Abstract
Phytochemical investigations on the ethyl acetate-soluble fraction of the whole plant of Buddleja crispa led to the isolation of the iridoids 1–7. Compound 2 displayed significant inhibitory potential against enzyme lipoxygenase in a concentration-dependant fashion with IC50 value of 39.7 ± 0.02μM, along with DPPH radical scavenging activity with IC50 value 0.638 mM.
Introduction
The genus Buddleja (synonym Buddleia), belonging to the family Buddlejaceae, is comprised of 100 species mainly distributed in East Asia, America and South Africa. In Pakistan it is represented by four species [Citation1]. Other authors classify this genus in the family Loganiaceae [Citation2] and Hegnauer and Kooiman classify the families Buddlejaceae and Loganiaceae in Scrophlariaceae [Citation3].
Various species of the genus Buddleja are used for the treatment of a variety of ailments such as ulcer, conjunctival congestion, clustered nebulae and skin disorders. Different parts of B. asiatica are used as antiinflamatories, abortifaciants, antifungals and also for the treatment of skin diseases [Citation4,Citation5]. The leaves and flowers of B. globasa are used for washing wounds and treatment of ulcers [Citation5].The flowers of B. officinalis are used in Chinese medicine for the treatment of conjunctival congestion and clustered nebulae [Citation5].
Previous studies on the genus Buddleja have resulted in the isolation of various compounds including glycosides of triterpenes, iridoids and flavonoids, sterols, aryl esters, phenolic fatty acid esters, alkaloids, lignans, neolignans, diterpenes and sesquiterpenes [Citation6].
Buddleja crispa is a densely tomentose shrub. The leaves are sessile or shortly petiolate, laneolate to ovate, 2.5–8 cm long, tomentose on both surfaces; margin crenate, dentate or stellate, white, evanescent on the upper surface. The flowers are sessile, purple, fragrant, in interrupted branched spikes. It is distributed in Pakistan, India and Afghanistan [Citation1].
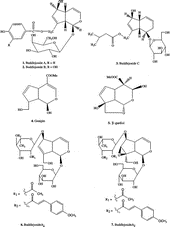
A methanolic extract of Buddleja crispa showed significant antioxidant and inhibitory activity against the lipoxygenase enzyme which prompted us to conduct phytochemical studies on this plant. As a result, seven iridoids, buddlejoside A (1), buddlejoside B (2), buddlejoside C (3), genipin (4), β-gardiol (5), buddlejoside A2 (6) and buddlejoside A5 (7) were isolated ().
Lipoxygenases (LOX, EC 1.13.11.12) constitute a family of non-haem iron containing dioxygenases that are widely distributed in animals and plants. In mammalian cells LOX are key enzymes in the biosynthesis of many bioregulatory compounds such as hydroxyeicosatetraenoic acids (HETEs), leukotrienes, lipoxins and hepoxylines [Citation7]. It has been found that these LOX products play a role in a variety of disorders such as bronchial asthma, inflammation [Citation7] and also have a profound influence on the development of several human cancers [Citation8]. LOX are therefore potential targets for rational drug design to discover mechanism–based inhibitors for the treatment of bronchial asthma, inflammation, cancer and autoimmune diseases.
Free radicals play an important role in carcinogenesis through their involment in breaking of the DNA strand [Citation9]. Numerous other pathological events are associated with the generation of reactive oxygen species (ROS) constituting a key mechanism for tissue injury. They have significant relevance in the inflammation process, cardiovascular disease [Citation10,Citation11,Citation12], arteriosclerosis, malaria, rheumatoid arthritis, neurodegenerative disease and the aging process [Citation13,Citation14].
In the current study we have described the lipoxygenase inhibitory and antioxidant activities of the iridoids (1–7) which were isolated from Buddleja crispa and their structures published previously by our research group [Citation6,Citation15,Citation16].
Materials and methods
In vitro lipoxygenase inhibition assay
Lipoxygenase inhibiting activity was measured by slightly modifying the spectrometric method developed by Tappel [Citation17]. Lipoxygenase (1.13.11.12) type I-B and linoleic acid were purchased from Sigma. All other chemicals were of analytical grade. Sodium phosphate buffer (160 μL, 100 mM, pH 8.0), 10 μL of test compound solution and 20 μL of lipoxygenase solution were mixed and incubated for 10 min at 25°C. The reaction was then initiated by the addition of 10 μL linoleic acid (substrate) solution, with the formation of (9Z, 11E)-(13S)-13-hydroperoxyoctadeca-9,11-dienoate, and the change of absorbance at 234 nm was followed for 10 min. Test compounds and the control were dissolved in MeOH. All the reactions were performed in triplicate in a 96-well micro-plate in SpectraMax 340 (Molecular Devices, USA).
Determination of IC50 values
The concentrations of the test compounds that inhibited the hydrolysis of the substrate (linoleic acid) by 50% (IC50) were determined by monitoring the effect of various concentrations of these compounds in the assays on the inhibition value. The IC50 values were then calculated using the EZ-Fit Enzyme Kinetics program (Perrella Scientific Inc., Amherst, USA).
DPPH (1, 1-diphenyl-2-picryl hydrazyl) free radical scavenging activity
The reaction mixture containing 5μL of test sample (1 mM in DMSO) and 95 μL of DPPH (Sigma, 300 μM) in ethanol.The reaction mixture was taken in a 96-well micro titer plate (Molecular Devices, USA) and incubated in Elisa at 37°C for 30 min., the absorbance was measured at 515 nm. Percent radical scavenging activity was determined by comparison with a DMSO-containing control (). IC50 values represent the concentration of compounds to scavenge 50% of DPPH radicals. BHA (3-t-butyl-4-hydroxyanisole) was used as a positive control. All the chemicals used were of analytical grade (Sigma, USA).
Table I. Lipoxygenase inhibitory and antioxidant activities of iridoids 1–7.
Results and discussion
Lipoxygenase inhibitory activities of iridoids 1–7
Compounds 2, 7 and 6 showed significant inhibitory activity against LOX with IC50 values of 39.7 ± 0.02, 40.4 ± 0.06 and 41.9 ± 0.04 μM, respectively. While compounds 1, 3, 4 and 5 displayed moderate inhibitory activity against LOX (), the standard inhibitor of LOX (baicalein) had an IC50 value of 22.5 ± 0.2 μM.
Antioxidant activities of iridoids 1–7
Compounds 1–7 and BHA scavenged the DPPH radical at a concentration of 1 mM, with the IC50 values ranging between 0.638–0.792μM, indicating significant activity (). Among these active substances, buddlejoside B (2) had a greater potential to scavenge the DPPH radicals. BHA was used as a standard (IC50 = 0.044 mM).
The compounds 1–7 could be lead compounds in the development of agents for treating inflammation, asthma, aging, tumor, angiogenesis and cancer. However, further in vivo study are needed to explore the pharmacological properties of these compounds.
References
- Abdullah P. Flora of west pakistan. 1974; 56: 1
- Kapoor VK, Chawla AS, Gupta YC, Passannanti S, Paternostro MP. Constituents of Buddleia species leaves. Fitoterpia 1982; 52: 235
- Hegnauer R, Kooiman P. The taxonomic significance of iridoids of Tubiflorae sensu Wettstein. Planta Med 1978; 33: 1
- Ahmad M, Sticher O. Isolation of acetacin-7-O-rutenoside and martynoside from Buddleja Davidii. J Chem Soc Pak 1988; 10: 117
- Liao Y-H, Houghton PJ, Hoult JRS. Novel and known constituents from Buddleja species and their activity against leukocyte eicosanoid generation. J Nat Prod 1999; 62: 1241
- Ahmad I, Malik A, Afza N, Fatima I, Tareen RB, Nawaz SA, Choudhary MI. Iridoid glucoside and aryl ester from Buddleja crispa. Pol J Chem 2006; 80: 1483–1491
- Steinhilber D. A target for anti-inflammatory drug revisited. Curr Med Chem 1999; 6: 71–85
- Ding XY, Tong WG, Adrian TE. Cyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancer. Pancreatol 2001; 1: 291–296
- Pathak MA, Joshi PC. The nature and molecular basis of cutaneous photosensitivity reactions to psoralens and coal tar. J Innvest Derm 1983; 80: 66
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet 1993; 342: 1007
- Moure A, Cruz J, Franco D, Dominguez M, Sineiro J, Dominguez H, Nunez J. Natural antioxidants from residual sources. Food Chem 2001; 72: 145
- Hollman PC, Hertog MG, Katan M. Analysis and health effects of flavonoids. Food Chem 1996; 57: 43
- Hunt EJ, Lester CE, Lester PA, Tackett RL. Effect of St. John's wort on free radical production. Life Sci 2001; 69: 181
- Meyer AS, Heiononen M, Frankel EN. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human ldl oxidation. Food Chem 1998; 61: 71
- Ahmad I, Afza N, Anis I, Malik A, Fatima I, Haq A, Tareen RB. Iridoid galactosides and a benzofuran type sesqueterpene from Buddleja crispa. Heterocycles 2004; 63: 1875–1881
- Ahmad I, Malik A, Afza N, Anis I, Fatima I, Nawaz SA, Tareen RB, Choudhary MI. Enzymes inhibitory constituents from Buddleja crispa. Z Naturforsch 2005; 60b: 341–346
- Tappel AL. Methods in Enzymology, Academic Press, New York 5 1962. p 539–542