overall management of surgical and postoperative pain and dental care [Citation10]. Ketamine, or ketamine hydrochloride, is a non-barbiturate used both in animals and humans and it also has been used in human medicine for pediatric burn cases and dentistry [Citation11].
Ketamine and bupivacaine are used world-wide as anesthetic drugs and there has been an increased use of regional anesthesia for vaginal and cesarean delivery [Citation12]. Owing to their wide use in both human and pergnant animals, we thought it was important to study the effect of these on LPO activity during lactation and breast milk and this is the first report effects on bovine LPO activity of anesthetic drugs.
Experimental
Purification of LPO
Bovine milk was centrifuged at 2500 rpm at 4°C for 15 min to remove fat. Amberlite CG resin (equilibrated with 5 mM sodium acetate pH 6.8) was added in the proportion of 22 g/L to the fresh raw skimmed bovine milk [Citation6,Citation13]. The supernatant was decanted and the resin was washed with distilled water and 20 mM sodium acetate (pH 6.8). The bound protein was eluted with 0.5 M sodium acetate pH 6.8. To the green-coloured mixture was gradually added solid ammonium sulfate (I. Precipitation; saturation 90%) over a period of 30 min while it was being stirred magnetically and the enzyme solution was dialyzed overnight against 5 mM sodium phosphate buffer pH 6.8.
The clear greenish supernatant obtained above was loaded onto a column of CM Sephadex C-50 (Fluka) (3x10 cm) previously equilibrated with 10 mM sodium phosphate buffer (pH 6.8). The column-bounded enzyme was washed with 100 ml of 10 mM phosphate buffer pH 6.8 containing 100 mM NaCl. The enzyme was eluted with a linear gradient 100–200 mM NaCl in 10 mM phosphate buffer pH 6.8 and subjected to ammonium sulfate precipitation (II, saturation 90%). Thereafter the enzyme solution was dialyzed overnight against 5 mM sodium phosphate buffer pH 6.8.
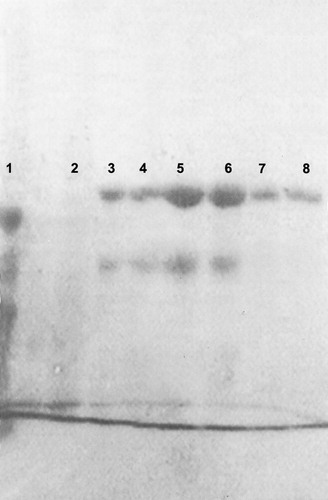
Lactoperoxidase enzyme obtained from the CM Sephadex C-50 column was applied to a column of Sephadex G 100 (Fluka) (2.5 × 100 cm). The column-bound enzyme was eluted with 0.1 M phosphate buffer pH 6.8, and salted out with ammonium sulfate precipitation (III, 90% saturation). The enzyme solution was dialyzed overnight against 0.5 M sodium phosphate buffer pH 6.0. Fractions were lyophilized and checked for purity by SDS-PAGE gel [Citation14] (). Protein concentration was determined according to the method of Lowry using bovine serum albumin as a standard [Citation15].
Figure 1 SDS-Polyacrylamide gel electrophoresis of purified LPO. (lane 1; Serum albumin (M.W 66.000) ovalbumine (M.W 45.000), carbonic anhydrase (M.W 30.000.) (Lane 2; Crude extract), (Lane 3–4: Ammonium sulfate precipitation), (Lane 5–6: purified bovine LPO in CM sephadex C-50 column), (Lane 7–8: purified bovine LPO in sephadex–G100 column).
Determination of LPO activity
Lactoperoxidase activities were determined by the procedure of Shindler and Bardsley with a slight modification [Citation16]. This method is based on oxidation of 2,2-azino-bis (3-ethylbenzthiazoline-6 sulfonic acid) diammonium salt (ABTS) as a choromogenic substrate by means of H2O2, and the color compound which occurs during reaction gives an absorbance at 412 nm. Briefly, 2.8 ml of 1 mM ABTS in phosphate buffer (0,1 M pH = 6.0) was mixed with 0.1 ml of enzyme in phosphate buffer 1 mM pH 6.8 and 0.1 ml of 3.2 mM H2O2 solution. The absorbance was taken at 412 nm as a function of time at every 15 s. One unit of activity is defined as the amount of enzyme catalyzing the oxidation of 1 μmol of ABTS min− 1 at 298°K (molar absorption coefficient; 32400 M− 1 cm− 1)
Effects on LPO activity of anesthetics drugs
To determine the effects of the anesthetic drugs on LPO, enzyme activities were measured in the presence of ketamine (0.095–0.469 mM), bupivacaine (0.184–0.363 mM) at these cuvette concentrations.Control cuvette activity in the absence of drug was taken as a 100%. For each drug an Activity-[Drug] graph was drawn. For two of these anesthetics (ketamine and bupivacaine) which had an inhibitory effect on the enzyme, the drug concentration that produced 50% (I50) was calculated from the graph ().
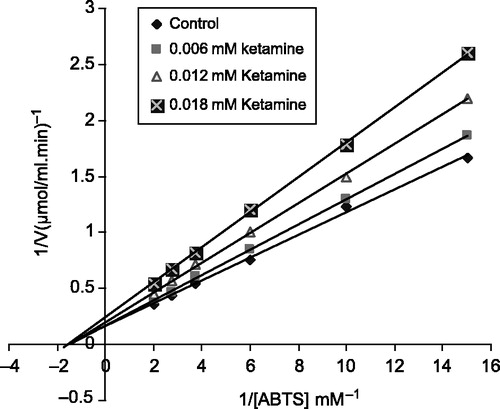
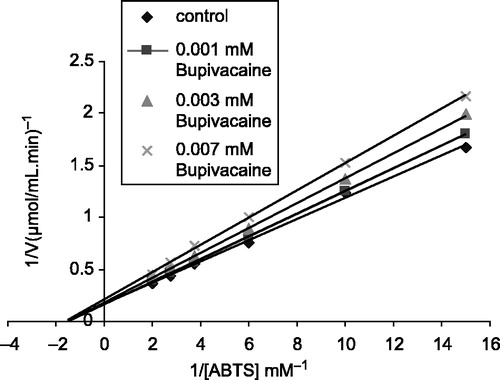
For determination of the Ki constant, three different inhibitor concentration (0.06, 0.012,0,018M for ketamine), and (0.001, 0.003, 0.007 mM for bupivacaine) were used. In these studies ABTS was used as substrate at six different concentrations (0.06–0.5 mM). From the Lineweaver-Burk graph (1/V-1/[S]) obtained for each inhibitor, Ki constant and inhibition type was determined from the graphs ( and ). Analysis of data obtained was made by t-test and they are given as X ± SD.
Results
LPO was eluated from CM sephadex C 50 ion exchange chromatography by measuring the Rz (A412/A280 nm) of fractions. Fractions with Rz values 0.7 or higher were pooled. The enzyme obtained from ion exchange chromatography was applied to a Sephadex G 100 gel filtration chromatography. Fractions with Rz values 0.7 or higher were pooled. As shown (), specific activity was calculated for the crude extract and purified enzyme solution, so yielding a purification of 11.2 fold and yield of 6.7 mg (Rz = 0.8) from 1 L bovine milk. Kinetic parameters at optimum pH, Km and Vmax were calculated by graphics for ABTS as substrate on LPO. Optimum pH value was found by means of activity-pH graphs, and was 6; the Km value at optimum pH was 0.62 mM, and the Vmax value at optimum pH was 6.1 μmol/mL min. All purification steps were controlled by SDS-PAGE (). Serum albumin, ovalbumin and carbonic anhydrase were used as standards and bovine lactoperoxidase had a molecular weight of about 80000 Da.
Table I. Purification steps of lactoperoxidase from bovine milk.
In this study, both Ki and I50 parameters for these drugs as inhibitors of bovine LPO were determined. Ki values were calculated from Lineweaver-Burk graphs ( and ). Ki constants of ketamine and bupivacaine were 0.019 ± 0.031 mM and 0.015 ± 0.021 mM, respectively. Ketamine and bupivacaine showed non-competitive inhibition. The inhibitor concentrations causing up to 50% inhibition were determined from % Activity-[drugs] graphs (). The obtained I50 values of ketamine and bupivacaine were 0.29 mM and 0.155 mM, respectively ().
Table II. I50 values, Ki constant, and inhibition types of Ketamine and Bupivacaine for bovine LPO.
Discussion
LPO is an oxidoreductase secreted into milk and plays an important role in protecting the lactating mammary gland and intestinal tract of newborn infants against pathogenic microorganism [Citation5]. The LPO system is the most significant microbial inhibitor in raw milk [Citation17]. LPO is naturally present in raw milk and together with thiocyanate and peroxide constitutes the LPO system. Thiocyanate is more variable in milk and depends on the feed for the animal (FAO,1999). The LPO system has been recommended for milk preservation. LPO system is one of the important host defence system in with oral activity and may exert some protective effects such as antimicrobial activity and removal of toxic peroxide both in the mammary gland and in the gut of infants throughout the lactation period [Citation5]. Human milk is known to contain several host defence factors including lactoferrin peroxidases and lysozyme.
Anesthetics are used as an adjunct to surgical procedures to make the patient unaware of and unresponsive to, painful stimulation. General anesthetics are usually given by inhalation or by intravenous injection [Citation18]. General anesthetics in cesarean section may cause problems such as maternal aspiration, difficulty in intubations, fetal distress and prolonged breast-feeding interval [Citation19]. Several studies concerning anesthetic drugs and erythrocytes enzymes have been published indicating a strong inhibitory effect [Citation20]. Local anesthetics have been reported to inhibit Ca2+-ATP as an Na+/Ca2+-exchanger of synaptosomal plasma membrane [Citation21]. The other study, anesthetics inhibited Na+,K+-ATPase, Mg2+-ATPase and acetylcholinesterase activity from rat cerebral cortex [Citation21]. There is no detailed study regarding the effect of anesthetic drugs on LPO activity. In this study, Anesthetic drugs using during lactation, were investigated for their inhibitory effects on LPO in vitro and kinetic constants (Ki and I50 values) reported. The study showed that ketamine and bupivacaine had strong inhibitory effects on LPO activity, effects not previously reported.
In conclusion, we think that the results of this study draw attention to using local and general anesthetic drugs throughout lactation the period since LPO has antimicrobial activity and contributes to the protective functions of milk; use of anesthetic drugs may decrease LPO activity in milk during lactation. If it is required to give these anesthetic drugs during lactation, their dosage should be very carefully considered to decrease the side-effects. Investigation of the in vivo effects of these drugs on LPO activity is more important from a clinical point of view and for this reason, detailed studies are required.
References
- Golhefors L, Marklundi S. Infect Immun 1975; 11: 1210–1215
- Shin K, Tomita M, Lönnerdal B. J Nutr Biochem 2000; 11: 95–101
- Morin DE, Rowan LL, Hurley WL. Small Ruminnat Res 1995; 17: 255–261
- Reiter B, Perraudin JP. Lactoperoxidase. Peroxidase Chemistry and Biology, J Everse, KE Everse, MB Grisham. CRC press, FL USA 1991
- Ueda T, Sakamaki K, Kuroki T, Yano I, Nagata S. Eur J Biochem 1976; 243: 32–41
- Kumar R, Bhatla KL. Acta Cryst 1995; D51: 1094–1096
- Dumonte C, Rousst B. J Biol Chem 1983; 258: 14166–14172
- Dewil JN, Van Hooydonk ACM. Netherlands milk dairy 1996; 50: 227–244
- Cariström A. Biochim Biophys Acta 1969; 23: 185–202
- Dunsky J, Moore PA. J Endodont 1984; 10: 457–460
- Dalgarno PJ, Shewan D. J Psychoactive drugs 1993; 28: 191–199
- Bond GM. Anaesthesia intensive care 1992; 20: 426,430
- Özdemir H, Aygül İ, Küfrevioğlu Öİ. Prep Biochem Biotech 2001; 31(2)125–134
- Laemmli DK. Nature 1970; 227: 680–685, (Lond.)
- Segel IE. Enzyme Kinetics. John Wiley and Sons. Toronto 1968; 403
- Shindler JS, Bardsley WG. Biochem Biophys Res Commun 1975; 67: 1307–1312
- Haddain MS, Ibrahim SA, Robinson RK. Food Control 1996; 7: 149–152
- Rang HP, Dale MM, Ritter JM. Pharmacology, 4th Edition. Churchill.Livinstone, China 1999; 1–830
- Trevor AJ, Miller RD. General anesthetic in basic and clinical pharmacology, BD Catzung. Appleton and Lange, USA 1989; 304–322
- Altıkat S, Çiftçi M, Büyükokuroğlu ME. Pol J Pharmacol 2002; 54: 67–68
- Sidek HM, Nayquist-Battie C, Vanderkooi G. Biochim Biophys Acta 1984; 801: 26–31

![Figure 2 % Activity-[drug] graphs for LPO for two drugs; (A) Bupivacaine and (B) Ketamine.](/cms/asset/da38e4f5-8ebb-47de-9f70-791b45dc5a70/ienz_a_122487_f0002_b.jpg)