Abstract
Several synthetic N-substituted N-nitrosohydroxylamines were found to inhibit mushroom tyrosinase in a pH-dependent manner regardless of the N-substituent. The inhibitory activity, or pI50 ( − log [IC50, M]) value, linearly decreased as the pH of the media increased. The inhibitory activities of tested N-substituted N-nitrosohydroxylamines at pH 6.8 and 5.8 were found to be almost 10 times and 100 times greater than at pH 7.8, respectively. The types of inhibition were different at pH 6.8 and 5.8. These results suggest that the inhibitory effect of N-substituted N-nitrosohydroxylamines is caused by the non-ionized form of the inhibitor. Furthermore, the mechanism of inhibition depends on the interaction between the inhibitor and the active site of tyrosinase at different pH values.
Introduction
Tyrosinase (EC 1.14.18.1), also known as polyphenol oxidase, is an enzyme that mediates the hydroxylation of monophenolic compounds to o-diphenols and the oxidation of o-diphenols to o-quinones. The enzyme is widely distributed in plants, fungi and mammals, and is known to be involved in enzymatic browning and melanin production [Citation1,Citation2]. Enzymatic browning of fruits and vegetables adversely changes the color, flavor and nutritional value, thereby lowering their market value. In addition, melanin hyperpigmentation is a serious problem in human health, especially in cases of melasma, ephelide, lentigo and the darkening of skin after overexposure to the sun. Therefore, tyrosinase inhibitors are useful in preventing enzymatic browning in the food industry, and in controlling the production of dermal melanin pigmentation in medical and cosmetic applications Citation3-5.
N-substituted N-nitrosohydroxylamines, classified as C-diazeniumdiolates, display a variety of interesting biological activities [Citation6]. For example, alanosine shows antitumor activity [Citation7], dopastin and nitrosoxacin are enzyme inhibitors [Citation8,Citation9] and fragin is a plant growth inhibitor [Citation10]. Recently, diazeniumdiolates were found to provide nitric oxide (NO) [Citation11]. In our previous paper, we showed that N-substituted N-nitrosohydroxylamines inhibit mushroom tyrosinase by chelating to the copper in the active site of the enzyme. We have also shown that the inhibitory activity [Citation12] and the mechanism of inhibition [Citation13] are dependent on the N-substituent.
Because the N-nitrosohydroxylamino moiety of these compounds is responsible for the inhibitory activity, they are classified as HA-type acid inhibitors. Benzoic acid, one of the typical HA-type acid tyrosinase inhibitors, has been reported to inhibit the enzyme in a pH-dependent manner [Citation14]. Here we have investigated the pH influence of tyrosinase inhibition by N-substituted N-nitrosohydroxylamines.
Materials and methods
Mushroom tyrosinase (specific activity 6800 U/mg) was purchased from Sigma-Aldrich Co. (St. Louis, MO) and used without further purification. N-cyclopentyl-N-nitrosohydroxylamine(1), N-dodecyl-N-nitrosohydroxylamine(2), N-(4′-hydroxybenzyl)-N-nitrosohydroxylamine(5), and N-(2′, 5′-dihydroxybenzyl)-N-nitrosohydroxylamine(6) were synthesized as ammonium salts by the method described previously [Citation12,Citation13]. All other chemical reagents were supplied by the Tokyo Chemical industry Co. (Tokyo, Japan).
Tyrosinase assay
The diphenolase assay for mushroom tyrosinase was performed by a spectrophotometric method with L-dopa as the substrate, using a UV-160A spectrophotometer (Shimadzu, Kyoto, Japan). The reaction mixture (total volume 3.2 mL) contained 0.54 mM L-dopa, 67 mM phosphate buffer, 0.1 mL of test sample in an aqueous solution and 0.1 mL of 80 units of mushroom tyrosinase in an aqueous solution (added last). The reaction mixture was incubated at 30°C for 5 min. The initial linear increase in absorbance at 475 nm, corresponding to the formation of dopachrome, was monitored.
The inhibitory action of the compounds was expressed as the concentration of inhibitor required for a 50% reduction in enzyme activity (IC50). The pH dependencies in potency are expressed as pI50 values, − log[IC50].
The pKa values of the synthesized compounds were obtained by a method described previously [Citation15], based on the absorption of 260 nm for the anionic form of the compounds.
Results
The effect of pH on the diphenolase activity of mushroom tyrosinase
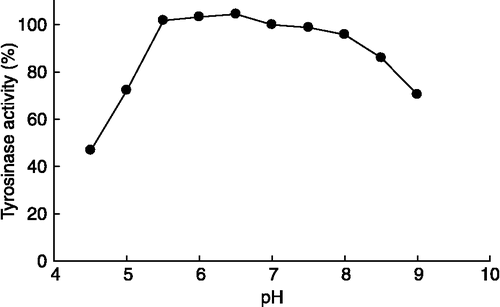
The activity of mushroom tyrosinase was studied in the pH range 4.5–9.0 at constant ionic strength and was expressed as a percentage of the value at pH 6.8 (). The activity was almost the same in the range of pH 5.5–8.0 (i.e. >95% of the value at pH 6.8).
The pH-dependent inhibition by N-substituted N-nitrosohydroxylamines
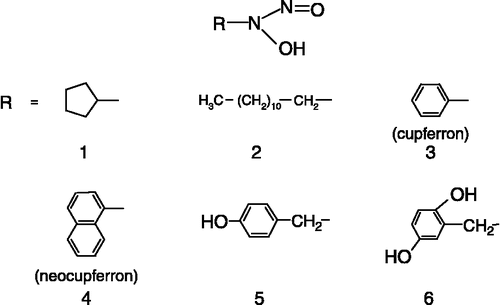
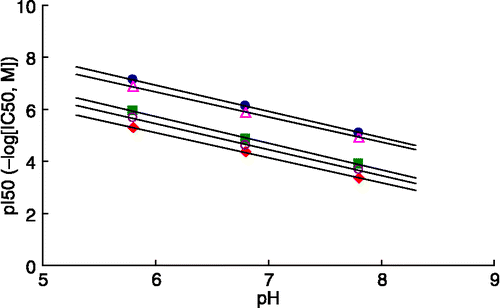
The chemical structures of the tested compounds, four synthesized N-substituted N-nitrosohydroxylamines (compounds 1, 2, 5 and 6), cupferron (3) and neocupferron (4) are shown in . Inhibition of the diphenolase activity of mushroom tyrosinase over the pH range of 5.5–8.0 was studied and pI50 values plotted against pH (). The observed inhibitory activity at pH 6.8 and 5.8 was approximately 10 times and 100 times greater than at pH 7.8. Interestingly, plots of the pI50 values versus pH gave a straight line for each compound with a slope value of almost − 1 (correlation coefficient of >0.999). Thus, the pH-dependency of inhibition was independent of the N-substituent of the nitrosohydroxylamine.
The inhibition effects of N-substituted N-nitrosohydroxylamines on the diphenolase activity of mushroom tyrosinase at pH 6.8 and 5.8
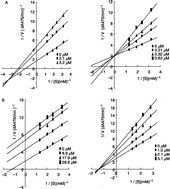
The inhibition of mushroom tyrosinase by compounds 1–6 was studied at both pH 6.8 and 5.8 (). The type of inhibition was determined by Lineweaver-Burk plot analysis. Typical Lineweaver-Burk plots for compounds 2 and 6 are shown in . Our results show that the IC50 values at pH 5.8 are almost one order of magnitude less than at pH 6.8. Although cupferron displayed competitive inhibition at both pH values, all the other N-substituted N-nitrosohydroxylamines showed a different type of inhibition at pH 6.8 and 5.8.
Table I. Inhibition effects of N-substituted N-nitrosohydroxylamines on mushroom tyrosinase at pH6.8 and 5.8.
The pH effect of the other HA-type acid inhibitors on copper chelation
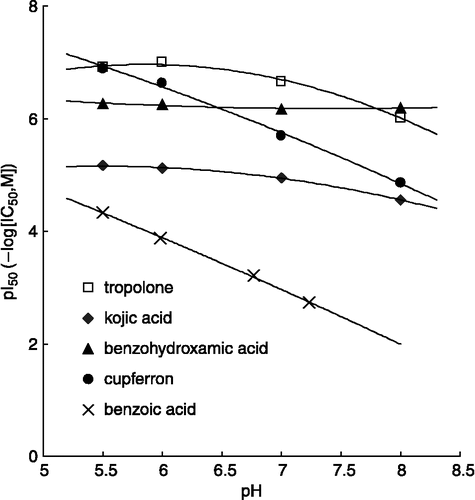
Tropolone [Citation16], kojic acid [Citation17], benzohydroxamic acid [Citation18] and benzoic acid [Citation19] are known to inhibit tyrosinase by chelating with copper. The chemical structures of these inhibitors are shown in . The pH-dependency for the inhibition of mushroom tyrosinase diphenolase activity is shown in . Our results show that benzoic acid acts in a similar manner to cupferron (compound 3), where the pI50 value decreases linearly as the pH is increased. By contrast, tropolone and kojic acid showed only a slight pH-dependence of tyrosinase inhibition, and the inhibition by benzohydroxamic acid was completely independent of pH.
Discussion
All N-substituted N-nitrosohydroxylamines tested in this study showed pH-dependent inhibition of mushroom tyrosinase diphenolase activity over the pH range of 5.5–8.0, where the enzyme is stable. Because the inhibitory activity depends on pH, the pI50 value decreased linearly as the pH increased. This linear relationship can be expressed as the slope value for a plot of pI50 versus pH, which was almost − 1 for all the compounds tested (). Thus, the dissociation of each compound in the reaction medium is involved in the inhibitory activity. These results suggest that the protonated form of N-substituted N-nitrosohydroxylamines (i.e. electrically neutral non-ionized species) is involved in the inhibitory activity by chelation to the the active site copper of tyrosinase.
N-nitrosohydroxylamines are classified as HA-type acid inhibitors, as is benzoic acid. Benzoic acid, which inhibits tyrosinase by a copper chelating mechanism, is known to show pH-dependent inhibition. We recognized that both N-substituted N-nitrosohydroxylamines and benzoic acid similarly inhibit mushroom tyrosinase activity in a pH-dependent manner ().
In the case of benzoic acid, the pH dependency has been interpreted by two different mechanisms. One mechanism involves the interaction between the non-ionized form of benzoic acid and copper in the active site of the enzyme. Indeed, deuterium isotope studies show that the proton of the carboxyl group in benzoic acid transfers to the oxygen, binding the copper in the enzyme [Citation14]. Alternatively, the pH dependency has been proposed to be the result of acid-base interaction with a histidine residue of the enzyme [Citation19,Citation20]. In this case, the inhibitory mechanism is due to the anionic form of benzoic acid interacting with the protonated form of the histidine residue in the active site of the enzyme. This hypothesis is supported by the fact that benzoic acid (pKa = 4.0) is fully dissociated over the pH range 5.5–8.0, and the midpoint of the range of pH effects is close to the pKa of the histidine imidazole groups (pKa = 6.0–6.3) in proteins. Therefore, the inhibitory activity would depend on the protonated versus deprotonated status of the histidine residue at the active site of the enzyme.
As far as N-substituted N-nitrosohydroxylamines are concerned, the pKa range of these compounds varies widely from 4.1 to 5.9, nearly that of the histidine residue (). Therefore the pH dependency did not support the acid-base interaction with the compounds and the histidine residue of the enzyme. Moreover, the types of inhibition of N-substituted N-nitrosohydroxylamines were different at pH 6.8 and 5.8. If the compounds interact with the histidine residue, the type of inhibition would be the same in spite of the N-substituent.
Our experimental data for other HA-type acid inhibitors show that benzoic acid inhibits in the same pH-dependent manner as cupferron. However, tropolone (pKa = 6.7), kojic acid (pKa = 7.9) and benzohydroxyamic acid (pKa = 8.7) displayed only a slight pH-dependency or none at all. This result suggested that the inhibitory activities of them might be dependent on the dissociation of the inhibitors not the enzyme. Therefore, it probably arises from their electrically neutral, non-ionized form. Thus, the inhibitory activity of N-substituted N-nitrosohydroxylamines might depend on the non-ionized species, which interacts with the copper at the active site of tyrosinase.
It is thought that the activation of mushroom tyrosinase at pH 5 is due to a conformational change of the enzyme [Citation4]. Therefore, the differences in the type of inhibition for N-substituted N-nitrosohydroxylamines at pH 6.8 and 5.8 might be caused by conformational changes of the enzyme over the pH range. The type of inhibition for compound 2 changed from the mixed-II type at pH 6.8, where the inhibitor (I) binds more readily to the enzyme-substrate (ES) complex than to the free enzyme (E), to a competitive type at pH 5.8, where the inhibitor can only bind the free enzyme. Compound 6 displays uncompetitive inhibition at pH 6.8, in which the inhibitor can only bind the enzyme-substrate complex, and non-competitive inhibition at pH 5.8, where the inhibitor binds equally to the free enzyme and enzyme-substrate complex. The other N-substituted N-nitrosohydroxylamines exhibited a similar pH-dependent change to the inhibition mechanism (). Thus, under acidic conditions (pH 5.8), the compounds can bind to the free enzyme much more readily than at higher pH values.
In conclusion, we have demonstrated that N-substituted N-nitrosohydroxylamines inhibit mushroom tyrosinase in a pH-dependent manner, regardless of N-substituent. These results indicate that the inhibitory activity is due to the non-ionized, electrically neutral form of the compound.
References
- Walker JRL. Enzymatic browning in fruits. ACS Symp Ser 1995; 600: 8–22
- Okombi S, Rival D, Bonnet S, Mariotte AM, Perrier E, Boumendjel A. Discovery of benzylidenebebzofuran-3(2H)-one (Aurones) as inhibitors of tyrosinase derived from human melanocytes. J Med Chem 2006; 49: 329–333
- Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci 2005; 62: 1707–1723
- Seo SY, Sharma VK, Sharma N. Mushroom tyrosinase: Recent prospects. J Agric Food Chem 2003; 51: 2837–2853
- Cabanes J, Chazarra S, Garcia-Carmoa F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitior of catecholase activity of tyrosinase. J Pharm Pharmacol 1994; 46: 982–985
- Hrabi JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (nitrosohydroxylamine) functional group and its oxygen-substituted derivatives. Chem Rev 2002; 102: 1135–1154
- Tyagi AK, Cooney DA. Biochemical pharmacology, metabolism and mechanism of action of L-alanosine, a novel, natural antitumor agent. Adv Pharmacol Chemother 1984; 20: 60–121
- Iinuma H, Matsuzaki M, Nagatsu T, Takeuchi T. Biochemical and biological studies on dopastin, an inhibitor of dopamine β-hydroxylase. Agric Biol Chem 1974; 38: 2107–2111
- Nishino M, Hasegawa M, Suzuki K, Sawada Y, Hook DJ. Nitrosoxacin-A, nitrosoxasin-B and nitrosoxacin-C, new 5-lipoxygenase inhibitors. J Antibiot 1993; 46: 193–195
- Murayama A, Hat K, Tamura S, Fragin A. A new biological active metabolite of a pseudomonas. Agric Biol Chem 1969; 33: 1599–1605
- McGill AD, Zhang W, Wittbrodt J, Wang J, Schlegel HB, Wang PG. para-Substituted N-nitroso-N-oxybenzenamine ammonium salts: A new class of redox-sensitive nitric oxide releasing compounds. Bioorg Med Chem 2000; 8: 405–412
- Shiino M, Watanabe Y, Umezawa K. Synthesis of N-substituted N-nitrosohydroxylamines as inhibitors of mushroom tyrosinase. Bioorg Med Chem 2001; 9: 1233–1240
- Shiino M, Watanabe Y, Umezawa K. Synthesis and tyrosinase inhibitory activity of novel N-hydroxybenzyl-N-nitrosohydroxylamines. Bioorg Chem 2003; 31: 129–135
- Conrad JS, Dawso SR, Hubbard ER, Meyers TE, Strothkamp KG. Inhibitor binding to the binuclear active site of tyrosinase: Temperature, pH and solvent deuterium isotope effects. Biochemistry 1994; 33: 5739–5744
- Buscarons F, Canela J. Analytical uses of some N-nitroso-N-alkyl(or -N-cycloalkyl)hydroxylamines. Analyt Chim Acta 1973; 67: 349–355
- Kahn V, Andrawis A. Inhibition of mushroom tyrosinase by tropolone. Phytochem 1985; 24: 905–908
- Kahn V, Lindner P, Zakin V. Effect of kojic acid on the oxidation of o-dihydroxyphenols by mushroom tyrosinase. J Food Biochem 1995; 18: 253–271
- Wilcox DE, Porras AG, Hwang YT, Lerch K, Winkler ME, Solomon EI. Substrate analogue binding to the coupled binuclear copper active site in tyrosinase. J Am Chem Soc 1985; 107: 4015–4027
- Rescigno A, Sollai F, Pisu B, Rinaldi A, Sanjust E. Tyrosinase inhibition: General and applied aspects. J Enz Inhib Med Chem 2002; 17: 207–218
- Prota G. Melanins and melanogenesis. Academic Press, San Diego, US 1992; 58–59