Abstract
Synthetic chalcones (SCs) having different side chains on the 1-(2-Hydroxy-3-(2-hydroxy-cyclohexyl)-4,6 dimethoxy-phenyl(-methanone structure were examined in-vitro for their antioxidant abilities by DPPH (2,2-diphenyl-1-picryl hydrazine) radical scavenging activity, reducing ability, OH radical scavenging activity, inhibition of polyphenol oxidase (PPO) and formation of diene conjugates. Overall, with few exceptions, all the SCs showed moderate biological activity in all the parameters examined. The SCs were found to be reactive towards DPPH radical and had considerable reducing ability. With few exceptions, all the test compounds under study were found to possess moderate to poor OH radical scavenging activity and inhibited PPO significantly and all were found to be effective inhibitors of hydroperoxide formation. These findings suggest that these SCs can be considered as potential antioxidant agents which might be further explored for the design of lead antioxidant drug candidates.
Introduction
There is increasing experimental, clinical and epidemiological evidence highlighting an involvement of free radicals and reactive oxygen species (ROS) in a variety of human diseases including cancer, inflammatory disorders and various degenerative ailments associated with aging [Citation1]. Antioxidants are chemical substances, which scaveng free radicals and ROS thereby minimizing the burden of oxidative stress generated in the body [Citation2]. Moreover, numerous experimental studies have suggested the importance of antioxidants as an alternative therapeutic approach for the treatment of several human ailments such as cardiovascular diseases, various types of cancer, and several inflammatory disorders Citation3-5. Interestingly, antioxidants can protect critical cell macromolecules (proteins, lipids & nucleic acids) from undergoing oxidation and thus help in health amelioration [Citation6].
Chalcones continue to attract considerable scientific attention because of their diverse biological activities. They are a class of polyphenolic compounds which are basically flavonoids lacking a heterocyclic C ring and are widely distributed in fruits and vegetables Citation7-8. Amongst the flavonoids, chalcones have been identified as interesting compounds possessing several biological activities. A study on natural chalcones is limited but Synthetic chalcones (SCs) have been reported to have a wide range of biological activities with improved therapeutic indices [Citation9]. A series of prenylated and nonprenylated SCs have been investigated in vitro for their antioxidant and prooxidant actions [Citation10]. Several SCs have been designed, synthesized and tested for inhibition of activation of mast cells, neutrophils, macrophages and microglial cells which are important players in the recruitment of inflammatory disorders [Citation9]. There are some interesting findings from a series of 2′,5′-dihydroxychalcones. Most of the chalcones exhibited cytotoxic activity against a variety of tumor cell lines as well as non-tumor endothelial cell lines at very low micromolar concentrations. Chalcones have also been implicated in the inhibition of angiogenesis, a process of formation of new blood vessels for growth and proliferation of solid tumors, suggesting their possible role as anticancer agents [Citation11]
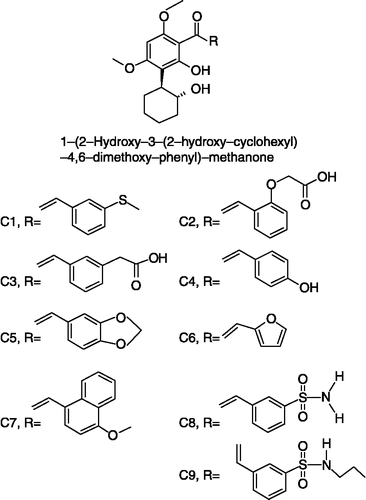
In the present investigation the antioxidant activities of selected SCs () containing 1-(2-hydroxy-3-(2-hydroxy-cyclohexyl)-4,6-dimethoxy-phenyl(-methanone as a basic nucleus with various substituents attached are described.
Materials and methods
Materials
The SCs under study were selected from the series of chalcones synthesized in the School of Chemical Sciences, S. R. T. M. University, Nanded (MS), India. The details of the synthetic methodology and characterization of the test SCs has been reported elsewhere [Citation12]. 2,2-Diphenyl-1-picrylhydrazine (DPPH) was obtained from Sigma-Aldrich Co. (St. Louis MO, USA), glutathione (GSH) and L-DOPA (3,4 dihydroxyphenyl L-alanine) were obtained from s. d. Fine Chemicals Ltd. Mumbai. Apples were purchased from the local market at Nanded City (MS) for extraction of PPO. The blood sample was collected from the slaughterhouse at Nanded City (MS) with the addition of EDTA (2 mg/mL) as an anticoagulant. Unless stated, all other chemicals used were of AR grade and were obtained from commercial sources.
DPPH radical scavenging assay [Citation13]
The DPPH radical scavenging potential of the individual SCs was conducted by mixing an equimolar concentration (1 mM in absolute ethanol) of individual SCs and DPPH. After 20 min reaction time the sample absorption was measured spectrophotometrically at 517 nm. Glutathione (1 mM) was used as a standard compound.
Determination of reducing power of SCs [Citation14]
The method is based on the ability of antioxidants to reduce Fe3 + of K3Fe(CN)6 to Fe2 + , the reducing power of the SCs being determined by the decrease in absorption of K3Fe(CN)6 at 420 nm. The reaction mixture contained 500 μL solution of individual SCs (1 mM in 0.5% v/v dimethyl sulfoxide) in 3 mL of 1 mM potassium ferricyanide solution and the absorbance was recorded at 420 nm after 10 min incubation. Glutathione (1 mM) was used as a reference compound.
OH radical scavenging assay [Citation15]
The ferric ion (Fe3 + )/ascorbic acid system was utilized for the generation of hydroxyl radicals, the OH radicals being detected by measuring the formaldehyde generated from the oxidation of dimethyl sulfoxide (DMSO). The reaction mixture consisted of mixing 0.1 mM EDTA, 167μM Fe3 + , 33 mM DMSO in phosphate buffer (50 mM, pH 7.4) and 0.1 mL individual SC (1 mM) solution. Ascorbic acid (150μL, 10 mM in phosphate buffer) was added finally to initiate the reaction. Trichloroacetic acid (17%, w/v) was used as reaction terminator. The formaldehyde produced was detected spectrophotomertically at 412 nm. Coumarin (1 mM) was used as a reference compound.
Polyphenol oxidase (PPO) inhibition assay [Citation16]
The enzyme PPO was extracted from apples by ammonium sulphate precipitation. The reaction mixture contained L-DOPA (1.5 mL, 2 mM), 0.75 mL enzyme, 1 mL solution of individual SC (1 mM) and citrate buffer (0.75 ml, pH 4.8, 0.1 M). After 10 min reaction time the brown chromophore (dopachrome) developed was recorded at 470 nm. L-cysteine (1 mM), a known PPO inhibitor, was used as a standard inhibitor.
Preparation of RBC membrane [Citation17,Citation18]
The blood sample, collected with addition of EDTA (2 mg/mL) as an anticoagulant, was centrifuged and the plasma aspirated. The blood cells were washed three times using saline (0.89%). To 0.5 mL of cells, 7 mL of ice-cold distilled water was added and left overnight at 0°C. The hemolysate was separated by centrifuging in a cooling centrifuge for 20 min. The pellet was washed twice with distilled water followed by centrifugation for 10 min and then suspended in a known volume of tris-HCL buffer (0.1 M, pH 7.4). The resultant solution was used as a membrane solution.
Assay of diene conjugates [Citation19]
The membrane solution (1.0 mL) was mixed with 5 mL of chloroform: methanol (2:1) followed by centrifugation at 1000 × g for 15 min to separate the two phases. The chloroform layer was removed and dried at 45°C in a water bath. The lipid residue was dissolved in 1.5 mL of cyclohexane and the hydroperoxides generated were detected at 233 nm against a cyclohexane blank. Acetylsalicylic acid (1 mM) was used as a standard drug.
The % activity in all the parameters was calculated by using the formula [Citation13]:
where T = absorbance of test sample and C = absorbance of control sample
Results and discussion
The results summarized in shows that all the SCs under study were effective towards the scavenging of DPPH radicals. The overall range of DPPH scavenging activity of all SCs was 43. 67- 14.27% as compared to the reference compound ascorbic acid (54.16%). The potency of the compounds was C4 > C2 > C3 > C8 towards the stabilization of DPPH radicals besides the moderate activity shown by other SCs. The DPPH radical scavenging assay has often been performed for evaluation of the anti-radical activity of antioxidants since DPPH possesses an odd electron responsible for giving a strong absorption peak at 517 nm [Citation13].
Table I. DPPH radical scavenging activity (% DPPH), reducing ability (% RA) and OH radical scavenging activity (% OH) of the selected SCs.
It is clear from the results in , that the SCs show moderate reducing activity in the range 51.23–12.18% as compared to glutathione (62.15%). Compound C4 and C3 were found to be most effective reducing agents as compared to the other SCs examined.
In the light of the structure-activity relationship, it seems that both DPPH radical scavenging activity and reducing potential are related with the degree of hydroxylation, which may be involved in donating electrons and thereby stabilizing the radical compounds.
The profile of OH radical scavenging activity was found to be poor (). Compounds C1, C5 and C7 did not react with OH radicals. Compound C4 (14.23%) was graded as the most potent OH radical scavenger followed by C8 (11.20%) and C3 (10.12%) as compared to a reference compound coumarin (4.6%).
OH radicals have been reported to be a key player in the physiological regulation and control of cell functions [Citation20]. The reaction rate constant for OH radicals is extremely high, wherein they react indiscriminately with almost every type of biomolecule within the cell and may deviate the normal physiological functions of the cell [Citation21]. It has also been reported that in many inflammatory disorders such as rheumatic arthritis, the reaction of nitric oxide with superoxide generates peroxynitrite which, under the acid conditions often found in regions of inflammation and ischemia, yields the hydroxyl radicals [Citation22]. The hydroxyl radicals thus generated in the above reaction have been blamed for membrane damage to the cells in the inflammatory region [Citation23].
The profile for the inhibition of PPO is shown in . Except for compounds C6 and C8, all the SCs inhibited the activity of PPO. The maximum PPO inhibition was shown by C1 (39.43%) followed by C2 (34.57%), while all other SCs inhibited PPO in the range 18.96–26.56% as compared to L-cysteine (87.94%) a known PPO inhibitor. The enzyme PPO (EC 1.14.18.1), a copper containing enzyme ubiquitously present in plants, has been studied as a model oxidizing enzyme since it contains metal ion (copper) and utilizes molecular oxygen [Citation24]. PPO catalyses the oxidation of a variety of phenols to the corresponding reactive ortho-quinones at the expense of molecular oxygen [Citation25]. Oxygen-induced transition metal-mediated generation of free radicals is attributed to the development of many diseases such as iron overload, rheumatoid arthritis and cancer [Citation21]. In general, a cellular system utilizing molecular oxygen and transition metal ions may generate free radicals and manifest altered physiological effects. A strategy for inhibition of this system may minimize the generation of oxygen-mediated free radicals. Flavonoids, especially chalcones like butein, have been reported to possess a copper chelation activity [Citation26]. The inhibition of PPO may be due to the chelation of copper, which is present in the active site of PPO.
Table II. Effect of SCs on PPO activity (% PPO) and on formation of hySdroperoxides (% OOH).
The effect of SCs on the formation of hydroperoxides (diene conjugates) has been summarized in . The experimental evidence indicates that all the SCs under study showed a good to excellent activity profile towards inhibition of formation of hydroperoxide, Compound C1 (70.65%) being the most effective. All other SCs showed % OOH inhibition within the range 45.27-18.69% as compared to acetylsalicylic acid (68.34%). Formation of diene conjugates (hydroperoxides) is one of the intermediate steps in membrane lipid peroxidation [Citation27]. The process of lipid peroxidation is thought to play a central role in many inflammatory disorders. During oxidation of lipids the polyunsaturated fatty acids in the lipoprotein are rapidly converted to lipid hydroperoxides and aldehydic breakdown products. The lipid peroxidation also results in the oxidative modification of the apoprotein, which plays a role in macrophage uptake and atherogenesis [Citation28]. It has been reported that chalcones inhibit the formation of diene conjugates and thereby reduce lipid peroxidation by virtue of their antioxidant activity [Citation10].
Conclusion
It can be concluded from the present findings that the SCs studied can be considered as potential antioxidant agents. Furthermore the 1-(2-hydroxy-3-(2-hydroxy-cyclohexyl)-4,6 dimethoxy-phenyl(-methanone scaffold can be subjected to optimization so as to design and develop a lead antioxidant drug candidate with an improved potency.
References
- Bandyopadhyay U, Das D, Banerjee RK. Curr Sci 1990; 77: 658–666
- Rani P, Unni M, Karthikeyan J. Ind J Clin Biochem 2004; 19(2)103–110
- Halliwell B. Nutr Rev 1994; 55: 522–544
- Borek C. Cancer Therap 2004; 3(4)333–341
- Salvatore C, Dennis PR, Achille PC, Daniel S. Pharmacol Rev 2001; 53: 135–159
- Nuttall SL, Kendall MJ, Martin U. Q J Med 1999; 92: 239–244
- Calliste CA, et al. Anticancer Res 2001; 21: 3949–3956
- Clifford MN. J Sci Food Agric 2000; 80: 1126–1137
- Won SJ, et al. Eur J Med Chem 2005; 40: 103–112
- Mirinda CL, et al. J Agric Food Chem 2000; 48: 3876–3884
- Nam NH, et al. J Med Chem 2003; 38: 179–187
- Kamble SG. Synthetic manipulation of biologically active molecules. Ph. D. Thesis. Swami Ramanand Teerth Marathwada University, Nanded (MS) India 2003
- Gulgun A, et al. J Enz Inhib Med Chem 2004; 19(2)129–135
- Sasaki K, Matsumoto I, Beppu M. Affinity chromatography. Tokyo Kagaku Dojin, Tokyo 1991; 117–130
- Christos K, Dimitra HL. J Enz Inhib Med Chem 2003; 18(1)63–69
- Gacche RN, Wrangkar SC, Ghole VS. J Enz Inhib Med Chem 2004; 19(2)175–179
- Dodge JT, Mitchell C, Hanaban DJ. Arch Biochem Biophys 1963; 100: 119–130
- Quist KH. Biochem Biophys Res Commun 1980; 92: 631–637
- Buege JA, Aust SD. Meth Enzymol 1978; 52: 302–305
- Drouge W. Physiol Rev 2002; 82: 47–95
- Barry H, John MCG. Biochem J 1984; 219: 1–14
- Brown GCH, Hall ND. Br J Rheumatol 1992; 31: 599–603
- Darligton LG, Stone TW. Brit J Nutr 2001; 85: 251–269
- Gacche RN, Dhole NA. J Pharm Biol 2006; 44(5)389–395
- Timothy DS, Kevin CV, Stephen OD. Phytochem 1991; 30(8)2499–2506
- Cheng ZJ, et al. Biochim Biophys Acta 1998; 1392: 291–299
- Shewfelt RL, Purvis AC. Hort Sci 1995; 30(2)213–218
- Esterbauer H, et al. Free Rad Biol Med 1992; 13: 341–390