Abstract
Some novel fused heterocyclic compounds of 2, 5-disubstituted-benzoxazole and benzimidazole derivatives, which were previously synthesized by our group, were investigated for their inhibitory activity on both eukaryotic DNA topoisomerase I and II in a cell free system. 2-Phenoxymethylbenzimidazole (17), 5-amino-2-(p-fluorophenyl)benzoxazole (3), 5-amino-2-(p-bromophenyl)benzoxazole (5), 5-nitro-2-phenoxymethyl-benzimidazole (18), 2-(p-chlorobenzyl)benzoxazole (10) and 5-amino-2-phenylbenzoxazole (2) were found to be more potent as eukaryotic DNA topoisomerase I poisons than the reference drug camptothecin having IC50 values of 14.1, 132.3, 134.1, 248, 443.5, and 495 μM, respectively. 5-Chloro-2-(p-methylphenyl)benzoxazole (4), 2-(p-nitrobenzyl)benzoxazole (6) and 5-nitro-2-(p-nitrobenzyl)benzoxazole (8) exhibited significant activity as eukaryotic DNA topoisomerase II inhibitors, having IC50 values of 22.3, 17.4, 91.41 μM, respectively, showing higher potency than the reference drug etoposide.
Introduction
DNA topoisomerases, which catalyze the interconversion of various topological states of DNA, were originally discovered to change the superhelical structure of closed circular DNAs. Depending on the nature of the reactants and reaction conditions, topoisomerases can catalyze DNA relaxation/supercoiling, catenation/decatenation and knotting/unknotting reactions [Citation1,Citation2]. Based on their functional mechanisms, DNA topoisomerases have been classified into two types. Type I DNA topoisomerase breaks and rejoins only one of the two strands during catalysis, while type II DNA topoisomerase acts on both strands for each DNA strand-passing reaction and it requires ATP for full activity [Citation3]. Since the activity of topoisomerases is essential for several cellular processes such as replication, transcription and chromosome condensation, investigation of inhibitory activities of eukaryotic topoisomerases is widely used in anticancer drug development.
Many antitumour agents poison topoisomerases by stabilising a topo-DNA cleavable complex to cause DNA breaks, thus shifting the reaction equilibrium towards cleavage, which leads ultimately to cell death. These compounds are called poisons because they convert this essential nuclear enzyme into a lethal poison. In contrast to the poisons, the catalytic inhibitors do not induce DNA breaks, instead they inhibit the catalytic activity of the topoisomerase at a step prior to the formation of the topo-DNA cleavable complex [Citation4].
Several of the anticancer agents in clinical use have been shown to be potent inhibitors of DNA topoisomerase II. Etoposide (VP-16), teniposide (VM-26), mitoxantrone, m-AMSA, adriamycin (doxorubicin), and daunomycin have all been demonstrated to possess significant activity as inhibitors of DNA topoisomerase II Citation5-7. In comparison to topoisomerase II inhibitors, however, there are fewer known topoisomerase I inhibitors and camptothecin is the most extensively studied mammalian topoisomerase I poison [Citation2,Citation8].
In recent years, detailed investigations of bi- and ter-benzimidazole derivatives revealed that these compounds constitute a new class of DNA topoisomerases I and II inhibitors Citation9-13. A derivative of camptothecin (Formula I) having a benzoxazole ring system within its structure was found to be significantly more potent than camptothecin as an inhibitor of DNA topoisomerase I [Citation14]. Work on such compounds indicates that a fused ring system in the structure is critical for the activity.
Currently, we investigated the inhibitory effects of some novel fused heterocyclic compounds on eukaryotic DNA topoisomerase II in a cell free system (Formula II) Citation15-17 and pointed out that in addition to the very well-known bi- and ter-benzimidazoles Citation10-13 compounds with single bicycled fused ring systems in their structure such as benzimidazole, benzoxazole, benzothiazole, and oxazolo(4,5-b)pyridine derivatives also exhibited significant DNA topoisomerase II inhibitory activity [Citation15].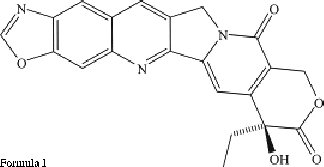
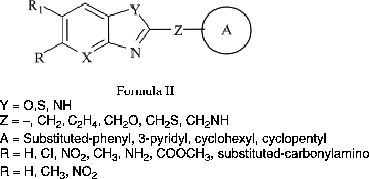
In this study, a new series of 2,5-disubstituted-benzoxazole and benzimidazole derivatives (), which was previously synthesized and tested for their antimicrobial activity by our group Citation18-20, has been investigated for their inhibitory activity on eukaryotic DNA topoisomerases I and II in a cell free system. The goal of this research, was that predictions from the structure-activity relationships of these tested compounds possibly could lead to the design of more potent new DNA topoisomerases I and II inhibitors.
Table I. IC50 values against eukaryotic DNA topoisomerase I and II for the tested compounds and the reference drugs (μM) .
.
Materials and methods
Materials
Eukaryotic DNA topoisomerases I and II from calf thymus were purchased from Amersham Biosciences UK. pBR322 plasmid DNA was obtained from MBI Fermentas Ltd. Fused heterocyclic test compounds were chemically synthesized as described previously Citation18-20 except compound 11 (see below). Solutions of the tested compounds in 20% dimethyl sulfoxide (DMSO) were freshly prepared. All the other chemicals were of analytical grade.
Synthesis of 5-amino-2-(p-methylbenzyl)benzoxazole (11)
A mixture of 2,4-diaminophenol dihydrochloride (0.01 mol) and p-methyl-phenylacetic acid (0.01 mol) was refluxed at 160–165°C in PPA (Polyphosphoric acid) (12.5 g) for 1–1.5 h. At the end of the reaction period the residue was poured into ice-water and neutralised with excess of 10% NaOH solution. The precipitate was collected, washed, dried and extracted with ethyl acetate to remove impurities. The combined ethyl acetate extracts were dried over anhydrous sodium sulfate and evaporated in vacuo. The crude product was recrystallised from ethanol and ethanol-water. Yield: 57%. MW: 238. mp:83–85°C. 1H-NMR (CDCl3): 2.70 (s, 3H, CH3), 4.45 (s, 2H, CH2), 6.65–6.80 (dd, 1H, C-6, J6,7 = 8.56, J6,4 = 2.19 6.95–7.00 (d, 1H, C-4, J4,6 = 2.10) 7.15–7.35 (m, 7H, C-2′, C-3′, C-4′, C-5′, C-6′, C-7, NH). IR (KBr disc cm–1): 3382, 1615, 1556, 1486, 1453, 1272, 1192. LC-MS(Scan ES+): 239 (M+).
DNA topoisomerase I assay
Relaxation activity of DNA topoisomerase I was determined by measuring the conversion of supercoiled pBR322 plasmid DNA to its relaxed form [Citation2,Citation21]. The reaction mixture contained 35 mM Tris-HCl (pH 8.0), 72 mM KCl, 5 mM MgCl2, 5 mM DTT, 5 mM spermidine, 0.01 mM bovine serum albumin (BSA), 0.3 μM pBR322 plasmid DNA, 1 U of enzyme and different concentrations of drugs in a total volume 12 μL. The mixture was incubated for 1 h at 37°C. After the incubation period, 3 μL of loading buffer containing in electrophoresis buffer (60 mM Tris, 30 mM acetic acid and 1.5 mM EDTA, pH 8.0) was added and mixture was subjected to electrophoresis on 1% agarose at 45 V for 3 h. After electrophoresis the gels were stained with ethidium bromide (1 μg/mL), photographed under UV light and band distribution was analyzed with a gel analysis system. The rate of formation of the newly formed bands was used as a measure of enzyme activity. Inhibitory activities were expressed as the micromolar concentration of test compound that caused 50% inhibition per unit of enzyme, under the assay conditions. Camptothecin was used as the reference drug.
DNA topoisomerase II assay
Relaxation activity of DNA topoisomerase II was determined by measuring the conversion of supercoiled pBR322 plasmid DNA to its relaxed form [Citation2,Citation21]. The reaction mixture contained 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 15 μL/mL bovine serum albumin (BSA), 1 mM ATP, 0.3 μM pBR322 plasmid DNA, 5 U of enzyme and different concentrations of drugs in a total volume 10 μl. The mixture was incubated for 1 h at 30°C. After incubation period, 3 μL of loading buffer in electrophoresis buffer TAE (60 mM Tris, 30 mM acetic acid and 1.5 mM EDTA, pH 8.0) was added and mixture was subjected to electrophoresis on 1% agarose at 45 V for 3 h. After the electrophoresis gels were stained with ethidium bromide (l μg/mL), photographed under light and band distribution was analyzed with a gel analysis system. The rate of formation of the newly formed bands was used as a measure of enzyme activity. Inhibitory activities were expressed as the micromolar concentration of test compound that caused 50% inhibition per unit of enzyme, under the assay conditions. Etoposide was used as the reference drug.
Results and discussion
Eukaryotic DNA topoisomerase I and II activities in cell-free systems were evaluated by the relaxation assay as described in above. The relaxation assay utilizes supercoiled plasmid as substrate and has been used by many investigators to study inhibition of DNA topoisomerase I and II activities. The supercoiled substrate and its relaxed product can easily be distinguished in ethidium bromide stained gels since relaxed isomers migrate more slowly than the supercoiled isomer [Citation22]. The change in the molecular shape without a change in the molecular weight can be differentiated, since more compact molecules move faster as compared to their more relaxed counterparts.
If the DNA molecules were completely relaxed by topoisomerases and there is equilibrium between the different topological forms of the DNA molecules, then, at the end of the electrophoresis several bands would be obtained. However, if the test compounds induce the formation of the topoisomerases-DNA cleavable complex, a single thick band (nicked DNA molecules) closest to the application point would be obtained after the electrophoresis. A typical electrophoresis pattern is seen in . On the other hand, if the catalytic activities of topoisomerases were inhibited by the test compounds and therefore all the DNA molecules were in the supercoiled form, a faster moving single band would be obtained ().
Figure 1 Electrophoregram showing the inhibitory effects of tested compound 17 and reference drug camptothecin on eukaryotic DNA topoisomerase I. Lane 1; supercoiled pBR322 plasmid DNA (0.3 μg) with incubation mixture without enzyme. Lane 2; plasmid DNA with 1 U of topo I enzyme (control). Lanes 3–6; plasmid DNA with 1 U of topo I enzyme in the presence of compound 17 at concentrations 0.1, 1, 5 and 10 μg/μL, respectively. Lane 7; plasmid DNA with compound 17 at a concentration of 10 μg/μL without enzyme. Lane 8; plasmid DNA with 1 U of topo I enzyme and a known DNA topoisomerase I inhibitor (camptothecin) at a concentration of 5 μg/μL. The relaxation assay in a cell-free system was performed as described in methods.
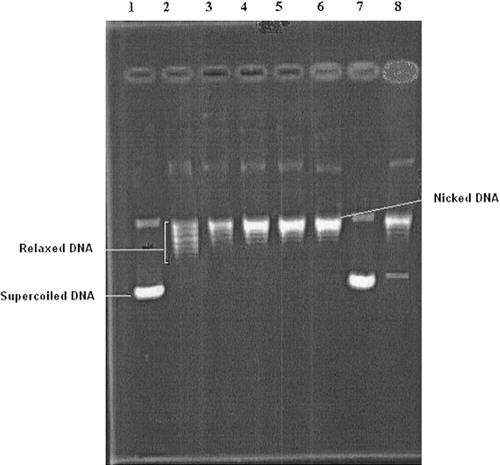
Figure 2 Electrophoregram showing the inhibitory effects of tested compound 6 and reference drug etoposide on eukaryotic DNA topoisomerase II. Lane 1; supercoiled pBR322 plasmid DNA (0.3 μg) with incubation mixture without enzyme. Lane 2; plasmid DNA with 5 U of topo II enzyme (control). Lanes 3–6; plasmid DNA with 5 U of topo II enzyme in the presence of compound 6 at concentrations 0.1, 1, 5 and 10 μg/μL, respectively. Lane 7; plasmid DNA with compound 6 at a concentration of 10 μg/μL without enzyme. Lane 8; plasmid DNA with 5 U of topo II enzyme and a known DNA topoisomerase II inhibitor (etoposide) at a concentration of 10 μg/μL. The relaxation assay in a cell-free system was performed as described in methods.
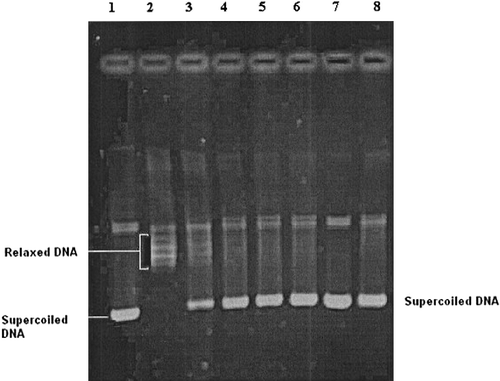
The rate of formation of the newly formed bands during the incubation period was used as a measure of the enzyme activity. The enzyme was incubated with four different concentrations (0.1, 1, 5, 10 μg/μL) of the test compounds. Decreases in the percentage of the activity compared with the activity obtained without using any agents was taken as the inhibitory activity of the tested compounds. Inhibitory activities of the compounds were presented as micromolar concentrations that cause 50% inhibition per unit of enzyme (IC50), under the assay conditions. From the plots obtained with four different concentrations of the drugs, IC50 values were obtained and the results are the average of two to three estimations. If inhibition was not obtained at any concentration of a tested compound it was assumed to have no inhibitory activity on eukaryotic DNA topoisomerase I and II.
The compounds were tested at a different range of concentrations to define their inhibitory activity where camptothecin and etoposide were used as the standard drug for DNA topoisomerase I and DNA topoisomerase II, respectively, to compare their activities (). Amongst the tested set of 18 compounds of 2,5-disubstituted-benzoxazole and benzimidazole derivatives (see ), compounds 2, 3, 4, 12, and 18 were found to inhibit both DNA topoisomerase I and II in a cell-free system using the relaxation assay.
Of these tested 18 compounds, 7 derivatives (2, 3, 4, 5, 10, 17, and 18) exhibited IC50 values in the 14.1–495 μM range for topoisomerase I inhibitory activity and were considered as positive DNA topoisomerase I poisons, which were more active than the reference drug camptothecin. 2-Phenoxymethylbenzimidazole (17) was the most potent DNA topoisomerase I poison (IC50 value = 14.1(μM) (). Moreover, 5-amino-2-(p-flourophenyl)benzoxazole (3), 5-amino-2-(p-bromophenyl)benzoxazole (5), 5-nitro-2-phenoxymethyl-benzimidazole (18), 2-(p-chlorobenzyl)benzoxazole (10), and 5-amino-2-phenylbenzoxazole (2) were found to be significant DNA topoisomerase I inhibitors having IC50 values of 132.3, 134.1, 258, 443.5, and 495 μM, respectively.
On the other hand, shows that compounds 4, 6, and 8 were significantly active as DNA topoisomerase II inhibitors having IC50 values 22.3, 17.4, 91.4 μM, respectively, showing more potency than the reference drug etoposide. Compound 6, 2-(p-nitrobenzyl)benzoxazole, was the most potent DNA topoisomerase II inhibitor with IC50 = 17.4 μM (), but shows no activity as a DNA topoisomerase I poison.
The structure-activity relationships for these tested compounds indicated that the benzoxazole ring was more important than the benzimidazole ring for DNA topoisomerase II inhibitory activity. In addition, substitution with a nitro- or a methyl group at position R1 enhanced the DNA topoisomerase II inhibitory properties. It could be pointed out that no apparent conclusion could be drawn concerning the preference of the fused ring system for DNA topoisomerase I poison activity when compared with the DNA topoisomerase II inhibition in these tested compounds.
In conclusion, while compound 17 was found as a significant inhibitor for DNA-topoisomerase I, compounds 4 and 6 showed a strong inhibition of DNA-topoisomerase II activity. Since DNA topoisomerases are considered as important targets for cancer chemotherapy, the present findings may provide future opportunities to design and develop new chemotherapeutic agents.
Acknowledgements
This study was supported by the Hacettepe University Research Fund (Grant no: 01.02.601.006), TUBITAK (Grant no: TBAG-2088, 101 T127) and Ankara University Research Fund (Grant No: 2005-0803049) for financial support for this study.
References
- Wang JC. DNA Topoisomerases. Ann Rev Biochem 1996; 65: 635–692
- Ting CY, Hsu CT, Hsu HT, Su JS, Chen TY, Tarn WY, Kuo YH, Jacqueline WP, Liu LF, Hwang J. Isodiospyrin as a novel human DNA topoisomerase I Inhibitor. Biochem Pharm 2003; 66: 1981–1991
- Nitiss JL. Investigating the biological function of DNA topoisomerases in eukaryotic cells. Biochim Biophys Acta 1998; 1400: 63–81
- Seaton A, Higgins C, Mann J, Baron A, Bailly C, Neidle S, Van Den Berg H. Mechanistic and anti-proliferate studies of two novel, biologically active bis-Benzimidazoles. Eur J Cancer 2003; 39: 2548–2555
- Bodley AL, Liu LF. Topoisomerases as novel targets for cancer chemotherapy. Biotechnology 1988; 6: 1315–1318
- Schneider E, Hsiang YH, Liu LF. DNA topoisomerase as anticancer drug targets. Adv Pharmacol 1990; 21: 149–183
- Chen AY, Liu LF. DNA topoisomerases: Essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol 1994; 34: 191–218
- Gallo RC, Whan-Peng J, Adamson RAH. Studies on the antitumor activity, mechanism of action, and cell cycle effects of camptothecin. J Natl Cancer Inst 1971; 46: 789–795
- Alper S, Temiz-Arpaci O, Aki-Sener E, Yalcin I. Some new bi- and terbenzimidazole derivatives as topoisomerase I inhibitors. Il Farmaco 2003; 58: 497–507
- Tolner B, Hartley JA, Hochhauser D. Transcriptal regulation of topoisomerase IIa at confluence and pharmacological modulation of expression by bis-benzimidazole drugs. Mol Pharmacol 2001; 59: 699–706
- Sun Q, Gatto B, Yu C, Liu A, Liu LF, LaVoie EJ. Synthesis and evaluation of terbenzimidazoles as topoisomerase I inhibitors. J Med Chem 1995; 38: 3638–3644
- Finlay GJ, Baguley BC. Potentiation by phenylbisbenzimidazoiles of cytotoxicity of anticancer drugs directed against topoisomerase II. Eur J Cancer 1990; 26: 586–589
- Rowe TC, Chen GL, Hsiang YH, Liu F. DNA damage by antitumor acridines mediated by mammalian DNA topoisomerase II. Cancer Res 1986; 46: 2021–2026
- Peel MR, Milstead MW, Sternbach DD, Besterman M, Leitner P, Morton B. Novel A-ring modified camptothecins as topoisomerase I inhibitors. Bioorg Med Chem Lett 1995; 5: 2129–2132
- Pinar A, Yurdakul P, Yildiz I, Temiz-Arpaci O, Acan NL, Aki-Sener E, Yalcin I. Some fused heterocyclic compounds as eukaryotic topoisomerase II Inhibitors. Biochem Biophys Res Comm 2004; 317: 670–674
- Temiz-Arpaci O, Tekiner-Gulbas B, Yildiz I, Aki-Sener E, Yalcin I. 3D-QSAR analysis on benzazole derivatives as eukaryotic topoisomerase II inhibitors by using comparative molecular field analysis method. Bioorg Med Chem 2005; 13: 6354–6359
- Tekiner-Gulbas B, Temiz-Arpaci O, Yildiz I, Aki-Sener E, Yalcin I. 3D-QSAR study on heterocyclic topoisomerase II inhibitors using CoMSIA. SAR QSAR Environ Res 2006; 17: 121–132
- Yalcin I, Sener E. QSARs of some novel antibacterial benzimidazoles, benzoxazoles and oxazolopyridines against an enteric gram-negative rod; K. pneumoniae. Int J Pharmaceutics 1993; 98: 1–8
- Yalcin I, Oren I, Sener E, Akin A, Ucarturk N. The synthesis and the structure-activity relationships of some substituted benzoxazoles, oxazolo(4,5-b)pyridines, benzothiazoles and benzimidazoles as antimicrobial agents. Eur J Med Chem 1992; 27: 401–406
- Yildiz IO, Yalcin I, Aki ES, Ucarturk N. Synthesis and structure-activity relationships of new antimicrobial active multisubstituted benzazole derivatives. Eur J Med Chem 2004; 39: 291–298
- Osheroff N, Shelton ER, Brutlag D. DNA topoisomerase II from drosophila melanogaster, relaxation of supercoiled DNA. J Biol Chem 1983; 258: 9536–9543
- Barrett JF, Sutcliffe JA, Gootz TD. In vitro assays used to measure the activity of topoisomerases. Antimicrob Agents Chemother 1990; 34: 1–7
