Abstract
A novel series of benzothiazole urea and thiourea derivatives was synthesized and evaluated for its in vitro cytotoxicity against MCF-7 breast cancer cells. The N1-(benzothiazol-2-yl)-N3-morpholinourea 3 displayed the highest cytotoxic activity in this series. A docked pose of 3 was obtained bound to G-quadruplex of human telomere DNA active site using the Molecular Operating Environment (MOE) module. Moreover, the synthesized compounds were screened for their antimicrobial activity against Mycobacterium tuberculosis H37Rv, E. coli, S. aureus and C. albicans. Again, 3 showed the best activity against M. tuberculosis H37Rv while other compounds were equipotent with ampicillin against S. aureus and E. coli.
Introduction
Cancer remains one of the most pressing health problems facing the world with breast cancer causing the most mortality among women. In recent years, the search for novel anticancer agents identified telomerase inhibitors as an ideal treatment not only for breast cancer but also for other cancers as well [Citation1,Citation2]. This reverse transcriptase enzyme (telomerase) is responsible for the maintenance of telomere length in over 80% of all tumor cells rendering them with an almost infinite capacity to divide and to be immortalized. Thus, the inhibition of telomerase activity will result in the loss of the divisional ability of tumor cells. On the other hand, the fact that the telomerase enzyme is not expressed in normal somatic cells made telomerase inhibition an ideal target for anticancer drug design. Therefore, many research groups recently reported a variety of telomerase inhibitors ranging from HIV reverse transcriptase inhibitors, to G-quadruplex stabilizers or others Citation3-9.
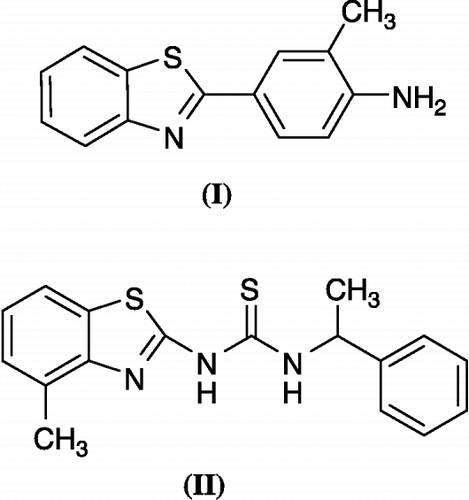
Various benzothiazoles (eg. I) () Citation10-12 as well as the urea and thiourea derivatives Citation13-15 have been reported to possess potent anticancer activities. The combination the urea or thiourea derivatives with benzothiazoles lead to inhibitors of DNA topoisomerase [Citation16,Citation17] or HIV reverse transcriptase (eg. II) () [Citation18,Citation19].
Figure 1 Some biologically active benzothiazoles: Antitumor agent (I) and HIV reverse transcriptase inhibitor (II).
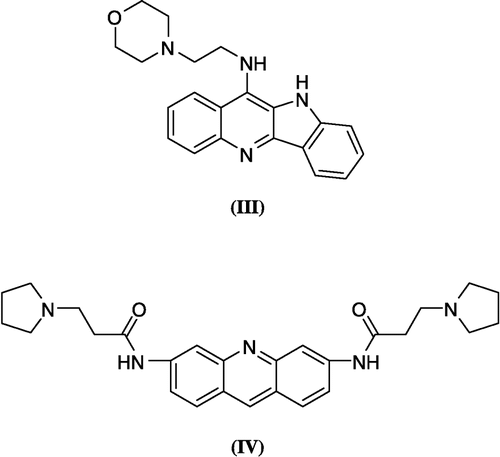
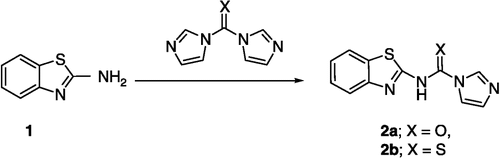
As previously mentioned, stabilization of the quadruplex conformation of telomeres by small molecules has been shown to inhibit telomerase, resulting in cancer cell death and many research groups have reported a variety of G-quadruplex stabilizers (eg. III, IV) (). [Citation8,Citation9].
Guided by this data, we suggested that benzothiazole urea and thiourea derivatives would possess anticancer activities possibly through inhibition of telomerase enzyme through G-quadruplex stabilization. To verify this hypothesis and as a continuation of our research program on the synthesis of novel anticancer agents [Citation20], herein we report here the design and synthesis of benzothiazole urea and thiourea derivatives and their cytotoxic evaluation against MCF-7 breast cancer cells.
Furthermore, the pronounced antimicrobial activity of urea and thiourea as well as benzothiazole derivatives Citation21-25 encouraged us to study the effect of the synthesized compounds against a variety of microorganisms such as Mycobacterium tuberculosis, Escherichia coli, Staphylococcus aureus and Candida albicans.
Materials and methods
Chemistry
Melting points were determined with an electrothermal apparatus (Stuart Scientific, England) and are uncorrected. Monitoring the chemical reactions and purity of the compounds was carried out using thin-layer chromatography (TLC) using silica gel 60 GF245 precoated sheets. Elemental analyses were performed on a “Analytischer Funktionstest vario EL Fab.-Nr. 11982027” (Germany). IR spectra were recorded as KBr disks on a Shimadzu-470 IR spectrophotometer (Japan). 1H-NMR spectra were carried out on a Bruker Avance 500 MHz, (USA) relative to TMS as internal standard. 13C-NMR spectra were recorded on a Bruker Avance at 125 MHz, (USA) using DMSO-d6 as internal standard. EI-MS spectra were recorded on a JEOL JMS 600 mass spectrometer.
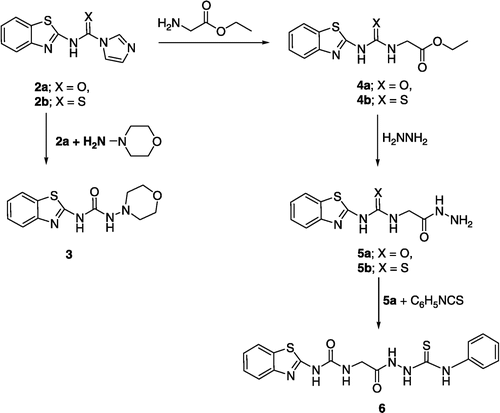
N-(Benzothiazol-2-yl)-1H-imidazole-1-carboxamide (2a)
A solution of 1,1′-carbonyldiimidazole (50 mmol) and 2-aminobenzothiazole (50 mmol) in acetonitrile (125 mL) was stirred at room temperature for 20 h. The resulting precipitate was collected by filtration. Yield 50%, mp: 250–253°C. IR cm− 1: 3410-3235 (NH), 1650 (C = O), 1610 (C = N), 1457, 1370; 1H NMR (DMSO-d6), δ ppm: 6.95–6.98 (m, 3H, ArH), 7.15–7.18 (t, 1H, J = 8 Hz, ArH), 7.28–7.30 (d, 1H, J = 8 Hz, ArH), 7.43 (s, 1H, NH), 7.59–7.62 (m, 2H, ArH). Anal. calcd. for C11H8N4OS: C, 54.09; H, 3.30; N, 22.94. Found: C, 53.91; H, 3.41; N, 23.07%.
N-(Benzothiazol-2-yl)-1H-imidazole-1-carbothioamide (2b)
As described for preparation of 2a but using 1,1′-thiocarbonyldiimidazole instead of 1,1′-carbonyldiimidazole as reported [Citation8,Citation9]. Yield: 45%, mp: 210–212°C. IR cm− 1: 3451-3240 (NH), 1634, 1613(C = N), 1225 (C = S); 1H NMR (DMSO-d6), δ ppm: 6.93–6.98 (m, 1H, ArH), 7.15–7.18 (m, 3H, ArH), 7.28–7.30 (d, 1H, J = 8 Hz, ArH), 7.43 (s, 2H, NH), 7.59–7.61 (d, 1H, J = 8 Hz, ArH), 8.02 (s, 1H, ArH).
N-(Benzothiazol-2-yl)-N′-morpholinourea (3)
A solution of 2a (8 mmol) and N-aminomorpholine (8 mmol) in DMF (10 mL) was stirred at 100°C for 1.5 h, the reaction mixture was cooled to room temperature and the solvent was evaporated under reduced pressure. The white precipitate obtained upon addition of cold water was collected and recrystallized from aqueous ethanol; Yield: 55%; mp: 262–265°C; IR cm− 1: 3450 & 3390 (NH), 1696 (C = O), 1607 (C = N), 1517, 1258, 1107; 1H NMR (DMSO-d6), δ ppm: 2.64–2.93 (dd, 4H, morphlino), 3.66–3.78 (dd, 4H, morphlino), 7.22–7.25 (t, 1H, J = 8 Hz, ArH), 7.36–7.39 (t, 1H, J = 8 Hz, ArH), 7.64–7.65(d, 1H, J = 8 Hz, ArH), 7.88–7.89 (d, 1H, J = 8 Hz, ArH), 8.65 (s, 1H, NH), 10.47(s, 1H, NH). 13C NMR (DMSO-d6), δ ppm: 56.00, 66.13, 120.37, 121.77, 123.33, 126.27, 174.35. EI-MS: m/z 278 [M]+(14.3%), 279 [M+1]+(3.4%), 176 (33.3%), 149 (100%), 101 (70.7%), Anal. calcd. for C12H14N4O2S. ½H2O: C, 50.16; H, 5.26; N, 19.50. Found: C, 49.44; H, 4.91; N, 19.32%.
Ethyl{[(benzothiazol-2-ylamino)carbonyl]amino}acetate (4a)
A mixture of 2a (8 mmol), glycine ethyl ester hydrochloride (8 mmol) and Triethylamine (8 mmol) in DMF (10 mL) was stirred at 100°C for 1.5 h, the reaction mixture was cooled to room temperature and the solvent was evaporated under reduced pressure. The white precipitate obtained upon addition of cold water was collected and recrystallized from ethanol; Yield: 60%; mp: 266–268°C; IR cm− 1: 3445 & 3390 (NH), 1726 (C = O, ester), 1695 (C = O, urea), 1625 (C = N), 1532, 1190; 1H NMR (DMSO-d6), δ ppm: 1.15–1.18 (t, 3H, J = 7 Hz, -CH2CH3), 3.92–3.93 (d, 2H, J = 5.7 Hz, -NHCH2), 4.06–4.11 (q, 2H, J = 7 Hz, -CH2CH3), 7.10 (t, 1H, J = 5.7 Hz, -NHCH2), 7.17–7.20 (t, 1H, J = 8 Hz, ArH), 7.31–7.34 (t, 1H, J = 8 Hz, ArH), 7.59–7.60 (d, 1H, J = 8 Hz, ArH), 7.83–7.84 (d, 1H, J = 8 Hz, ArH), 11.00 (s, 1H, NH). 13C NMR (DMSO-d6), δ ppm: 14.42, 41.84, 61.05, 121.75, 123.21, 126.26, 170.52. EI-MS: m/z 279 [M]+(3.5%), 280 [M+1]+(1.6%), 176 (43.4%), 149 (100%). Anal. calcd. for C12H13N3O3S: C, 51.60; H, 4.69; N, 15.04. Found: C, 51.10; H, 4.56; N, 14.92%.
Ethyl{[(benzothiazol-2-ylamino)thiocarbonyl]amino}acetate. (4b)
Prepared as described for 4a but starting from 2b. Recrystallized from ethanol; Yield: 58%; mp: 148–150°C; IR cm− 1: 3470 & 3395 (NH), 1731 (C = O), 1634, 1613, 1544, 1506, 1211(C = S); 1H NMR (DMSO-d6), δ ppm: 1.16–1.19 (t, 3H, J = 7 Hz, -CH2CH3), 4.09–4.13 (q, 2H, J = 7 Hz, -CH2CH3), 4.35–4.36 (d, 2H, J = 5 Hz, -NHCH2), 7.23–7.26 (t, 1H, J = 8 Hz, ArH), 7.37–7.40 (t, 1H, J = 8 Hz, ArH), 7.60–7.61 (d, 1H, J = 8 Hz, ArH), 7.85–7.87 (d, 1H, J = 8 Hz, ArH), 10.10 (bs, 1H, NH), 12.20 (bs, 1H, NH). 13C NMR (DMSO-d6), δ ppm: 14.44, 46.17, 61.17, 122.25, 124.14, 126.83, 169.31. Anal. calcd. for C12H13N3O2S2: C, 48.79; H, 4.44; N, 14.23. Found: C, 48.60; H, 3.98; N, 14.16%.
{[(Benzothiazol-2-ylamino)carbonyl]amino}acetic acid hydrazide (5a)
A solution of 4a (5 mmol) and hydrazine hydrate (10 mmol) in ethanol (10 mL) was refluxed for 2 h, the reaction mixture was cooled to room temperature and the solvent was evaporated under reduced pressure. The white precipitate obtained was collected and recrystallized from aqueous ethanol; Yield: 85%; mp: 210–212°C; IR cm− 1: 3410 & 3250 (NH), 1696 (C = O, urea), 1675 (C = O, hydrazide), 1607 (C = N). 1H NMR (DMSO-d6), δ ppm: 3.76–3.77 (d, 2H, J = 5 Hz, -NHCH2), 4.20–4.40 (bs, 2H, -NHNH2), 7.04 (bs, 1H, NH), 7.15–7.18 (t, 1H; J = 8 Hz, ArH), 7.30–7.33 (t, 1H; J = 8 Hz, ArH), 7.57–7.59 (d, 1H, J = 8 Hz, ArH), 7.81–7.83 (d, 1H; J = 8 Hz, ArH), 9.20 (bs, 1H, NH), 10.80 (bs, 1H, NH). 13C NMR (DMSO-d6), δ ppm: 41.70, 120.04, 121.71, 123.13, 126.22, 131.68, 149.29, 154.32, 160.14, 168.70. Anal. calcd. for C10H11N5O2S: C, 45.27; H, 4.18; N, 26.40. Found: C, 44.96; H, 4.08; N, 26.35%.
{[(Benzothiazol-2-ylamino)thiocarbonyl]amino}acetic acid hydrazide (5b)
Prepared as described for 5a but starting from 4b. Recrystallized from aqueous ethanol; Yield: 80%; mp: 195–196°C; IR cm− 1: 3410 & 3230 (NH), 1654 (C = O), 1610 (C = N), 1466, 1368, 1228 (C = S). 1H NMR (60 MHz, DMSO-d6), δ ppm: 4.30–4.40 (d, 2H, J = 5 Hz,-NHCH2), 4.50–4.70 (bs, 2H, -NHNH2), 7.50–7.55 (s, 1H, NH), 7.60–8.50 (m, 4H, ArH), 9.20–9.30(bs, 1H, NH), 10.70–10.90 (bs, 1H, NH). 13C NMR (DMSO-d6), δ ppm: 41.70, 120.04, 121.71, 123.13, 126.22, 131.68, 149.29, 154.32, 160.14, 168.70. Anal. calcd. for C10H11N5OS2: C, 45.27; H, 4.18; N, 26.40. Found: C, 44.96; H, 4.08; N, 26.35%.
1-({[(Benzothiazol-2-ylamino)carbonyl]amino}acetyl)-4-phenythiosemi- carbazide (6)
A solution of 5a (0.005 mmol) and phenyl isothiocyanate (0.005 mmol) in ethanol (10 mL) was refluxed for 2 h, the reaction mixture was cooled to room temperature and the solvent evaporated under reduced pressure. The white precipitate obtained was collected and recrystallized from aqueous ethanol; Yield: 75%; mp: 197–198°C; IR cm− 1: 3390, 3275 (NH), 1703(C = O, urea), 1659 (C = O, hydrazide), 1540, 1206 (C = S). 1H NMR (DMSO-d6), δ ppm: 3.92–3.93 (d, 2H, J = 5 Hz, -NHCH2), 7.09 (bs, 1H, NH), 7.14–7.20 (m, 3H, ArH), 7.30–7.34 (m, 3H, ArH), 7.40–7.42 (d, 1H, J = 8 Hz, ArH), 7.58–7.60 (d, 1H, J = 8 Hz, ArH), 7.81–7.82 (d, 1H, J = 8 Hz, ArH), 9.48 (bs, 1H, NH), 9.68 (s, 1H, NH), 10.26 (s, 1H, NH), 11.00 (bs, 1H, NH). Anal. calcd. for C17H16N6O2S2: C, 50.98; H, 4.03; N, 20.98. Found: C, 50.11; H, 3.60; N, 20.77%.
In vitro cytotoxicity screening
Human MCF-7 breast cancer cells were from ATCC (Manassas, VA, USA). MTT kit was obtained from Sigma (St. Louis, MO, USA).
Procedures
Human MCF-7 breast cancer cells were cultured in RPMI medium supplemented with 10% fetal calf serum and 1 μg/mL kanamycin at 37°C in 5% CO2 in humidified air. In vitro cytotoxicity was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [Citation26]. This assay measures the reduction of the tetrazolium salt to the formazan salt by mitochondrial dehydrogenases in living cells. After being cultured overnight to allow for cell attachment, the cells, plated in 96-well culture plates (3 × 103 cells/well), were incubated in the presence of different test compounds at 10, 100 μM for 96 h. The spectrophotometric absorbance of the formazan salt was measured at 580 nm using a microplate reader. Pirarubicin (Pharmacia-Upjohn) was used as a positive control. The cell survivals were calculated as percentages of the absorbency of treated cells over untreated controls.
Modeling studies
Computer-assisted simulated docking experiments were carried out under an MMFF94X force field on telemetric DNA G-quadruplex structures (PDB ID: 1L1H) using Chemical Computing Group's Molecular Operating Environment (moe-dock 2005) software, Montréal, Canada.
Methodology
The coordinates of the X-ray crystal structure of IV bound to the G-quadruplex were obtained from Protein Data Bank (PDBID: 1L1H). The ligand molecules were constructed using the builder module and were energy minimized. The active site of G-quadruplex was generated using the MOE-Alpha Site Finder, and then ligands were docked within this active site using the MOE-Dock. The lowest energy conformation was selected and the ligand interactions (hydrogen bonding and hydrophobic interaction) with G-quadruplex were determined.
Antimycobacterial assay
The primary antimycobacterial evaluation was performed at the National Hansen's Disease Programs (NHDP) TAACF facilities, Baton Rouge, LA, USA. The screening was conducted at a single concentration of 6.25 μg/mL against Mycobacterium tuberculosis H37Rv (ATCC 27294) in BACTEC 12B medium using the Microplate Alamar Blue Assay (MABA). Compounds exhibiting fluorescence were tested in the BACTEC 460-radiometric system [Citation27]. Compounds effecting < 90% inhibition in the primary screen were not evaluated further.
Antimicrobial assay
The microdilution susceptibility test in Müller-Hinton Broth (Oxoid) and Sabouraud Liquid Medium (Oxoid) were used for the determination of antibacterial and antifungal activity [Citation28]. The utilized test organisms were: Escherichia coli ATCC 25922 as an example of Gram-negative bacteria, Staphylococcus aureus ATCC 19433 as an example of Gram-Positive bacteria and Candida albicans as yeast-like fungi. Ampicillin trihydrate and clotrimazole were used as standard antibacterial and antifungal agents, respectively. Solutions of the test compounds, ampicillin trihydrate and clotrimazole were prepared in DMSO at a concentration of 1600 μg mL− 1. The two-fold dilutions of the compounds were prepared (800, 400,.6.25 μg mL− 1). The microorganism suspensions at 106 CFU mL− 1 (Colony Forming Unit/mL) concentrations were inoculated to the corresponding wells. Plates were incubated at 36°C for 24 h to 48 h. The incubation chamber was kept sufficiently humid. At the end of the incubation period, the minimal inhibitory concentrations (MICs) were determined which were defined as the minimum concentrations of a compounds that visually inhibits the growth of tested microorganisms.
Results & discussion
Chemistry
Benzothiazolyl urea and thiourea derivatives 3–6 were prepared according to the procedure depicted in Schemes , . The precursors 2a and 2b were prepared by analogy with a previously reported method [Citation18,Citation19] by reaction of 2-aminobenzothiazole 1 with 1,1′-carbonyldiimidazole or 1,1′-thiocarbonyldiimidazole, respectively. Reaction of 2a with N-aminomorpholine afforded 3 while reaction of 2a and 2b with ethyl glycinate yielded the esters 4a and 4b, respectively. When these esters reacted with hydrazine hydrate, the corresponding hydrazides 5a, 5b were obtained (Scheme ). Further reaction of the hydrazide 5a with phenyl isothiocyanate yielded the thiosemicarbazide 6. The identities of the compounds obtained were confirmed by elemental analyses, IR, 1H-NMR, 13C-NMR and mass spectral data. The IR spectra of the synthesized compounds generally show the characteristic bands corresponding to the carbonyl and the thiocarbonyl functions, in addition to the NH moieties. The 1H-NMR spectra of 1–6 showed the benzothiazole nucleus protons at the aromatic region and broad exchangeable singlets due to NH urea or thiourea protons. All other aromatic and aliphatic protons were observed in the expected regions.
Cytotoxicity study
Out of the synthesized benzothiazole derivatives, 2a, 2b, 3, 4a, 4b, and 5a were evaluated for their cytotoxic activity against MCF-7 (Breast cancer cell lines) using (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) MTT assay [Citation26] with pirarubicin as a positive control. Compounds were tested at 100 μM and 10 μM concentrations and the culture was incubated for 96 h. The percent survival for each compound was reported as the percent of growth of the treated cells when compared to the untreated control cells. For each compound, the procedures were repeated four times and the mean value was calculated. As shown in , at 100 μM, all the tested compounds reduced the growth of MCF-7 cell lines to the 32–75% range. On the other hand, at 10 μM, 3 was the most active compound reducing the growth of MCF-7 cells to 76%.
Table I. Primary in vitro growth inhibition assay results for compounds at 100 and 10 μM concentration.
Docking of 3 with G-quadruplex of human telomere DNA
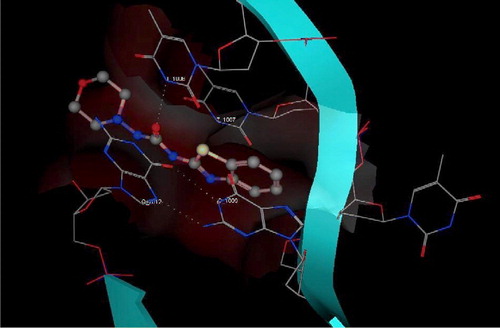
In an attempt to understand the reason for the observed cytotoxic activity of 3, we performed a molecular modeling study using the Molecular Operating Environment (MOE) module.
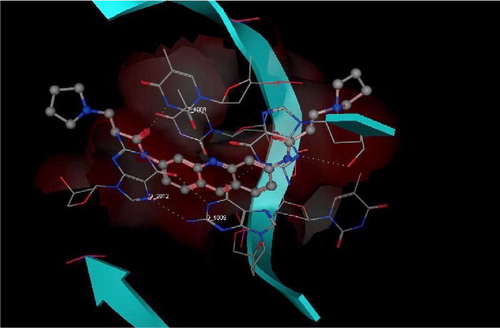
A structural similarity between 3 and the reported G-quadruplex stabilizers (eg. III, IV) (.), encouraged us to study a possible G-quadruplex stabilization action of 3. To test this hypothesis, as a starting point, we used the crystal structure of IV with G-quadruplex (PDB ID: 1H1L) () [Citation29]. Docking of the energy minimized conformation of 3 into the G-quadruplex of human telomere DNA () showed a very close pattern of binding to the G-quadruplex of human telomere DNA as that resulting from the crystal structure of IV. In both cases (IV and 3) a hydrogen bond interaction can be observed between a carbonyl group of the ligand and the thiamine 1006 base. From this data, 3 may provide a starting point for the design of unique compounds with high affinity and selectivity for human telomeric DNA, leading to enhanced telomerase inhibition.
Figure 3 3D View from a molecular modeling study, of the minimum-energy structure of the complex of IV docked in the G-quadruplex of human telomere DNA (PDB ID: 1H1L). White dashed lines depict hydrogen bond interactions. Viewed using Molecular Operating Environment (MOE) module. (See colour online)
Antimycobacterial activity
Compounds 2–6 were tested for their primary antimycobacterial activity against M. tuberculosis H37Rv at a single concentration of 6.25 μg/mL [Citation27]. As shown in , the imidazole derivatives 2a, 2b were the least active in this series while there was no observed variations of the biological activities of the tested esters 4a, 4b, hydrazides 5a, 5b or the thiosemicarbazide derivative 6. Again, 3 was the most effective in this series with a 37% growth inhibition. From the above results we can conclude that the benzothiazole urea or thiourea derivatives appear to be a useful scaffold for the antimycobacterial agents.
Table II. Antimycobacterial in vitro activity of test compounds.
Antimicrobial activity
Compounds 2–6 were tested for their in vitro antimicrobial against E. coli ATCC 25922, S. aureus ATCC 19433, and C. albicans [Citation28]. Ampicillin trihydrate and clotrimazole were used as standard antibacterial and antifungal agents, respectively. As shown in ., the MIC values of the tested compounds are generally within the range of 12.5 to more than 200 μg mL− 1 against all evaluated strains. Against E. coli, 6 showed similar activity to ampicillin, while the other compounds are only moderately active. Only 5b showed similar activity against S. aureus as ampicillin. None of the tested compounds showed any activity against C. albicans when compared to clotrimazole. Further studies on these types of compounds are needed to find out a possible mechanism of action and will be the subject of future reports.
Table III. Minimal inhibitory concentrations (MIC) in μg mL−1 of test compounds.
Conclusions
In conclusion, a series of benzothiazole urea and thiourea derivatives was synthesized as potential cytotoxic and antimicrobial agents. Compound 3 was the most effective against MCF-7 breast cancer cell lines and Mycobacterium tuberculosis H37Rv strain. On the other hand, 6 and 5b were the most effective derivatives against E. coli and S. aureus, respectively.
Acknowledgements
Antimycobacterial data were provided by the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) through a research and development contract with the U.S. National Institute of Allergy and Infectious Diseases.
The authors also wish to thank Dr Adnan A. Bekhit, and Dr. Elsayed Aboulmagd, Faculty of Pharmacy, University of Alexandria, Egypt, for the in vitro antimicrobial screenings of the tested compounds.
References
- Gellert GC, Jackson SR, Dikmen ZG, Wright WE, Shay JW. Telomerase as a therapeutic target in cancer. Drug Discov Today: Disease Mech 2005; 2: 159–164
- Herbert B-S, Wright WE, Shay JW. Telomerase and breast cancer. Breast Cancer Res 2001; 3: 146–149
- Olaussen KA, Dubrana K, Domont J, Spano J-P, Sabatier L, Soria J-C. Telomeres and telomerase as targets for anticancer drug development. Crit Rev Oncol/Hematol 2006; 57: 191–214
- Harrison RJ, Reszka AP, Haider SM, Romagnoli B, Morrell J, Read MA, Gowan SM, Incles CM, Kellandc LR, Neidle S. Evaluation of by disubstituted acridone derivatives as telomerase inhibitors: the importance of G-quadruplex binding. Bioorg Med Chem Lett 2004; 14: 5845–5849
- Rossetti L, Franceschin M, Schirripa S, Bianco A, Ortaggi G, Savino M. Selective interactions of perylene derivatives having different side chains with inter- and intramolecular G-quadruplex DNA structures. A correlation with telomerase inhibition. Bioorg Med Chem Lett 2005; 15: 413–420
- Huang H-S, Chou C-L, Guo C-L, Yuan C-L, Lu Y-C, Shieh F-Y, Lin J-J. Human telomerase inhibition and cytotoxicity of regioisomeric disubstituted amidoanthraquinones and aminoanthraquinones. Bioorg Med Chem 2005; 13: 1435–1444
- Schultes CM, Guyen B, Cuesta J, Neidle S. Synthesis, biophysical and biological evaluation of 3,6-bis-amidoacridines with extended 9-anilino substituents as potent G-quadruplex-binding telomerase inhibitors. Bioorg Med Chem Lett 2004; 14: 4347–4351
- Zhou J-L, Lu Y-J, Ou T-M, Zhou J-M, Haung Z-S, Zhu X-F, Du X-Z, Ma L, Gu L-Q, Li Y-M, Chan AS-C. Synthesis and evaluation of quindoline derivatives as G-quadruplex inducing and stabilizing ligands and potential inhibitors of telomerase. J Med Chem 2005; 48: 7315–7321
- Burger AB, Dai F, Schultes CM, Reszka AP, Moore MJ, Double JA, Neidle S. The G-quadruplex-interactive molecule BRACO-19 inhibits tumor growth, consistent with telomere targeting and interference with telomerase function. Cancer Res 2005; 65: 1489–1496
- Mortimer C, Wells G, Crochard J-P, Stone EL, Bradshaw TD, Stevens MFG, Westwell AD. Antitumor benzothiazoles 26. 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothizole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. J Med Chem 2006; 49: 179–185
- Yoshida M, Hayakawa I, Hayashi N, Agatsuma T, Oda Y, Tanzawa F, Iwasaki S, Koyama K, Furukawa H, Kurakata S, Sugano Y. Synthesis and biological evaluation of benzothiazole derivatives as potent antitumor agents. Bioorg Med Chem Lett 2005; 15: 3328–3332
- Vicini P, Geronikaki A, Incerti M, Busonera B, Poni G, Cabras CA, La Colla P. Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg Med Chem 2003; 11: 4785–4789
- Sanmartin C, Echeverria M, Mendıvil B, Cordeu L, Cubedo E, Garcia-Foncillas J, Fontc M, Palop JA. Synthesis and biological evaluation of new symmetrical derivatives as cytotoxic agents and apoptosis inducers. Bioorg Med Chem 2005; 13: 2031–2044
- Hallur G, Jimeno A, Dalrymple S, Zhu T, Jung MK, Hidalgo M, Isaacs JT, Sukumar S, Hamel E, Khan SR. Benzoylphenylurea sulfur analogues with potent antitumor activity. J Med Chem 2006; 49: 2357–2360
- Moreau E, Fortin S, Desjardins M, Rousseau JLC, Petitclerc EC, Gaudreault RC. Optimized N-phenyl-N′-(2-chloroethyl)ureas as potential antineoplastic agents: Synthesis and growth inhibition activity. Bioorg Med Chem 2005; 13: 6703–6712
- Esteves-Souza A, Pissinate K. Nascimento Md-G, Grynberg NF, Aurea E. Synthesis, cytotoxicity, and DNA-topoisomerase inhibitory activity of new asymmetric ureas and thioureas. Bioorg Med Chem 2006; 14: 492–499
- Choi SJ, Park HJ, Lee SK, Kim SW, Han G, Choo H-YP. Solid phase combinatorial synthesis of benzothiazoles and evaluation of topoisomerase II inhibitory activity. Bioorg Med Chem 2006; 14: 1229–1235
- Venkatachalam TK, Mao C, Uckun FM. Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds. Bioorg Med Chem 2004; 12: 4275–4284
- Lind PT, Morin JM, Jr, Noreen R, Ternansky RJ. Thiourea derivatives and methods for inhibition of HIV and related viruses. 119: 160110, WO 9303022, (1993). Through CA
- Aboraia AS, Abdel-Rahman HM, Mahfouz NM, EL-Gendy MA. Novel 5-(2-hydroxyphenyl)-3-substituted-2,3-dihydro-1,3,4-oxadiazole-2-thione derivatives: promising anticancer agents. Bioorg Med Chem 2006; 14: 1236–1246
- Seth P, Ranken R, Robinson DE, Osgood SA, Risen LM, Rodgers EL, Migawa MT, Jefferson EA, Swayze EE. Aryl urea analogs with broad-spectrum antibacterial activity. Bioorg Med Chem Lett 2004; 14: 5569–5572
- Phetsuksiri B, Baulard AR, Cooper AM, Minnikin DE, Douglas JD, Besra GS, Brennan PJ. Antimycobacterial activities of isoxyl and new derivatives through the inhibition of mycolic acid synthesis. Antimicrob Agents Chemother 1999; 43: 1042–1051
- Wilson LJ, Morris TW, Wu Q, Renick PJ, Parker CN, Davis MC, McKeever HD, Hershberger PM, Switzer AG, Shrum G, Sunder S, Jones DR, Soper SS, Dobson RLM, Burt T, Morand KL, Stella M. The identification and characterization of hydrazinyl urea-based antibacterial agents through combinatorial chemistry. Bioorg Med Chem Lett 2001; 11: 1149–1152
- Sriram D, Yogeeswari P, Madhu K. Synthesis and in vitro antitubercular activity of some 1-[(4-sub)phenyl]-3-(4-{1-[(pyridine-4-carbonyl)hydrazono]ethyl}phenyl) thiourea. Bioorg Med Chem Lett 2006; 16: 876–878
- Takayama W, Shirasaki Y, Sakai Y, Nakajima E, Fujita S, Sakamoto-Mizutani K, Inoue J. Synthesis and PDF inhibitory activities of novel benzothiazolylidenehydroxamic acid derivatives. Bioorg Med Chem Lett 2003; 13: 3273–3276
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assay. J Immunol Meth 1983; 65: 55–63
- Collins L, Franzblau SG. Microplate alamer blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacyerium tuberculosis and mycobacterium avium. Antimicrob Agents Chemother 1997; 41: 1004–1009
- Henderson B. Textbook of Immunopharmacology. MM Dale, JC Foreman, T-PD Fan. Blackwell Scientific Publications, London 1994; 16, and 193
- Haider SM, Parkinson GN, Neidle S. Structure of a G-quadruplex-Ligand complex. J Mol Biol 2003; 326: 117–125