Abstract
The synthesis and activity of a new series of non-steroidal inhibitors of 17β-hydroxysteroid dehydrogenase that are based on a 1,5-benzodiazepine scaffold are presented. Their inhibitory potential was screened against 17β-hydroxysteroid dehydrogenase from the fungus Cochliobolus lunatus (17β-HSDcl), a model enzyme of the short-chain dehydrogenase/reductase superfamily. Some of these compounds are potent inhibitors of 17β-HSDcl activity, with IC50 values in the low micromolar range and represent promising lead compounds that should be further developed and investigated as inhibitors of human 17β-HSD isoforms, which are the enzymes associated with the development of many hormone-dependent and neuronal diseases.
Introduction
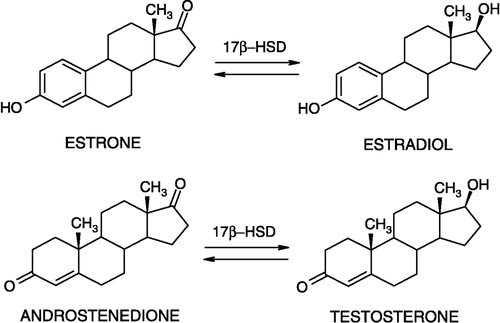
The 17β-hydroxysteroid dehydrogenases (17β-HSDs) are enzymes that belong to the short-chain dehydrogenase/reductase (SDR) and aldo-keto reductase (AKR) superfamilies [Citation1,Citation2]. They catalyze the conversion of inactive 17-keto-steroids into their active 17β-hydroxy-forms (such as estradiol, testosterone and dihydrotestosterone), and vice versa, using NAD(P)H or NAD(P)+ as cofactors () [Citation3,Citation4]. To date, 13 types of human 17β-HSDs have been described and they differ in their tissue distributions, substrate and cofactor specificities, subcellular localizations, and mechanisms of regulation [Citation5]. Due to their involvement in the final step of the biosynthesis of the sex hormones, they have key roles in modulation of their biological potencies. For this reason, the 17β-HSDs constitute emerging therapeutic targets for the treatment of hormone-dependent diseases, such as breast, prostate and endometrial cancers, and disorders of reproduction and neuronal diseases [Citation5].
Over the last decade, numerous potent inhibitors of the 17β-HSDs have been reported [Citation6]. The development of 17β-HSD inhibitors that consist of a non-steroidal core appears especially attractive, as these compounds are devoid of residual steroidogenic activity, which can cause many side effects. Among the non-steroidal inhibitors of the 17β-HSDs, attention has recently been focused on the phytoestrogens, and especially the flavonoids, such as the flavones and chalcones Citation7-13. We are using 17β-HSD from the filamentous fungus Cochliobolus lunatus (17β-HSDcl) as a model enzyme for the SDR superfamily [Citation14,Citation15]. We have recently shown that flavonoids also inhibit 17β-HSDcl, and that the structural features of these flavonoids are very similar to those reported for phytoestrogen inhibitors of human 17β-HSD types 1 and 2 [Citation16]. We have also synthesized a series of cinnamic acid esters and amides that are related to flavones and chalcones which inhibit 17β-HSDcl at low micromolar concentrations [Citation17,Citation18].
Besides the phytoestrogens, some other non-steroidal compounds are also able to inhibit the HSDs from the AKR and SDR superfamilies. For example, 1,4-benzodiazepines are potent inhibitors of the AKR1C1-AKR1C4 HSDs from the AKR superfamily [Citation19,Citation20]. Here, we present the synthesis, inhibition of 17β-HSDcl, and docking studies of some of our new 1,5-benzodiazepines, which represent a novel, so far untested, scaffold for the design of inhibitors of the SDR superfamily of enzymes.
Experimental
Chemistry
All of the reactions were carried out under dry conditions and with magnetic stirring. Chemicals were purchased from Acros and used without further purification. Solvents were used without purification or drying, unless otherwise stated. Reactions were monitored using analytical TLC plates (Merck, silica gel 60 F254) with sulphuric acid staining. Silica gel grade 60 (70-230 mesh, Merck) was used for column chromatography. NMR spectra were obtained on a Bruker Avance DPX 300 instrument. 1H-NMR spectra were recorded at 300.13 MHz with tetramethylsilane as an internal standard. Mass spectra were obtained with a VG-Analytical Autospec Q mass spectrometer with EI or FAB ionization (MS Centre, Jožef Stefan Institute, Ljubljana). IR spectra were recorded on a Perkin-Elmer FTIR 1600 spectrometer. Elemental analyses were performed by the Department of Organic Chemistry, Faculty of Chemistry and Chemical Technology, Ljubljana, on a Perkin Elmer elemental analyzer 240 C. Melting points were determined using a Reichert hot-stage microscope and are uncorrected.
2-((2-Aminophenylamino)methylene)malononitrile (3)
1,2-Diaminobenzene (8.845 g, 80.2 mmoles) (2) was suspended in dry dichloromethane (100 mL) and heated until dissolved. The solution was then allowed to cool to room temperature and ethoxymethylenemalononitrile (10.0 g, 80.2 mmoles) (1) was added in small portions over a period of 5 min. After the addition of this reagent, a pale-yellow suspension began to form. The suspension was stirred for 3 h and the precipitate was filtered, washed with cooled dichloromethane, and dried under vacuum. The product (3) was a yellow-brown solid. Yield: 76%; m.p.: 102–104°C (lit. m.p.: 100°C) [Citation21].
4-Amino-1H-1,5-benzodiazepine-3-carbonitrile hydrochloride (4)
Ethanol (150 mL) was cooled to 0°C and acetylchloride (4.6 mL, 63.3 mmoles) was added drop-wise over a period of 30 min. The solution was heated to 35°C and compound 3 (10.60 g, 5.75 mmoles) was added. The resulting suspension was then heated at reflux overnight. The red precipitate (4) was filtered and dried under vacuum. Yield: 92%; m.p.: 272°C (dec.; lit. m.p.:280°C) [Citation22].
General procedure for the synthesis of compounds 5, 7a-b
To a stirred solution of the appropriate acid (3.0 mmoles) and compound 4 (0.728 g, 3.3 mmoles) in dry DMF (15 mL), diphenylphosphorylazide (0.75 mL, 3.4 mmoles) and triethylamine (1.39 mL, 10.0 mmoles) were added at 0°C. Stirring was continued for 5 h at 0°C, and then overnight at room temperature. Ethyl acetate (100 mL) was added, and the solution was extracted with 10% citric acid (2 × 30 mL), H2O (30 mL), saturated NaHCO3 solution (2 ×30 mL), H2O (30 mL), and saturated NaCl solution (2 ×30 mL).The organic phase was dried (Na2SO4) and evaporated in vacuo. The residue was purified by column chromatography on a silica gel column (eluent: 5–10% methanol in chloroform).
N-(3-Cyano-1H-1,5-benzodiazepin-4-yl)-4-nitrobenzamide (5)
Yield: 59%; m.p.: 300–305°C; IR (KBr) ν 2223, 1636, 1594, 1511, 1345, 1220, 838, 720 cm− 1; 1H-NMR (300 MHz, DMSO-d6) δ 6.64 (dd, J = 6.9 Hz, 2.4 Hz, 1H, ArH), 6.74 (dd, J = 6.9 Hz, 2.4 Hz, 1H, ArH), 6.92-7.01 (m, 2H, ArH), 7.23 (s, 1H, CH), 8.33 (s, 4H, ArH), 10.23 (s,1H, NH), 11.78 (s, 1H, CO-NH); FAB MS m/z 334 [M + H]; EI HRMS Calcd. for C17H11N5O3 m/z [M+] 333.087050, found 333.086189. Anal. Calcd for C17H11N5O3 ×H2O: C, 58.12; H, 3.73; N, 19.93. Found: C, 58.33; H, 4.09; N, 19.87%.
(E)-N-(3-Cyano-1H-1,5-benzodiazepin-4-yl)-3-phenyl-2-propenamide (7a)
Yield: 51%; m.p.: 197–200°C; IR (KBr) ν 3443, 3083, 2212, 1631, 1447, 1339, 1218, 751 cm–1; 1H-NMR (300 MHz, DMSO-d6): δ 6.56–6.61 (m, 1H, ArH), 6.65 (d, J = 15.8 Hz, 1H, CH), 6.73–6.78 (m, 1H, ArH), 6.88-7.00 (m, 2H, ArH), 7.20 (s, 1H, CH), 7.38-7.48 (m, 3H, ArH), 7.61-7.69 (m, 2H, ArH), 7.73 (d, J = 15.8 Hz, 1H, CH), 10.12 (br s, 1H, NH), 11.90 (s, 1H, CO-NH). FAB MS m/z 315 [M + H]+; EI HRMS Calcd. for C19H14N4O m/z: [M]+314.116761, found 314.117350. Anal. Calcd. for C19H14N4O ×H2O: C, 68.66; H, 4.85; N, 16.86. Found: C, 68.60; H, 4.74; N, 16.36%.
N-(3-Cyano-1H-1,5-benzodiazepin-4-yl)-2-oxo-2H-chromene-3-carboxamide (7b)
Yield: 27%; m.p.: 247–251°C; IR (KBr) ν 3414, 2925, 2210, 1738, 1639, 751 cm–1; 1H-NMR (300 MHz, DMSO-d6) δ 6.59-6.68 (m, 1H, ArH), 6.71-6.79 (m, 1H, ArH), 6.88-7.01 (m, 2H, ArH), 7.22 (s, 1H, CH), 7.36-7.48 (m, 2H, ArH), 7.68-7.83 (m, 2H, ArH), 7.95 (s, 1H, CH), 8.75 (s, 1H, NH), 11.22 (s, 1H, CO-NH). FAB MS m/z 357 [M + H]+; EI HRMS Calcd. for C19H14N4O m/z: [M]+356.090940, found 356.091100. Anal. Calcd. for C20H12N4O3 ×2H2O: C, 61.22; H, 4.11; N, 14.28. Found: C, 61.19; H, 4.42; N, 14.36%.
4-[(4-Nitrobenzoyl)amino]-1H-1,5-benzodiazepine-3-carboxamide hydrochloride (6)
5 mL 37% HCl was poured over 5 (0.215 g, 0.65 mmoles) and heated to 40°C. The dark-brown suspension was stirred for 1 h and cooled to room temperature. Water (30 mL) was added to produce a yellow-brown precipitate 6. Yield 85%; dec >300°C; IR (KBr) ν 3271, 3159, 1655, 1520, 1343, 1270, 1076, 836, 712 cm− 1; 1H-NMR (300 MHz, DMSO-d6) δ 6.72 (d, J = 6.9 Hz, 1H, ArH), 6.77 (d, J = 7.8 Hz, 1H, ArH), 6.85 (t, J = 7.5 Hz, 1H, ArH), 6.98 (t, J = 7.5 Hz, 1H, ArH), 7.25 (s,1H, CH), 8.28 (d, J = 9.0 Hz, 2H, ArH), 8.40 (d, J = 9.0 Hz, 2H, ArH), 9.86 (s, 1H, NH), 12.76 (bs, 1H, CO-NH); FAB MS m/z 352 [M + H]+; EI HRMS Calcd. for C17H13N5O4 m/z [M+] 351.097320, found 351.096754. Anal. Calcd for C17H14N5O4Cl: C, 52.65; H, 3.64; N, 18.06. Found: C, 52.40; H, 3.80; N, 17.98%.
N-(3-Cyano-1H-1,5-benzodiazepin-4-yl)benzamide (7c)
To a suspension of compound 4 (0.540 g, 2.1 mmoles) in 30 mL of dry tetrahydrofuran, triethylamine (0.9 mL, 6.2 mmoles) was added and the resulting solution was cooled to 0°C. Benzoyl chloride (0.3 ml, 2.6 mmoles) was then added drop-wise to the stirring solution, over a period of 1 min and the mixture was stirred at room temperature overnight. The resulting precipitate was filtered off and 200 mL of ethyl acetate added to the remaining solution. The organic phase was then washed with 10% citric acid (2 ×50 mL), saturated NaHCO3 (2 ×50 mL) and brine (2 ×50 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified on a silica column (5% methanol in chloroform) to give 7c. Yield: 53%; m.p.: 256–260°C; IR (KBr) ν 3263, 3099, 2206, 1639, 1569, 1348, 1219, 925, 707 cm− 1; 1H-NMR (300 MHz, DMSO-d6) δ 6.64 (dd, J = 7.2 Hz, 1.8 Hz, 1H, ArH), 6.75 (dd, J = 7.2 Hz, 1.8 Hz, 1H, ArH), 6.90-7.00 (m, 2H, ArH), 7.22 (s, 1H, CH), 7.49 (t, J = 6.9 Hz, 2H, ArH), 7.59 (t, J = 7.5 Hz, 1H, ArH), 8.17 (d, J = 6.9 Hz, 2H, ArH), 10.15 (s, 1H, NH), 11.94 (s, 1H, CONH); EI MS m/z 288 [M]+; EI HRMS Calcd. for C17H12N4O m/z: [M+] 288.101850, found 288.101111. Anal. Calcd for C17H12N4O: C, 70.82; H, 4.20; N, 19.43. Found: C, 70.49; H, 4.18; N, 19.47%.
N-(3-Cyano-1H-1,5-benzodiazepin-4-yl)-3-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)propanamide (7d)
Compound 4 (0.7 g, 2,7 mmoles) was suspended in dry N,N-dimethylformamide (25 mL), and triethylamine (0.4 ml, 2.8 mmoles) was added. The resulting solution was cooled to − 5°C and then the 3-phthalimidopropanoic acid (0.461 g, 2.25 mmoles), HOBt (0.4 g, 3.00 mmoles), triethylamine (0.9 ml, 6.4 mmoles) and EDC (0.575 g, 3.00 mmoles) were added. The mixture was left stirring overnight while the temperature was gradually increased to room temperature. The reaction mixture was then poured into water (200 mL) and extracted with ethylacetate (3 ×70 mL). The organic fraction was rinsed with 10% citric acid (3 ×70 mL), water (70 mL), a saturated solution of NaHCO3 (3 ×70 mL) and brine (2 ×70 mL), and then dried over sodium sulphate, filtered and concentrated in vacuo. The residue was purified through a silica gel column (5% methanol in chloroform) to give 7d. Yield: 46%; m.p.: 150–156°C; IR (KBr) ν 2205, 1718, 1633, 1369, 999, 717 cm− 1; 1H-NMR (300 MHz, DMSO-d6) δ 2.69 (t, J = 7.8 Hz, 2H, CH2), 3.85 (t, J = 7.2 Hz, 2H, CH2), 6.55 (dd, J = 6.9 J = 2.1 Hz, 1H, ArH), 6.73-6.76 (m,1H, ArH), 6.93-6.95 (m, 2H, ArH), 7.16 (s,1H, CH), 7.81-7.88 (m, 4H, ArH), 10.11 (s, 1H, NH), 11.20 (s, 1H, CO-NH); FAB MS m/z 386 [M + H]+; EI HRMS Calcd. for C21H15N5O3 m/z [M+] 385.118950, found 385.117490; Anal. Calcd for C21H15N5O3x0.9 H2O: C, 62.81; H, 4.22; N, 17.44. Found: C, 63.06; H, 4.24; N, 17.14%.
3H-1,5-Benzodiazepine-2,4-diamine (8)
Compound 4 (11.5 g, 52.1 mmoles) was suspended in 3.0 M NaOH (150 mL) and slowly heated to 100°C. The suspension was then cooled to room temperature, filtered and washed with water. The orange solid was dissolved in 1.0 M HCl (50 mL) and the product was precipitated with 0.5 M Na2CO3. The orange precipitate (8) was filtered, washed with water and dried under vacuum. Yield: 49%; m.p.: >260°C (lit. m.p.: >260°C) [Citation22].
(E)-3-Phenyl-N-(4-{[(E)-3-phenyl-2-propenoyl]amino}-3H-1,5-benzodiazepin-2-yl)-2-propenamide (9)
To a stirred solution of the appropriate acid (6.0 mmoles) and compound 4 (3.3 mmoles) in dry DMF (15 mL), diphenylphosphorylazide (1.5 mL, 6.8 mmoles) and triethylamine (1.40 mL, 10.1 mmoles) were added at 0°C. Stirring was continued for 5 h at 0°C, and then overnight at room temperature. Ethyl acetate (100 mL) was added and the solution was extracted with 10% citric acid (2 × 30 mL), H2O (30 mL), saturated NaHCO3 solution (2 × 30 mL), H2O (30 mL), and saturated NaCl solution (2 ×30 mL). The organic phase was dried (Na2SO4) and evaporated in vacuo. The residue was purified on a silica gel column (ethyl acetate/hexane = 1/1) to give 9. Yield: 22%; m.p.: 196–198°C; IR (KBr) ν 3450, 1618, 1356, 1153, 756 cm–1; 1H-NMR (300 MHz, DMSO-d6) δ 4.04 (s, 2H, CH2), 6.86 (d, J = 15.8 Hz, 2H, 2x CH), 7.21-7.27 (m, 2H, ArH), 7.28-7.34 (m, 2H, ArH), 7.40-7.49 (m, 6H, ArH), 7.58-7.64 (m, 4H, ArH), 7.66 (d, J = 15.8 Hz, 2H, 2x CH), 10.85 (s, 2H, 2x CO-NH). FAB MS m/z 435 [M + H]+. Anal. Calcd. for C27H22N4O2x2/3H2O: C, 72.63; H, 5.27; N, 12.55. Found: C, 72.28; H, 5.32; N, 12.90%.
2,4-bis(4-Methyl-1-piperazinyl)-3H-1,5-benzodiazepine (10)
Compound 8 (2.0 g, 11.2 mmoles) was suspended in a mixture of toluene (15 mL), DMSO (15 mL) and 1-methylpiperazine (10 mL, 90 mmoles) and heated at 125°C overnight. The resulting solution was concentrated in vacuo, cooled and the precipitated product 10 was filtered off, washed with isopropyl acetate and dried. Yield: 79%; m.p.: 227–228°C; IR (KBr) ν 2936, 2794, 1600, 1550, 1455, 1290, 1143, 1006, 756 cm− 1; 1H-NMR (300 MHz, DMSO-d6) δ 2.21 (s, 6H, 2x CH3), 2.35 (m, 8H, 4x CH2), 3.04 (s, 2H, CH2), 3.53 (m, 8H, 4x CH2), 6.87 (m, 2H, ArH), 7.02 (m, 2H, ArH); EI MS m/z 340 [M]+; EI HRMS Calcd. for C19H28N6 m/z [M+] 340.238330, found 340.237545; Anal. Calcd for C19H28N6: C, 67.03; H, 8.29; N, 24.68. Found: C, 66.90; H, 8.11; N, 24.77%.
Inhibition studies
Compounds were tested for their inhibitory activities towards homogenous recombinant 17β-HSDcl [Citation14]. 17β-HSDcl catalyzes the oxidation of 4-estrene-17β-ol-3-one to 4-estrene-3,17-dione in the presence of NADP+, and reduction of 4-estrene-3,17-dione to 4-estrene-17β-ol-3-one in the presence of coenzyme NADPH. The reaction was followed spectrophotometrically by measuring the difference in NADPH absorbance (ελ340 = 6270 M− 1cm− 1) in the absence and presence of the compounds. Assays were carried out in a 0.6-mL volume in 100 mM phosphate buffer (pH 8.0) containing 1% DMF as co-solvent, as described previously [Citation16,Citation17]. The concentrations of substrate and coenzyme were each 100 μM, and the compounds were tested from 0.5 μM to 100 μM; the enzyme concentration was 0.5 μM. Initial velocities of the enzymatic reactions in the absence (v0) or presence (vi) of inhibitor were measured. Percentage inhibition (% inh.) was given by 100-((vi/v0) ×100). The IC50 values were determined graphically from plots of % inhibition versus log (inhibitor conc.) using GraphPad Prism Version 4.00 (GraphPad Software, Inc.).
Molecular docking
Automated docking was used to locate the potential binding orientations of the inhibitors within the active site of 17β-HSDcl. The genetic algorithm method implemented in the program AutoDock 3.0 was used [Citation24]. The structures of the inhibitors were prepared using HyperChem 7.5 (HyperChem, version 7.5 for Windows. Hypercube, Inc.: Gainesville, FL, 2002). The homology built model of 17β-HSDcl was retrieved from the web side http://www2.mf.uni-lj.si/∼ stojan/stojan.html and the steroid ligand was removed. Polar hydrogen atoms were added and Kollman charges [Citation25], and atomic solvation parameters and fragmental volumes were assigned to the protein using AutoDock Tools (ADT). For docking calculations, Gasteiger-Marsili partial charges [Citation26] were assigned to the coenzyme molecule and the ligands and non-polar hydrogen atoms were merged. All torsions were allowed to rotate during docking. The grid map, which was generated with the auxiliary program AutoGrid, was large enough to cover the inhibitors and the active site of the enzyme. Lennard-Jones parameters 12-10 and 12-6, supplied with the program, were used for modeling H-bonds and van der Waals interactions, respectively. The distance-dependent dielectric permittivity of Mehler and Solmajer [Citation27] was used for the calculation of the electrostatic grid maps. For all ligands, random starting points, and random orientation and torsions were used. The translation, quaternion and torsion steps were taken from the default values in AutoDock. The Lamarckian genetic algorithm and the pseudo-Soils and Wets methods were applied for minimization, using the default parameters. The number of docking runs was 100, the population in the genetic algorithm was 250, the number of energy evaluations was 500 000, and the maximum number of iterations was 27 000.
Results and discussion
As the first step, 2-[(aminoanilino)methylene]malononitrile (3) was obtained after mixing ethoxymethylenemalononitrile (1) with 1,2-diaminobenzene (2) in dichloromethane for two hours (Scheme ) [Citation21,Citation22]. After isolation of the yellow-brown precipitate, compound 3 was immediately added to a solution of acetyl chloride in anhydrous ethanol, and the reaction mixture was heated under reflux for 20 hours, giving 4-amino-1H-1,5-benzodiazepine-3-carbonitrile hydrochloride (4) [Citation22]. This key intermediate 1,5-benzodiazepine was then N-acylated with different carboxylic acids using either the acid chloride method or the free carboxylic acids activated with the coupling reagents (diphenylphosphorylazide (DPPA) or N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC)/1-hydroxybenzotriazole (HOBT) mediated coupling) to yield the amides 5 and 7a-d. In addition, the cyano group of 4′-nitrobenzoyl derivative 5 was hydrolysed to a carboxamido group using 37% hydrochloric acid, to give compound 6.
Scheme 1 Synthesis of the 1,5-benzodiazepine derivatives. a) CH2Cl2, 1 h, rt, then 2 h, 8–12°C; b) EtOH, AcCl, 0°C, then 20 h, reflux; c) nitrobenzoic acid, DPPA, Et3N, DMF, 0°C, 5 h, then rt overnight; d) 37% HCl, 1 h, 40°C; e) RCOOH, DPPA, Et3N, DMF, 0°C, 5 h, then rt, overnight; or benzoyl chloride, Et3N, rt, overnight; or EDC, HOBt, Et3N, DMF, − 5°C and then rt overnight; f) H2O, NaOH, rt to 100°C, 2 h; g) cinnamic acid, DPPA, Et3N, DMF, 0°C, 5 h, then rt overnight; h) 1-methylpiperazine, DMSO, toluene, 12 h, 125°C.
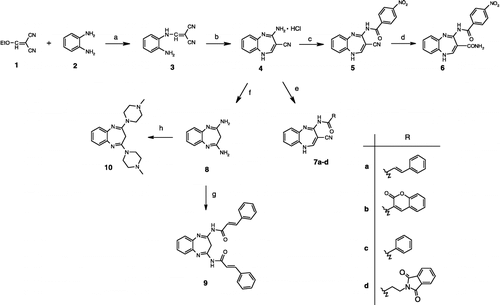
Compound 4 was also converted to the diamino derivative 8 by reflux in 3 M aqueous NaOH [Citation22]. Diamide 9 was then obtained from the reaction of compound 8 with two equivalents of cinnamic acid and the DPPA reagent, while the 2,4-bis(4-methyl-1-piperazinyl)-3H-1,5-benzodiazepine (10) was prepared by heating a solution of compound 8 and 1-methylpiperazine in a mixture of toluene and DMSO (1:1) to 125°C overnight.
All of these 1,5-benzodiazepines were evaluated for inhibition of the oxidative reaction catalyzed by 17β-HSDcl, a model enzyme of the SDR superfamily [Citation14,Citation15]. It is interesting to note that both of the free amines (compounds 4 and 8) do not inhibit 17β-HSDcl (). However, acylation with different aromatic carboxylic acids resulted in compounds 5-7 and 9, which showed very promising activities. When compound 4 was acylated on the free amino group (position 4) by 4-nitrobenzoic acid, inhibitor 5 was obtained that had an IC50 of 44 μM. In addition, when the 3-cyano group of compound 5 was partially hydrolysed to the 3-carbamoyl group, the activity of compound 6 increased 4-fold (IC50 = 10 μM). The best inhibitors in the series were compounds 7a (IC50 = 3 μM) and 7c (IC50 = 4 μM), where the 1,5-benzodiazepine core was substituted with cinnamic and benzoic acid, respectively. Also, the activities of conjugates with coumarin-3-carboxylic acid (7b, IC50 = 40 μM) and 3-phthalimidopropanoic acid (7d, IC50 = 19 μM) were in the low micromolar range.
Table I. 1,5-Benzodiazepines as inhibitors of 17β-HSDcl.
Comparison of the activities of compounds 5 and 7c, leads to the conclusion that the introduction of a nitro group in position 4 of the benzoyl substituent decreases the activity by one order of magnitude. Cinnamic acid and its rigid derivative coumarin-3-carboxylic acid were selected for acylation of the 1,5-benzodiazepine core on the basis of our previous results, where we demonstrated that some cinnamates, cinnamamides and coumarin-3-carboxylates can inhibit 17β-HSDcl [Citation17,Citation18]. It is interesting to note that also in the present study the rigid coumarin-3-carboxylic acid derivative is less active than were more flexible derivatives of cinnamic acid (compare compounds 7a and 7b).
Cinnamic acid was used to also acylate 3H-1,5-benzodiazepine-2,4-diamine (8), and the resulting diamide 9 is a promising inhibitor of 17β-HSDcl (IC50 = 43 μM) as well. Additionally, 2,4-bis(4-methyl-1-piperazinyl)-3H-1,5-benzodiazepine (10) was prepared in one step from the intermediate 8, but the compound was devoid of any inhibitory activity. Also, this observation is comparable to our previous results, where we found that introduction of a non-aromatic ring into the target inhibitor resulted in a decrease in inhibitory activity [Citation17].
The most promising inhibitors of the oxidative reaction were also evaluated for inhibition of the reductive reaction catalyzed by 17β-HSDcl (). Compounds 5, 7d and 9 are moderate inhibitors of the reductive reaction. Also here, the best inhibitors were compounds 6 (IC50 = 28 μM), 7a (50% inhibition at 25 μM conc. of inhibitor; due to poor solubility we were unable to determine IC50 value) and 7c (IC50 = 10 μM).
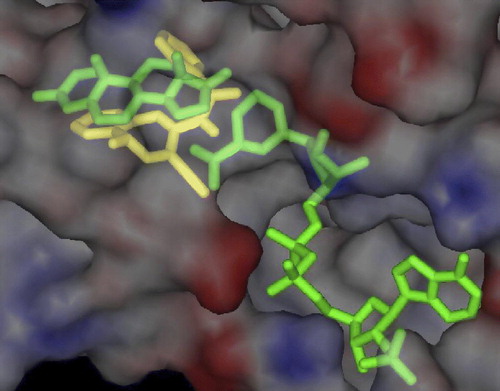
To investigate the possible binding modes of our inhibitors, compound 7c was docked into the active site of the homology-built model of 17β-HSDcl [Citation23], using AutoDock 3.0 with the Lamarckian genetic algorithm [Citation24]. AutoDock calculated that compound 7c occupies the substrate-binding region of the active site in a similar position to that previously suggested for androstenedione () [Citation23]. It is bound to the hydrophobic cavity that is defined by Val107, Thr155, Phe205 and Tyr212. The amide carbonyl group is oriented similarly to the 17-keto group of androstenedione, and points towards the catalytic amino-acid residues Tyr167 and Ser153, and the nicotinamide ring of the coenzyme.
Figure 2 Superimposition of the computer modeling of compound 7c (in yellow) on the homology-built model of androstenedione and NADPH (both in green) bound to 17β-HSDcl. The highest ranked position of the inhibitor, as calculated by AutoDock 3.0, is presented. For clarity, only the surface of the protein is shown. (See colour online)
To conclude, we have synthesized a series of new 1,5-benzodiazepine derivatives that inhibit 17β-HSDcl. These compounds are interesting lead compounds that should be further developed and investigated as inhibitors of the human 17β-HSD isoforms, which are implicated in many hormone-dependent and neuronal diseases.
Acknowledgements
This work was supported by the Ministry of Education, Science and Sport of the Republic of Slovenia. The authors wish to thank Dr Chris Berrie for critical reading of the manuscript.
References
- Adamski J, Jakob JF. A guide to 17β-hydroxysteroid dehydrogenases. Mol Cell Endocrinol 2001; 171: 1–4
- Mindnich R, Möller G, Adamski J. The role of 17 beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol 2004; 218: 7–20
- Penning TM. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocrinol Rev 1997; 18: 281–305
- Penning TM, Ricigliano JW. Mechanism based inhibition of hydroxysteroid dehydrogenases. J Enz Inhib 1991; 5: 165–198
- Lukacik P, Kavanagh KL, Opperman U. Structure and function of human 17β-hydroxysteroid dehydrogenases. Mol Cell Endocrinol 2006; 248: 61–71
- Poirier D. Inhibitors of 17β-hydroxysteroid dehydrogenases. Curr Med Chem 2003; 10: 453–477
- Le Lain R, Nicholls PJ, Smith HJ, Mahrlouie FH. Inhibitors of human and rat testes microsomal 17beta-hydroxysteroid dehydrogenase (17beta-HSD) as potential agents for prostatic cancer. J Enz Inhib 2001; 16: 35–45
- Le Lain R, Barrell KJ, Saeed GS, Nicholls PJ, Simons C, Kirby A, Smith HJ. Some coumarins and triphenylethene derivatives as inhibitors of human testes microsomal 17beta-hydroxysteroid dehydrogenase (17beta-HSD type 3): further studies with tamoxifen on the rat testes microsomal enzyme. J Enz Inhib 2002; 17: 93–100
- Le Bail JC, Pouget C, Fagnere C, Basly JP, Chulia AJ, Habrioux G. Chalcones are potent inhibitors of aromatase and 17β-hydroxysteroid dehydrogenase activities. Life Sci 2001; 68: 751–761
- Makela S, Poutanen M, Lehtimaki J, Kostian ML, Santii R, Vihko R. Estrogen-specific 17β-hydroxysteroid oxidoreductase type I as a possible target for the action of phytoestrogens. Proc Soc Exp Biol Med 1995; 208: 51–59
- Makela S, Poutanen M, Kostian ML, Lehtimaki N, Strauss L, Santii R, Vihko R. Inhibition of 17beta-hydroxysteroid oxidoreductase by flavonoids in breast and prostate cancer cells. Proc Soc Exp Biol Med 1998; 217: 310–316
- Krazeisen A, Breitling R, Moeller G, Adamski J. Phytoestrogens inhibit human 17β-hydroxysteroid dehydrogenase type 5. Mol Cell Endocrinol 2001; 171: 151–162
- Krazeisen A, Breitling R, Moeller G, Adamski J. Human 17beta-hydroxysteroid dehydrogenase type 5 is inhibited by dietary flavonoids. Adv Exp Med Biol 2002; 505: 151–161
- Lanišnik Rižner T, Möller G, Thole HH, Žakelj-Mavrič M, Adamski J. A novel 17β-hydroxysteroid dehydrogenase in the fungus Cochliobolus lunatus: New insights into the evolution of the steroid-hormone signalling. Biochem J 1999; 337: 425–431
- Kristan K, Lanišnik Rižner T, Stojan J, Gerber JK, Kremmer E, Adamski J. Significance of individual amino acid residues for coenzyme and substrate specificity of 17β-hydroxysteroid dehydrogenase from the fungus Cochliobolus lunatus. Chem Biol Interact 2003; 143/144: 493–501
- Kristan K, Krajnc K, Konc J, Gobec S, Stojan J, Lanišnik Rižner T. Phytoestrogens as inhibitors of fungal 17β-hydroxysteroid dehydrogenase. Steroids 2005; 70: 626–635
- Gobec S, Sova M, Kristan K, Lanišnik Rižner T. Cinnamic acid esters as potent inhibitors of fungal 17β-hydroxysteroid dehydrogenase – a model enzyme of the short-chain dehydrogenase/reductase superfamily. Bioorg Med Chem Lett 2004; 14: 3933–3936
- Kristan K, Starčević Š, Brunskole M, Lanišnik Rižner T, Gobec S. Cinnamates and Cinnamamides inhibit fungal 17β-hydroxysteroid dehydrogenase. Mol Cell Endocrinol 2006; 248: 239–241
- Usami N, Yamamoto T, Shintani S, Higaki Y, Ishikura S, Katagiri Y, Hara A. Substrate specificity of human 3(20)α-hydroxysteroid dehydrogenase for neurosteroids and its inhibition by benzodiazepines. Biol Pharm Bull 2002; 25: 441–445
- Higaki Y, Usami N, Shintani S, Ishikura S, El-Kabbani O, Hara O. Selective and potent inhibitors of human 20α-hydroxysteroid dehydrogenase (AKR1C1) that metabolizes neurosteroids derived from progesterone. Chem Biol Interact 2003; 143/144: 503–513
- von Stahl PH, Barchet R, Merz KW. Umsetzungen von aromatischen und heterocyclischen o-Diaminen mit Äthoxymethylenemalonester und Äthoxymethylencyanessigester. Arzneimittelforschung 1968; 18: 1214–1219
- Okamoto Y, Ueda T. Preparation and hydrolysis of 4-amino-1H-1,5-benzodiazepine-3-carbonitrile. Chem Pharm Bull 1975; 23: 1391–1395
- Lanišnik Rižner T, Adamski J, Stojan J. 17β-Hydroxysteroid dehydrogenase from the fungus Cochliobolus lunatus: Model structure and substrate specificity. Arch Biochem Biophys 2000; 384: 255–262
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comp Chem 1998; 19: 1693–1662
- Weiner SJ, Kollman PA, Case DA, Singh UC, Ghio C, Alagona G, Profeta S, Weiner P. A new force-field for molecular mechanical simulation of nucleic acids and proteins. J Am Chem Soc 1984; 106: 765–784
- Gasteiger J, Marsili M. Iterative partial equalization of orbital electronegativity – a rapid access to atomic charges. Tetrahedron 1980; 36: 3219–3228
- Mehler EL, Solmajer T. Electrostatic effects in proteins – comparison of dielectric and charge models. Protein Eng 1991; 4: 903–910