Abstract
Quantitative structure–activity relationship (QSAR) studies have been performed on a combined series of 2-sulfonylphenyl-3-phenyl-indoles and 2-phenyl-3-sulfonylphenyl-indoles with a common 2,3 vicinal diaryl indole scaffold, recently reported as selective COX-2 inhibitors. This study is aimed to throw light on this, special class of diaryl heterocyclic family of selective COX-2 inhibitors. A preliminary Fujita-Ban analysis on 32 compounds provided valuable insights about the role of different substituents R1 and R2 around the 2,3 vicinal diaryl rings and R3, at position-5 of the central indole moiety in explaining their in vitro COX-2 inhibitory activity. The contribution of R1, R2, R3 towards COX-2 inhibitory activity resulted in statistically significant linear multiple regression equation with r = 0.942, r2 = 0.888, s = 0.532 and F = 7.92, q2 = 0.516 for 29 compounds. Fujita-Ban model shows a negative contribution of SO2NH2 and SO2CH3 at the R1 position; a negative contribution of 4-Cl, 2-Cl, 3-Cl, 3-CH3, 4-SO2CH3, 4-Br and a positive contribution of 4-OCH3, 4-CH3 substituents at the R2 position. At the R3 position a negative contribution of F, Br and a positive contribution of Cl, CH3 is encountered. In the light of our preliminary investigation that electron donating groups at the para position of R2 are conducive for COX-2 inhibitory activity from the Fujita-Ban model, we attempted to correlate the COX-2 inhibitory activity with quantum chemical descriptors of semi-empirical AM1 optimized geometries of the title compounds. Correlation analysis showed the molecular electronic descriptor, MOPAC total energy as crucial in governing COX-2 inhibitory activity of all the reported 41 compounds.
Introdution
Conventional non steroidal anti-inflammatory drugs (NSAIDs) have been used over a century for the treatment of pain, inflammation and arthritis or arthritis-associated disorders [Citation1]. The rate limiting step in the synthesis of prostaglandins and thromboxanes is the conversion of arachidonate to prostaglandin H2 which is catalyzed by cyclooxygenase (COX) enzymes. The conventional NSAIDs inhibit both isoforms of prostaglandin synthase namely cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). COX-1 is constitutively expressed and is responsible for the maintenance of bodily homeostasis. COX-2 is induced by inflammatory stimuli and is responsible for the progression of inflammation. Despite the fact that NSAIDs have been widely used in the effective management of pain and inflammation, their chronic use has been associated with gastro-intestinal toxicity including ulceration, bleeding and perforation. Thus the treatment of inflammation-associated disorders urges for the design and development of selective inhibition of COX-2 over COX-1.Several selective COX-2 inhibitors also called coxibs have been developed successfully and marketed as new generation NSAIDs. Some of them are celecoxib, rofecoxib, valdecoxib and parecoxib. X-ray crystallographic studies [Citation2,Citation3] suggest that it is a single amino acid difference that is primarily responsible for the selectivity of most selective COX-2 inhibitors: at position 523 is an isoleucine molecule in COX-1 and valine in COX-2.The amino acid valine, which is smaller than isoleucine by a single methyl group in COX-2, allows access to a side pocket, the binding site of most selective COX-2 inhibitors, Whereas the bulkier isoleucine in COX-1 blocks access to that side pocket. Compounds with a central heterocyclic or carbocyclic core bearing two vicinal aryl rings have been studied to a greater extent for selective COX-2 inhibition. Substitution of one of the aromatic rings with a sulphonamido or methyl sulphonyl group is crucial for selective COX-2 inhibition. The central heterocyclic core is essential in properly orienting the aromatic rings in the COX binding site. The common heterocycles used as the central core includes pyrrole, thiazole, oxazole, furan, imidazole, isooxazole, pyrimidine, thiophene, indoles and other fused ring sytems. Ever since the development of indolalkanoic acids as potent and selective cyclooxygenase-2 inhibitors by Black et al. [Citation4] the structural modification of classical non-selective indomethacin to coxibs emerged as a fruitful target in this area. In reference 4, the benzoyl group of indomethacin has been replaced with a 4-bromobenzyl group, and the acetic acid side chain has been extended to get selective COX-2 inhibitors. A series of 1,3-diaryl-4,5,6,7-tetrahydro-2H-isoindole derivatives in which a sulfonyl group is not a structural requisite has been reported as potent and selective COX-2 inhibitors by Portevin et al. [Citation5], Woods et al. [Citation6] reported thiazole analogs of indomethacin as selective COX-2 inhibitors. In this, the carboxyl group of the NSAID indomethacin was replaced with a variety of substituted thiazoles to obtain a series of potent, selective COX-2 inhibitors. Additional substitutions were made at the 1-position and 5-position of the indole of indomethacin. A rational design of 6-methylsulfonylindoles as selective cyclooxygenase-2 inhibitors have been recently reported by Campbell et al. [Citation7]. The introduction of 3-arylmethyl, 3-aryloxy and 3-arylthio moieties into a 6-methylsulfonylindole framework using rational drug design by the authors led to potent, selective COX-2 inhibitors. 3-Arylthioindoles with indoles bearing a 6-MeSO2 and either a 2-methyl or 2-carboxymethyl substituent and 2-cyano-6-MeSO2-3-arylmethylindoles were reported as selective COX-2 inhibitors by Campbell et al. [Citation8,Citation9]. A series of novel N-substituted indole carboxylic, acetic and propionic acid esters have been prepared and studied as possible COX-2 enzyme inhibitors by Olgen et al. [Citation10]. Synthesis and receptor docking studies of N-substituted indole-2-carboxylic acid esters for COX-2 selective enzyme inhibitors were reported by Olgen et al. [Citation11].
Materials and methods
Forty one recently reported compounds including 2-sulfonylphenyl-3-phenyl-indoles [Citation12] and 2-phenyl-3-sulfonylphenyl-indoles [Citation13] reported by Hu et al., were considered as a part of our ongoing research efforts Citation14-20 in exploring COX-2 selectivity requirements. The substitution pattern of these compounds among the parent nucleus prompted us to adopt Fujita-Ban analysis rather than the Hansch approach. Fujita-Ban analysis [Citation21,Citation22] is based on the assumption that the biological activity of a molecule is the sum of the activity contributions of definite substructures, example of the unsubstituted whole molecule and the corresponding substituents. COX-2 inhibitory activity was reported as IC50 in nM units, this being the drug concentration required to inhibit 50% of the enzyme. All the biological activities retained for the study were obtained by the same cellular assay using freshly harvested mouse peritoneal macrophages. For the present QSAR study the reported IC50 was converted to negative logarithm (pIC50) in molar units. QSAR models were built using the regression analysis module of systat version 10.2.The correlation matrix was used to correlate the biological activity with the various physicochemical and structural predictor variables. Descriptors with inter correlation above |r|>0.5 are not considered while deriving QSAR models. The predictor variables with a p value greater than 0.05 were eliminated whilst deriving the QSAR models in order to assure their statistical reliability. The QSAR models were evaluated by using the statistical parameters viz., correlation coefficient (r) or coefficient of determination (r2), standard error of estimate (s), Fischer F-value and student's t-distribution. The latter is used to assess the significance of the individual regression terms. The figures within the parentheses following the coefficient terms are the standard error of the regression terms and the constant. Durbin-Watson (DW) test [Citation23] was employed to check the serial correlation in residuals. A data point is considered as an outlier if it has a large magnitude (when the residual value exceeds twice the standard error of estimate of the model). Self-consistency of the derived models is ensured using the leave-one-out (loo) process and the predictability of each model was assessed using cross-validated r2 or q2.The computational works were performed on a Pentium IV workstation using ChemOffice 2001 molecular modeling software, version 6.0 supplied by Cambridge Soft Corporation, USA. The structures and the biological activity data are given in . The molecules were sketched using Chem Draw Ultra version 6.0, cleaned copied and pasted into Chem3D ultra version 6.0.The resulting three-dimensional (3D) structures were subjected to energy minimization process using a semi empirical quantum mechanics module implemented on a molecular orbital package (MOPAC) version. Austin Model-1 (AM1) [Citation24] Hamiltonian method, closed shell restricted wave function was adopted for the energy minimization process. Dipole moment, electronic energy, HOMO energy, LUMO energy, repulsion energy and total energy (the sum of MOPAC electronic energy and the MOPAC repulsion energy) were calculated from the MOPAC server of Chem 3D. Other descriptors calculated were Connolly solvent accessible surface area, Connolly molecular surface area, Connolly solvent excluded volume, ovality, principal moments of inertia (X, Y, Z), LogP, molar refractivity, and heat of formation.
Table I. Structure, observed and predicted COX-2 inhibitory activity through derived QSAR model-5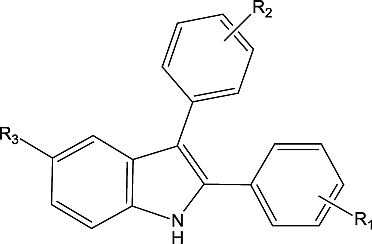
Results and discussion
In order to use the Fujita-Ban approach for this data set, the substituent (at positions R1, R2 and R3) shifts of the activity should be additive. A thorough analysis of the reported activity data () shows that little additivity of the substituent shifts exist. For example, if we start from compound 3 with R1 = 4-SO2NH2, R2 = 4-F, R3 = H, PIC50 = 8.29 and change R1 = 4-SO2NH2 to R1 = 4-SO2CH3, we get compound 4 with PIC50 = 10.70 (with Δ PIC50 = +2.41). On the contrary if we move from compound 17 to 18 by changing R1 = 4-SO2NH2 to R1 = 4-SO2CH3 we obtain Δ PIC50 = − 2.2.This clearly shows that the substituent shifts of the PIC50 value associated with the R1 change from 4-SO2NH2 to SO2CH3 are considerably different. In another instance, if we move from compound 1 with R1 = 4-SO2NH2, R2 = R3 = H to compound 3 with R1 = 4SO2NH2, R2 = 4F and R3 = H the Δ PIC50 = − 1.76.But if we move from compound 2 with R1 = 4-SO2CH3, R2 = R3 = H, to compound 4 R1 = 4-SO2CH3, R2 = 4-F, R3 = H the Δ PIC50 = +1.48.This again illustrates that substituent shifts of the PIC50 value associated with the R2 change from H to 4-F are also different. Also in moving from compound 17 with R1 = 4-SO2NH2, R2 = 4-Cl and R3 = Cl, to compound 19 with R1 = 4-SO2NH2, R2 = 3-Cl, R3 = Cl the Δ PIC50 is − 0.77 and while going from compound 18 with R1 = 4-SO2CH3, R2 = 4-Cl and R3 = Cl, to compound 20 with R1 = 4-SO2CH3, R2 = 3-Cl and R3 = Cl, the Δ PIC50 is +2.03.This shows that substituent shifts of the PIC50 value associated with the R2 change from 4-Cl to 3-Cl are also different. These investigations suggest that the substituent effects are highly coupled and may not yield significant Fujita-Ban QSAR models. Nevertheless, we adopted this method just to find the influence of various kinds of repeated substituent units on these congeners on COX-2 inhibitory activity with the objective of using those structural variables in a better meaningful QSAR model with physico–chemical descriptors.
All the reported 41 compounds could be considered as structural modifications of 2,3 vicinal diaryl indoles at three positions viz., R1, R2, around the vicinal diaryl rings and R3, at position-5 of the indole nucleus. R1 varies from 4-SO2CH3, 4-SO2NH2, 4-SO2NHAc, 2-F, 2-OCOCH3, 4-Cl, 4-CH3, 4-OCH3 and 4-OCOCH3.R2 varies from 4-F, 4-Cl, 4-Br, 4-OCH3, 4-CH3, 4-OH, 4-SO2CH3, 4-SO2NH2, 3-CH3, 3-Cl, 2-Cl. R3 varies from Cl, F, Br, CH3 at position-5 of the indole nucleus. The structure and the observed and predicted COX-2 inhibitory activity through derived QSAR models are given in . Since the frequency of occurrence of certain groups () in compound 15, 4-OH; compound 16, 4-SO2NHAc; compound 32, 2-F; compound 33, 4Cl; compound 34, 4-CH3; compound 35, 4-OCH3; compound 36, 2-OCOCH3 at R1 and in compound 41, 4-SO2NH2 at R2 is only one, these compounds were not included in the Fujita-Ban analysis. The Fujita-Ban matrix of those compounds used for the analysis is given in . In spite of the feeling that little additivity of the substituent shifts exist among the compounds considered for an effective Fujita-Ban model, QSAR model-1 developed for 32 compounds resulted in a reasonably good correlation with statistical parameters, n = 32, r = 0.864, r2 = 0.747, s = 0.78, F = 3.142, DW = 2.392 and q2 = 0.033.QSAR model 1 is not used further in deriving significant conclusions due to its very poor q2 value. The Fujita-Ban contribution of substituents at R1, R2 and R3 and the parent moiety towards COX-2 inhibition are given in . Three compounds, 17, 3 and 4, were sequentially eliminated as outliers while deriving QSAR model-2. The corresponding rows in the Fujita-Ban matrix and the column showing the presence or absence of 4-F group at R2 were deleted due to the removal of the afore mentioned compounds. The regression analysis of the resulting matrix resulted in QSAR model-2 with excellent statistical parameters, n = 29, r = 0.942, r2 = 0.888, s = 0.532, F = 7.92, DW = 2.731, q2 = 0.516.Unlike QSAR model-1, the q2 value shows that the QSAR model-2 is not over-trained with too many descriptors. Nevertheless it is highly dangerous to derive conclusions from QSAR model-1 and QSAR model-2 due to the feeling that the substituent effects are highly coupled (as discussed above with compounds 1, 2, 3, 4, 17, 18, 19, 20). Hence, substituents 4-Cl, 4-OCH3 and 2-Cl that are statistically significant as discerned through their less standard error rendering their t-value statistically significant above 95% confidence interval are derived from QSAR model-2 for further analysis. Overall, the Fujita-Ban model shows a negative contribution of SO2NH2 and SO2CH3 at the R1 position; a negative contribution of 4-Cl, 2-Cl, 3-Cl, 3-CH3, 4-SO2CH3, 4-Br and a positive contribution of 4-OCH3, 4-CH3 substituents at the R2 position. At the R3 position a negative contribution of F, Br and a positive contribution of Cl, CH3 is obtained. Statistically significant terms were derived from the QSAR model-2 for the R2 position and the following QSAR model-3 is accomplished.
Table II. Position and frequency of occurrence of substituents
Table III. Fujita-Ban matrix of 2,3 diaryl indoles
pIC50 = − 2.118( ± 0.414) 4-Cl – 1.745 ( ± 0.496) 2-Cl +1.715 ( ± 0.496) 4-OCH3 +9.245 ( ± 0.143)
n = 29, r = 0.825, r2 = 0.680, s = 0.672, F = 17.72, p = 0.000, q2 = 0.590, DW = 1.984
QSAR model-3 is a tri-parametric equation and explains 68.0% variance in COX-2 inhibitory activity. All the coefficients of QSAR model-3 are statistically significant above 95% confidence interval as indicated by their t values. Since the DW value is greater than 1.4, there is probably not any serious autocorrelation in the residuals. The electron donating OCH3 group at the para position of R2 of the 3-aryl ring of vicinal diaryl indoles is favorable for COX-2 inhibitory activity whereas the electron withdrawing group Cl at the para and ortho positions are detrimental for COX-2 inhibitory activity. The predictability of QSAR model-3 is also fairly high as indicated by the cross validated q2 value.
In the light of our preliminary investigation that electron donating groups at the para position of R2 are conducive for COX-2 inhibitory activity from the Fujita-Ban model, we attempted to calculate molecular electronic descriptors as described under the Materials and Methods section. Molecular electronic descriptors like energies of highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO), dipole moment, total Mopac energy (MOPAC_ET), electronic energy were calculated. ClogP and CMR and other physicochemical descriptors were also included to account for the hydrophobic and steric effects of COX-2 enzyme inhibition. Correlation analysis of all the reported 41 compounds resulted in the following QSAR model-4.
pIC50 = − 1.364( ± 0.385) 4-Cl – 1.363( ± 0.535) 2-Cl +1.876( ± 0.528) 4-OCH3 +0.001( ± 0.000) MOPAC_ET+13.129 ( ± 1.553)
n = 41, r = 0.741, r2 = 0.549, s = 0.726, F = 10.98, p = 0.000, q2 = 0.384, DW = 2.136
QSAR model-4 predicts a very low activity for compound 17 than the observed activity. Hence, the following QSAR model-5 is derived upon eliminating compound 17 as outlier. QSAR model -5 is a better QSAR model than models 1, 2 and 3 as it involves all the reported compounds together with MOPAC total energy.
pIC50 = − 1.964( ± 0.389) 4-Cl – 1.334( ± 0.476) 2-Cl +1.876( ± 0.470) 4-OCH3 +0.001( ± 0.000) MOPAC_ET+13.655 ( ± 1.391)
n = 40, r = 0.808, r2 = 0.653, s = 0.645, F = 16.44, p = 0.000, q2 = 0.567, DW = 2.291
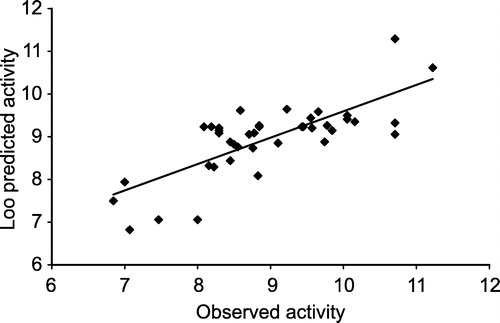
Compound 17 has a chlorine atom at the R3 position and a 4-SO2NH2, 4-Cl at the R1 and R2 positions respectively. The outlying behavior of compound 17 may be probably due to unfavorable orientation of the polar atom at the R3 position towards the hydrophobic amino acid residues at the COX-2 active site. SAR data as reported by Hu et al., [Citation12] also suggests that methyl substitution at the R3 position for example, compounds 29 and 30 are more potent than the unsubstituted compounds 1 and 2, illustrating the importance of a hydrophobic functional group at R3. QSAR model-5 explains 65.3% variance in COX-2 inhibitory activity. QSAR model-5 shows the MOPAC_ET as crucial for COX-2 inhibitory activity of the 40 substituted 2,3 diaryl indoles. MOPAC_ET is the sum of electronic and nuclear energy. Total energy of compounds is one of the quantum chemical descriptors widely used in QSAR studies [Citation25]. All the coefficients of QSAR model-5 are statistically significant above the 95% confidence interval as indicated by their t values. The inter correlation among the descriptors used in QSAR model-5 is given in . Since the DW value is greater than 1.4, there is probably not any serious autocorrelation in the residuals. The predictability of the model is also fairly high as reflected by its q2 value. The correlation between the observed and COX-2 activity predicted by QSAR model-5 is given in .
Table IV. Inter correlation matrix of descriptors of QSAR model-5
In conclusion, two combined series of 2-sulfonylphenyl-3-phenyl-indoles and 2-phenyl-3-sulfonylphenyl-indoles with a common 2,3 vicinal diaryl indole scaffold, recently reported as selective COX-2 inhibitors were investigated using QSAR analysis. Our preliminary investigations using the Fujita-Ban approach suggested that a 4-OCH3 group at the R1 position of the parent structure studied is conducive for COX-2 inhibitory activity. The 2-Cl and 4-Cl groups show negative effects toward COX-2 inhibitory activity. The quantum chemical descriptor MOPAC_ET is found to be crucial for COX-2 inhibitory activity among these congeners. The results drawn together here may be helpful in further exploitation of this lead structure to design more potent COX-2 inhibitors in the future.
Acknowledgements
Authors S. P and E. M thank the University Grants Commission (UGC), New Delhi, India for the financial support for this research. Author S.P wishes to thank the anonymous reviewer whose valuable comments and suggestions greatly helped in improving the manuscript.
References
- Vane JR, Bakhle YS, Botting RM. Annu Rev Pharmacol Toxicol 1998; 38: 97–120
- Picot D, Loll PJ, Gravito RM. Nature 1994; 367: 243–249
- Hawkey CJ. Lancet 1999; 353: 307–314
- Black WC, Bayly C, Belley M, Chan C-C, Charleson S, Denis D, Gauthier YR, Gordon J, Guay D, Kargman S. Bioorg Med Chem Lett 1996; 6: 725–730
- Portevin B, Tordjman C, Pastoureau P, Bonnet J, De Nanteuil G. J Med Chem 2000; 43: 4582–4593
- Woods KW, McCroskey RW, Michaelides MR, Wada CK, Hulkower KI, Bell RL. Bioorg Med Chem Lett 2001; 11: 1325–1328
- Campbell JA, Bordunov V, Broka CA, Browner MF, Kress JM, Mirzadegan T, Ramesha C, Sanpablo BF, Stabler R, Takahara P. Bioorg Med Chem Lett 2004; 14: 4741–4745
- Campbell JA, Bordunov V, Broka CA, Dankwardt J, Hendricks RT, Kress JM, Walker KAM, Jin-Hai Wang. Tetrahedron Lett 2004; 45: 3793–3796
- Campbell JA, Broka CA, Gong L, Walker KAM, Jin-Hai Wang. Tetrahedron Lett 2004; 45: 4073–4075
- Olgen S, Nebioglu D. Farmaco 2002; 57: 677–683
- Olgen S, Akaho E, Nebioglu D. Eur J Med Chem 2001; 36: 747–770
- Hu W, Guo Z, Chu F, Bai A, Yi X, Cheng G, Li J. Bioorg Med Chem 2003; 11: 1153–1160
- Hu W, Guo Z, Yi X, Guo C, Chu F. and Cheng, G. Bioorg Med Chem 2003; 11: 5539–5544
- Prasanna S, Manivannan E, Chaturvedi SC. Bioorg Med Chem Lett 2005; 15: 2097–2102
- Prasanna S, Manivannan E, Chaturvedi SC. Bioorg Med Chem Lett 2005; 15: 313–320
- Prasanna S, Manivannan E, Chaturvedi SC. Bioorg Med Chem Lett 2004; 14: 4005–4011
- Prasanna S, Manivannan E, Chaturvedi SC. QSAR Comb Sci 2004; 23: 621–628
- Prasanna S, Manivannan E, Chaturvedi SC. Arch Pharm Pharm Med Chem 2004; 337: 440–444
- Prasanna S, Manivannan E, Chaturvedi SC. Indian J Chem 2004; 43B: 2249–2253
- Manivannan E, Prasanna S, Chaturvedi SC. Indian J Biochem Biophys 2004; 41: 179–184
- Fujita T, Ban T. J Med Chem 1971; 14: 148–152
- Kubinyi H, Kehrhahn OH. J Med Chem 1976; 19: 1040–1049
- Durbin J, Watson GS. Biometrika 1951; 38: 159–178
- Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP. J Am Chem Soc 1985; 107: 3902–3909
- Karelson M, Lobanov VS, Katritzky AR. Chem Rev 1996; 96: 1027–1044