Abstract
Given the importance of protein phosphorylation in the context of cellular functions, abnormal protein phosphatase activity has been implicated in several diseases, including cancer. These critical roles of protein phosphatases qualify them as potential targets for the development of medicinal compounds that possess distinct modes of action such as violacein. In this work, studies with this natural indolic pigment at a concentration of 10.0 μmol L− 1 demonstrated a 20% activation of total protein phosphatase extracted from human lymphocytes. Although no alteration was observed on protein tyrosine phosphatase (CD45), 30% of inhibition was achieved in cytoplasmatic protein phosphatase activity after incubation with 10.0 μmol L− 1 violacein. Additionally, 5.0 μmol L− 1 of violacein inhibited by 50% the serum tartrate-resistant acid phosphatase activity. Violacein presented toxic effect on lymphocytes with IC50 values of 3 and 10 μmol L− 1 for protein content and protein phosphatase activity, respectively. These findings suggest an important role for protein phosphatases in the mechanisms controlling proliferation and cell death.
Introduction
Violacein (), the major pigment produced by Chromobacterium violaceum, is an indole derivative characterized as 3- (1,2-dihydro-5- (5-hydroxy-1H-indol-3-yl)-2-oxo-3H-pyrrol-3-ilydene)-1,3-dihydro-2H-indol-2-one [Citation1]. The biosynthesis and biotransformation of violacein has been extensively studied and further optimized its production, extraction and purification [Citation1,Citation2].
This pigment has been reported to possess several activities, including bactericidal [Citation3,Citation4], trypanocidal [Citation5,Citation6], mycobactericidal [Citation7], as well as antiviral [Citation8], antiulcerogenic [Citation9] and antitumoral properties Citation10-14.
Protein phosphorylation-dephosphorylation is probably the most crucial chemical reaction taking place in living organisms [Citation15]. In an average eukaryotic cell, every third protein undergoes reversible phosphorylation [Citation15,Citation16]. This dynamic equilibrium is a ubiquitous element of intracellular signal transduction pathways that are essential for regulating cell growth, differentiation and metabolism, and survival. Abnormal changes in the activities of these enzymes can lead to severe consequences, including cancer and diseases resulting from immunological disorders [Citation17].
Protein tyrosine phosphatases constitute a large family of enzymes that can be divided into three major subfamilies: tyrosine-specific, dual-specific, and low molecular weight phosphatases. Unlike the tyrosine-specific and low molecular weight phosphatases, which strictly target pTyr-containing proteins, dual-specificity phosphatases utilize protein substrates that contain pTyr as well as pSer and pThr. Protein tyrosine-specific phosphatases (PTPs) can be further divided into two groups: receptor-like (e.g. CD45) and intracellular PTPs. Genetic and biochemical studies have implicated various PTPs as potential targets for drug development in diverse areas such as cancer, hematopoiesis and immune function Citation18-20.
The significant effect of violacein on different tumor cell lines prompted us to evaluate its effect on normal human lymphocytes viability as well as its action on two important phosphatases from these cells, CD45 and low-molecular-weight PTP. T cell express approximately 50–60 different PTPs. CD45, SH2 (Src homology 2 SH2-domain-containing PTP2) and low-molecular-weight PTP have positive roles in signaling through the T cell receptor [Citation21]. The preferential cytotoxic activity of violacein in myeloid leukemia cells alludes to the potential of violacein to be developed as an anti-leukemic agent [Citation14]. Therefore, the establishment of its cytotoxic effects on untransformed cells is of major importance. Given that the active site pockets of phosphatases are very similar among various enzymes in each family, it would be challenging to develop specific inhibitors to probe specific physiological roles played by individual phosphatases in this process. It was therefore of interest to us, in the course of the search for new therapeutic agents from natural origin, to study the effects of violacein on protein phosphatases extracted from different sources and also investigate their involvement in violacein cytotoxicity to human blood lymphocytes. The observation of violacein action on these molecular targets, would not only contribute to the elucidation of its mechanism of action, but also promote a rational and safe use of this natural product for new therapeutics.
Materials and methods
Violacein
Chromobacterium violaceum (CCT 3496) was cultivated on cotton, in modified 1 litre Roux bottles on a surface tray bioreactor according to Rettori and Durán [Citation1]. Violacein {3-[1,2-dihydro-5-(5-hydroxy-1H-indol-3-yl)-2-oxo-3H-pyrrol-3-ilydene]-1,3-dihydro-2H-indol-2-one} was isolated from C. violaceum, purified by Soxhlet extraction and characterized by proton and carbon-13 NMR spectroscopies, mass spectrometry, UV-VIS spectroscopy and infrared spectroscopy as previously described [Citation1,Citation4].
Cells
Peripheral blood was obtained by venipuncture from healthy adult donors and mononuclear cells were isolated by Ficol1-Hypaque gradient separation. Lymphocytes were washed and resuspended in RPMI medium supplemented with 10% fetal bovine serum.
Cytotoxicity assays
Lymphocytes were washed, resuspended in RPMI medium supplemented with 10% fetal bovine serum and plated (1x106 cells mL− 1) in the presence of 5 μg mL− 1 phytohemagglutinin. After incubation for 48 h at 37°C and 5% CO2, the cells were treated with different concentrations of violacein (0.1–20 μmol L− 1) for 24 h. Cytotoxicity of violacein was determined by the MTT assay, phosphatase activity and total protein content evaluation as previously described Citation22-26.
Protein phosphatases extraction from human lymphocytes
Briefly, 1.0 × 106 cells mL− 1 were centrifuged at 2000 rpm for 2 min and washed with saline solution. The cells were lysed with 1 mmol L− 1 acetate buffer, pH 5.5 for 20 min with periodic agitation. The supernatant containing soluble phosphatases, was obtained after centrifugation at 20000 rpm for 30 min. The precipitate was resuspended in 1 mmol L− 1 acetate buffer, pH 5.5 in Triton X-100 and agitated for 20 min for the extraction of CD45.
Tartrate-resistant acid phosphatase (TRAP) partial purification from human blood serum
TRAP was partly purified from fresh human serum of healthly adult volunteers through ammonium sulphate precipitation.
Effect of violacein on protein phosphatases
The assay conditions were utilized as previously described by Freire et al. (2003) [Citation27], using p-nitrophenyl phosphate (pNPP) as the substrate for the enzyme cell extract. Violacein was added to the assay medium and the enzyme activity determined after different periods of incubation. In the absence of violacein the activity was considered to be 100%.
Statistical analysis
All experiments were performed in triplicate and the results shown in the graphs represent the means ± standard deviation (SD). To verify significant differences among groups, analysis of variance (ANOVA) was used. When significant difference was obtained, the Tukey test was used to evaluate the minimal differences among the groups.
Results and discussion
Cytotoxicity of violacein against human lymphocytes
Based on the important events that protein phosphatases mediate in the control of cell growth and differentiation, Aoyama et al. (2000) [Citation25] and Freire et al. (2003) [Citation27] have proposed the use of phosphatase activity as a tool to evaluate cellular viability in cytotoxicity studies. This complementary parameter was also shown to be useful for studying cellular adaptation to apoptosis and oxidative stress among other conditions. Recently, the use of protein phosphatase activity as a cytotoxic parameter has been successfully applied to natural products [Citation27,Citation28]. Lymphocytes were treated with gradually increasing concentrations of violacein. According to , cytotoxic effect was observed on cells (IC50 >10 μmol L− 1) when protein phosphatase activities were evaluated. Kinetic studies with enzymatic extract from HL60 cells and lymphocytes revealed that the predominant phosphatase is a protein tyrosine phosphatase (PTP) [Citation18]. Since all PTPs have SH residues at the active site [Citation27,Citation29], it is possible that violacein inhibitory effect was due to modification of sulphydryl groups. It is also possible that hydrophobic interactions between violacein and specific domains of the enzyme may contribute to the observed effect.
Figure 2 Cytotoxicity of violacein in human lymphocytes after 24 hours of incubation. The curves show the effects of violacein on MTT reduction, total protein content and protein phosphatase activity. The inhibition was expressed relative to normal cell viability (100%) and each point represents the mean ± SD of at least three experiments run in quadruplicate.
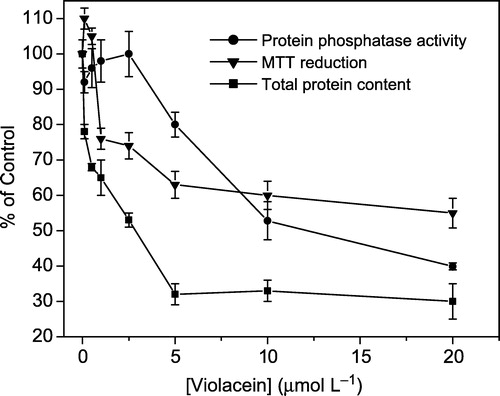
The cytotoxicity of violacein on human lymphocytes stimulated in vitro was also examined by assessing its effect on MTT reduction and protein content (). These assays give information about the integrity and susceptibility of different cellular organelles [Citation30,Citation31]. The cleavage of the yellow tetrazolium salt MTT to purple formazan by metabolic cells reveals the mitochondrial function through the action of succinate dehydrogenase, involving the pyridine nucleotide cofactors NADH and NADPH [Citation32]. The decreased lymphocyte proliferation resulted in a decrease in the total metabolic activity of the cells, as shown in . The IC50 value obtained (>10 μmol L− 1) is in agreement with that determined by the phosphatase assay.
shows the total protein contents measured after the treatment of lymphocytes with different concentrations of violacein and in this case, an IC50 of 3 μmol L− 1 was found. The protein content provides an index of the total cell number based on the determination of the macromolecule content. Thus, it is clear that the decrease in lymphocyte proliferation was due to an inhibitory effect of violacein. It is important to note that a reduced cytotoxic effect of violacein on normal lymphocytes relative to leukemia cells (IC50 < 1.0 μmol L− 1) [Citation11] was observed, suggesting violacein as a promising agent in leukemia therapy.
Effects of violacein on protein phosphatases extracted from human lymphocytes and blood serum
In order to compare violacein action on different sources of phosphatases, total protein phosphatase as well as tartrate-resistant acid phosphatase were extracted from human lymphocytes and blood serum, respectively, and tested in the presence of violacein.
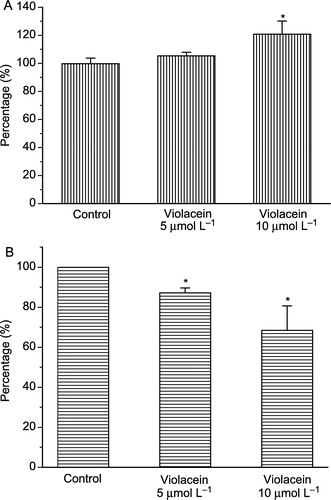
Protein phosphatases (PPs) are important control elements in cell signaling with particular relevance in human diseases, including cancer. Thus, PPs represent promising new targets for drug discovery. In this work, 20% activation of total protein phosphatase activity from human lymphocytes was observed after 20 minutes of pre-incubation with 10.0 μmol L− 1 violacein (). Since the active site (the pTyr-binding site) is highly conserved among the PTPs, next it was evaluated the effects of violacein on isolated receptor-like transmembrane tyrosine phosphatase (CD45) and intracellular tyrosine phosphatases. At these same conditions, 30% inhibition was achieved for intracellular protein tyrosine phosphatases activity (), whereas no alteration was observed on isolated CD45 (data not shown). Alteration of CD45 conformation provoked by its isolation from membrane lipids (lipid rafts), thus affecting the sensitivity of this protein to violacein, may explain why activation of total protein phosphatase from human lymphocytes was observed, despite the inhibition of intracellular protein tyrosine phosphatases and the absence of violacein effect on CD45. Recently, Zhang and collaborators have demonstrated that CD45 function can be affected when its interaction with lipid rafts is disrupted [Citation33]. All PTPs share a common catalytic mechanism to effect catalysis, utilizing the active-site cysteine as a nucleophile in the formation of a thiophosphoryl covalent enzyme intermediate. Despite this fact and unlike receptor-like transmembrane tyrosine phosphatases, which usually contain a tandem repeat of PTP catalytic domains and one of them commonly inactive, intracellular tyrosine phosphatases contain a single catalytic domain and various amino- or carboxy-terminal extensions, including SH2 domains that may have targeting or regulatory functions. This suggests that violacein exerts diverse in vitro effects on these proteins and that the control of intracellular protein tyrosine phosphatases in vivo could affect signal transduction pathways on lymphocytes. Considering the higher cytotoxic effect of violacein on human myeloid leukemia cells (HL60) and its ability to induce differentiation and apoptosis in this cell line [Citation11], it would be interesting to evaluate the roles of PPs in violacein-induced cell death of leukemic cells.
Figure 3 Effect of violacein on total phosphatases (A) and soluble phosphatases (B) from human lymphocytes. *p < 0.01 compared to control (ANOVA, Tukey test).
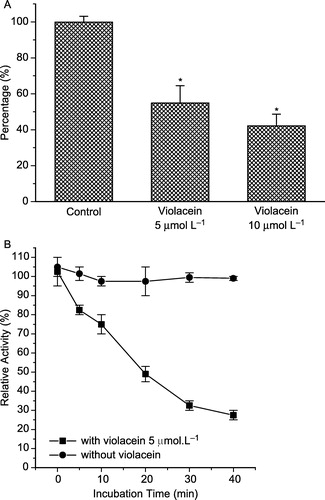
Osteoclasts, the multinucleated cells that resorb bone during bone remodeling and participate in Ca2+ homeostasis, are terminally differentiated cells derived from hematopoietic progenitors. The importance of PTK activity in osteoclast function was underscored by gene-deletion experiments. Other results found that PTP inhibitors suppress in vitro osteoclast differentiation and bone resorption by freshly isolated osteoclasts [Citation34,Citation35]. Osteoclasts express high amounts of tartrate-resistant acid phosphatase (TRAP), whose role in osteoclastogenesis has also been well documented, and secrete it into circulation [Citation35]. Thus, the effect of violacein on TRAP extracted from human blood serum was studied using different concentrations of violacein. A dose-dependent effect on TRAP activity was observed, with 50% inhibition after incubation with 5.0 μmol L− 1 of violacein for 20 minutes at 37°C (). In addition, the violacein effect was also time-dependent as shown in , when an IC50 of 5.0 μmol L− 1 was obtained after 20 minutes of incubation.
Figure 4 Effect of violacein on tartrate-resistant acid phosphatase (TRAP) extracted from human blood serum (A). The enzyme activity was also measured with and without pre-incubation with violacein (B).
Since PTP activity is essential for osteoclast function and that measurement of the level of bone-specific alkaline phosphatase can be used as an index of bone formation and overall bone turnover, we can suggest that TRAP could be a molecular target for violacein to be explored in future experiments.
The findings of this work showed the importance of evaluating the activity of phosphatases from different sources in toxicological studies of new compounds. Further studies are necessary to clarify the mechanisms underlying the effects of violacein on specific PPs involved in cell cycle progression and cell death and their roles in the differential cytotoxicity exerted by violacein in myeloid leukemia cells and lymphocytes.
Acknowledgements
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- Rettori D, Durán N. Production, extraction and purification of violacein: an antibiotic pigment produced by Chromobacterium violaceum. World J Microb Biot 1998; 14: 685–688
- Bromberg N, Durán N. Violacein transformation by peroxidases and oxidases: implications on its biological properties. J Mol Catal B-Enzym 2001; 11: 463–467
- Lichstein HC, Van de Sand VF. Violacein, an antibiotic pigment produced by Chromobacterium violaceum. J Infec Dis 1945; 76: 47–51
- Durán N, Erazo S, Campos V. Bacterial Chemistry-II: antimicrobial photoproduct from pigment of Chromobacterium violaceum. An Acad Bras Cien 1983; 55: 231–234
- Haun M, Pereira MF, Hoffman ME, Joyas A, Campos V, Filardi LDS, De Castro SL, Durán N. Bacterial chemistry VI. Biological activities and cytotoxicity of 1, 3-dihydro-2H-indol-2-one derivatives. Biol Res 1992; 25: 21–25
- Durán N, Antônio RV, Haun M, Pilli RA. Biosynthesis of a Trypanocide of Chromobacterium violaceum. World J Microb Biot 1994; 10: 686–689
- De Souza A, Aily DCG, Sato DN, Durán N. Atividade da Violaceína in vitro sobre Mycobacterium tuberculosis H37RA. Rev Inst Adolfo Lutz 1999; 58: 59–62
- Andrighetti-Frohner CR, Antonio RV, Creczynski-Pasa TB, Barardi CRM, Simões CMO. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Mem Inst Oswaldo Cruz 2003; 98: 843–848
- Durán N, Justo GZ, Melo PS, De Azevedo MBM, Souza-Brito ARM, Almeida ABA, Haun M. Evaluation of the antiulcerogenic activity of violacein and its modulation by the inclusion complexation with β-cyclodextrin. Can J Physiol Pharm 2002; 81: 387–396
- Melo PS, Justo GZ, Xavier AL, De Azevedo MBM, Durán N, Haun M. Apoptosis induction by free co-formulated violacein in HL60 human promyelocytic leukemia cells. FASEB J 2001; 15: A172
- Melo PS, Justo GZ, De Azevedo MBM, Durán N, Haun M. Violacein and its beta-cyclodextrin complexes induce apoptosis and differentiation in HL60 cells. Toxicology 2003; 186: 217–225
- Melo PS, Maria SS, Vidal BC, Haun M, Durán N. Violacein cytotoxicity and induction of apoptosis in V79 cells. In vitro Cell Dev B 2000; 36: 539–543
- Durán N, Menck CFM. Chromobacterium violaceum: A review of pharmacological and industrial perspectives. Crit Rev Microbiol 2001; 27: 201–222
- Ferreira CV, Bos CL, Versteeg HH, Justo GZ, Duran N, Peppelenbosch MP. Molecular mechanism of violacein-mediated human leukemia cell death. Blood 2004; 104: 1459–1464
- Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol 2000; 60: 1225–1235
- Garcia A, Cayla X, Guergnon J, Dessauge F, Hospital V, Rebollo MP, Fleischer A, Rebollo A. Serine/threonine protein phosphatases PP1 and PP2A are key players in apoptosis. Biochimie 2003; 85: 721–726
- Zhang ZY. Protein tyrosine phosphatases: prospects for therapeutics. Curr Opin Chem Biol 2001; 5: 416–423
- Davidson D, Veillette A. PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocytes activation by targeting a unique set of substrates. EMBO J 2001; 20: 3414–3426
- Harrod TP, Justement LB. Evaluating function of transmembrane protein tyrosine phosphatase CD148 in lymphocyte biology. Immunol Res 2002; 26: 153–166
- Ma XZ, Jin T, Sakac D, Fahim S, Zhang X, Katsman Y, Bali M, Branch R. Abnormal splicing of SHP-1 protein tyrosine phosphatase in human T cells: Implications for lymphomagenesis. Exp Hematol 2003; 31: 131–142
- Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nature Rev Immunol 2005; 5: 43–57
- Sodeoka M, Baba Y. Development of protein phosphatases inhibitors: A focused library approach. J Syn Org Chem Jpn 2001; 59: 1095–1102
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications of the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986; 89: 272–277
- Aoyama H, Melo PS, Granjeiro PA, Haun M, Ferreira CV. Cytotoxicity of okadaic acid and kinetic characterization of protein tyrosine phosphatase activity in V79 fibroblasts. Pharm Pharmacol Commun 2000; 6: 331–334
- Hartree EF. Determination of protein: A modification of the Lowry method that gives a linear photometric response. Anal Biochem 1972; 48: 422–427
- Freire ACG, Aoyama H, Haun M, Ferreira CV. Relationship between phosphatase activity and cytotoxic effect of two protein phosphatase inhibitors, okadaic acid and pervanadate, on human myeloid leukemia cell line. J Enz Inhib Med Chem 2003; 18: 425–429
- Freire ACG, Melo PS, Aoyama H, Haun M, Durán N, Ferreira CV. Cytotoxic effect of the diterpene lactone dehydrocrotonin from Croton cajucara on human promyelocytic leukemia cells. Planta Med 2003; 69: 67–69
- Zhang ZY, Dixon JE. Active site labeling of the Yersinia protein tyrosine phosphatase: The determination of the pKa of the active site cysteine and the function of the conserved histidine 402. Biochemistry 1993; 32: 9340–9345
- Jones PA, Bracher M, Marenus K, Kojima H. Performance of the neutral red uptake assay in the COLIPA international validation study on alternatives to the rabbit eye irritation test. Toxicol In Vitro 1999; 13: 325–333
- Rodriguez JA, Haun M. Cytotoxicity of transdehydro-crotonin from Croton cajucara on V79 cells and rat hepatocytes. Planta Med 1999; 65: 522–526
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys 1993; 303: 474–482
- Zhang M, Moran M, Round J, Low TA, Patel VP, Tomassian T, Hernandez JD, Miceli MC. CD45 signals outside of lipid rafts to promote ERK activation, synaptic raft clustering, and IL-2 production. J Immunol 2005; 174: 1479–1490
- Schmidt A, Rutledge SJ, Endo N, Opas EE, Tanaka H, Wesolowski G, Leu CT, Ramachandaran C, Rodan SB, Rodan GA. Protein-tyrosine phosphatase activity regulates osteoclast formation and function: Inhibition by alendronate. Proc Natl Acad Sci 1996; 93: 3068–3073
- McAllister TN, Du T, Frangos JA. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun 2000; 270: 643–6448