Abstract
The structural and spectroscopic properties of novel five-coordinated dimeric-Cu(II) system have been investigated. The biocidal activities of all eight compounds, ligands, cupric nitrate and standard drugs against six bacteria and three fungi were determined. The DNA interaction activity of complexes was studied using spectrophotometry and electrophoresis. The superoxide dismutase (SOD)-like activity of the complexes was compared with previously reported monomeric- and dimeric copper complexes. The results support the five-coordinated dimeric square pyramidal geometry for the quinolone-Cu(II) system.
Introduction
The fluoroquinolone drugs are well known for their indistinguishable property of coordination with several transition metals [Citation1]. Several spectrophotometry methods have been reported for the determination of fluoroquinolones in human blood and urine via metal chelation Citation2-4. There is a drastic change in the biochemical properties of copper on chelation with drugs and it possess modified toxicological and pharmacological properties. Copper(II) is biologically essential and its compounds have proved beneficial in diseases like tuberculosis, rheumatoid arthritis, gastric ulcer, and cancer Citation5-7. Some advanced copper-containing compounds have been reported as anti-inflammatory, antiamoebic, antimalerial, cytotoxic under hypoxic condition, and antiradical, antifungal Citation8-16. Some dimeric Cu-proteins have an advanced application in oxygen activation and reduction [Citation17]. Several monomeric and dimeric Cu(II) compounds are known to be biologically stable, membrane permeable and nontoxic as SOD mimics [Citation18,Citation19]. In the present study, eight dimeric Cu(II) compounds were synthesized and studied for different biochemical and thermal aspects.
Experimental
Materials and methods
All the chemicals used were of analytical grade. Cupric nitrate, 3-chloro aniline, cyclohaxanone, o-phenylenediamine, benzaldehyde, ethylenediamine, p-anisaldehyde and benzil, were purchased from the E. Merck (India) Ltd, Mumbai. Ciprofloxacin hydrochloride from Bayer AG (Wyppertal, Germany). Thiophene-2-carboxaldehyde, 2,2′-bipyridylamine, 2-aminopyridine, 1,8-diaminonaphthalene were from, Lancaster, England. Luria broth and agar-agar were from Hi-media Laboratories Pvt. Ltd., India. Agarose and ethidium bromide were from Sigma Chemical Co., India. Bromophenol blue, xylene cyanol FF, tris(hydroxymethyl)methylamine, sucrose, acetic acid and EDTA were from Qualigens Fine Chemicals, India. NADH, nitroblue tetrazolium, phenazine methosulphate, and sperm herring DNA were from Sigma Chemical Co., USA. The organic solvents were purified by recommended methods [Citation20]. Infrared spectra were recorded on a FT-IR Shimadzu spectrophotometer as KBr pellets in the range 4000–400 cm–1. Carbon, hydrogen, nitrogen, and/or sulfur elemental analyses were performed with a model 240 Perkin Elmer elemental analyzer. The metal contents of the complexes were analyzed by EDTA titration [Citation21] after decomposing the organic matter with a mixture of HClO4, H2SO4, and HNO3 (1:1.5:2.5). Thermogravimetric analyses and the differential scanning calorimetric study were done with a model 5000/2960 SDTA, TA instrument (USA). The 1H NMR and 13C NMR was recorded on a Bruker Avance (400 MHz). The diffuse reflectance spectra of the complexes were recorded in the range 1700–350 nm (as MgO discs) on a Beckman DK-2A spectrophotometer while electronic spectra were recorded on a Shimadzu UV-VIS spectrophotometer. The magnetic moments were measured by Gouy's method using mercury tetrathiocyanatocobaltate(II) as the calibrant (χg = 16.44 × 10− 6 cgs units at 20°C) on a Citizen Balance. The diamagnetic correction was made using Pascal's constant [Citation22]. All the complexes were insoluble in water, methanol and dimethyl formamide, but were soluble in dimethyl sulphoxide.
Synthesis of schiff bases
The Schiff bases were synthesized according to reported methods and are summarized in Citation23-26.
Synthesis of complexes:
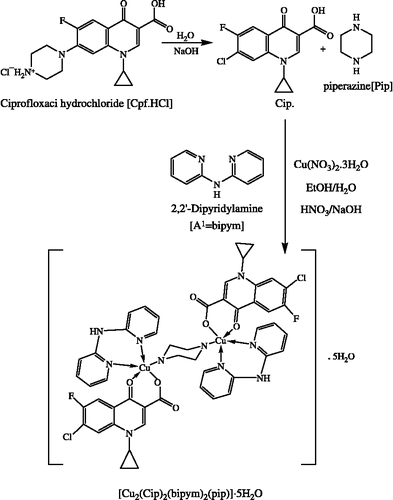
[Cu2(Cip)2(bipym)2(pip)]·5H2O (I):
A methanolic solution of Cu(NO3)2·3H2O (2.41 g, 10 mmol) was added to (2,2′bipyridylamine)bipym (1.71 g, 10 mmol) in methanol, followed by addition of a previously prepared solution of (ciprofloxacin)Cpf·HCl (3.67 g, 10 mmol) in water. The pH was adjusted to 6 ∼ 7.5 pH with dilute HNO3 or NaOH solution. The resulting blue solution was refluxed for 0.5 h., evaporated down to half volume, and kept overnight. The crystalline product obtained was washed with ether and dried over a vacuum desiccator. Yield: 80%, m.p.: 260°C, Found: C, 49.72, H, 3.42, N, 11.50; Cu, 10.49. C50H42Cl2Cu2F2N10O11 (1204.92) requires: C, 49.84, H, 3.51, N, 11.62; Cu, 10.54%.
[Cu2(cip)2(bap)2(pip)]5h2o (Ii):
Prepared from bap (1.82 g, 10 mmol). Yield: 75%, m.p.: 293°C, Found: C, 52.81, H, 4.39, N, 9.05; Cu, 10.34. C54H54Cl2Cu2F2N8O11 (1226.97) requires: C, 52.86, H, 4.43, N, 9.13; Cu, 10.35%.
[Cu2(cip)2(tca)2(pip)]·5h2o (Iii)
Prepared from tca (1.87 g, 10 mmol. Yield: 70%, m.p.: 349°C, Found: C, 50.51, H, 4.25, N, 6.81; Cu, 10.25. C52H52Cl2Cu2F2N6O11S2 (1237.05) requires: C, 50.48, H, 4.39, N, 6.79; Cu, 10.27%.
[Cu2(cip)2(bendan)2(pip)]·5h2o (Iv)
Prepared from bendan (3.34 g, 10 mmol). Yield: 63%, m.p.: 273°C, Found: C, 61.19, H, 4.45, N, 7.19; Cu, 8.18. C78H70Cl2Cu2F2N8O11 (1531.35) requires: C, 61.17, H, 4.60, N, 7.31; Cu, 8.29%.
[Cu2(Cip)2(benen)2(pip)]·5H2O (V)
Prepared from benen (2.36 g, 10 mmol). Yield: 75%, m.p.: 273°C, Found: C, 55.73, H, 5.01, N, 8.41; Cu, 9.47. C62H66Cl2Cu2F2N8O11 (1335.15) requires: C, 55.77, H, 4.97, N, 8.39; Cu, 9.51%.
[Cu2(cip)2(bmbbd)2(pip)]·5h2o (Vi)
Prepared from bmbbd (3.44 g, 10 mmol). Yield: 58%, m.p.: 284°C, Found: C, 57.11, H, 4.72, N, 7.16; Cu, 8.05. C74H74Cl2Cu2F2N8O15 (1551.34) requires: C, 57.29, H, 4.80, N, 7.22; Cu, 8.19%.
[Cu2(cip)2(bcpded)2(pip)]·5h2o (Vii)
Prepared from bcpded (4.29 g, 10 mmol). Yield: 50%, m.p.: 288°C, Found%: C, 57.18, H, 4.07, N, 6.41; Cu, 7.23. C82H70Cl6Cu2F2N8O11 (1721.20) requires: C, 57.22, H, 4.09, N, 6.50; Cu, 7.38%.
[Cu2(cip)2(dcbd)2(pip)]·5h2o (Viii)
Prepared from dcbd (2.68 g, 10 mmol). Yield: 60%, m.p.: 251°C, Found: C, 56.49, H, 5.88, N, 7.97; Cu, 8.97. C66H82Cl2Cu2F2N8O11 (1399.32) requires: C, 56.65, H, 5.90, N, 8.00; Cu, 9.08%.
Preparation of stock solution
A stock solution of 2.5 ppm was made by dissolving 0.25 mg of each compound in 5% dimethyl sulphoxide solution.
Determination of MIC value
The biocidal screening was estimated by the minimal inhibitory concentration (MIC). MIC was determined using the method of progressive double dilution in liquid media containing 1ppm to 50ppm of the compound being tested. The biological screening of all the compounds was carried out at MIC (2.5 μg/mL) and results are expressed as zone of inhibition in mm. The biocidal activity of the ofloxacin, levofloxacin, flucanozole, ligands, Cu(NO3)2·3H2O, and its complexes were analyzed against various gram-negative and gram-positive bacterial cultures of Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Salmonella typhi, Escherichia coli and Serratia marcescens and three fungal cultures namely Aspergillus niger, Aspergillus flavus, Lasiodiplodia theobromae using the Agar-Plate technique [Citation27].
Preparation of agar plates
The media was made up by dissolving bacteriological agar (20 gm) and Luria broth (20 gm) in 1 L distilled water. The mixture was autoclave for 15 min at 120°C and then dispensed into sterilized petri dishes, allowed to solidify, and then used for inoculation.
Procedure of inoculation
The target microorganism cultures were prepared separately in 15 mL of liquid LB medium for activation. Inoculation was done with the help of a micropipette with sterilized tips; 100μL of activated strain was placed onto the surface of an agar plate, and spread evenly over the surface by means of a sterile, bent glass rod. Then two wells in each plate having diameter 10 mm were made with the help of a sterilized borer.
Application of discs
Sterilized stock solutions (2.5μg/mL) were used for application in the well of previously inoculated agar plates. When the discs were applied, they were incubated at 37°C for 24 h. The zone of inhibition was then measured (in mm) around the disc and the results are represented in . Control experiments were performed where only the equivalent volume of solvent without added test compound was applied to the petri dishes. All experiments were performed in triplicate and ofloxacin, levofloxacin, and fluconozole were used as standard drugs. The growth was compared with the control and is expressed as zone of inhibition vs control.
Table I. Biocidal activity data of compounds.
Super oxide dismutase activity
Measurement of SOD-mimic activity
The SOD-mimic activity of the copper(II) complexes was evaluated according to Viswanathan et al., [Citation28], which was first proposed by McCord and Fridovich [Citation18]. This in vitro method is based on the competing reactions of the tested compounds and nitroblue tetrazolium (NBT), phenazine methosulphate with NADH solution in distilled water. The reaction product is a blue formazan compound. The decrease in concentration of NBT is proportional to the SOD mimic activity of the tested compounds. The reaction solution consisted of a solution of tested compound in DMSO and NBT, phenazine methosulphate and NADH in distilled water. The formazan product was subsequently estimated at 560 nm using a Shimadzu UV-VIS spectrophotometer. The actual concentration of formazan dye produced was measured continuously at time intervals of 30 s.
DNA interaction absorption titration
A DNA interaction study was performed on a UV-VIS spectrophotometer. Absorption titration of compounds in DMSO, and whole system in buffer (phosphate, pH 7.2) were done by keeping fixed the amount of the copper compounds (where compound: I = 12.04, II = 12.26, III = 12.37, IV = 15.31, V = 13.35, VI = 15.51, VII = 17.21, VIII = 13.99 μgm) and the amount of DNA variable i.e. 2 to 7 μgm. Compound-DNA solutions were employed to record absorption spectra.
Gel analyses and quantification
The inspection of super coiled pBR322 was done in TAE [tris(hydroxymethyl)methylamine, acetic acid and EDTA] buffer pH 8.0. The pattern of inspection was designated as DNA alone (control), DNA in presence of ligands and DNA in presence of complex. Nuclease activity experiments conducted by mixing pBR322 (50μM) in TE [40 mM Tris acetate and 1 mM EDTA] buffer (pH 8.0), and ligand or complex (50 μM). The reaction mixture was incubated at room temperature for 1 h and then amended with 6 × loading buffer (40% sucrose, 0.02% bromophenol blue and 0.02% xylene cyanol FF) and loaded onto 0.8% agarose gel. Electrophoresis was carried out at constant voltage (100 V) in a Submarine Electrophoresis Unit (Genie, Banglore, India). The gel was stained with ethidium bromide. The same experimental conditions were maintained in control assays. The gels were viewed on a UV transilluminator, and images were captured with an attached camera and estimated using AlphaDigiDoc™ RT. Version V.4.1.0 PC-Image software.
Results and discussion
The ligands A2–A8 were prepared by the condensation reaction of aldehyde/ketone with amine in ethanol. The isolated ligands were characterized by elemental analyses, IR, 1H and 13C NMR spectroscopy. The copper(II) complexes of quinolone-3-carboxylic acid (cpf.HCl) were isolated by metathesis reaction of cupric nitrate and variable ligands A1–A8 in a 1:1:1 ratio. The copper(II) is coordinated to the carboxylate and pyridone oxygen of the quinolone-3-carboxylic acid to form a six membered ring. Copper complexes assumed five coordinated square pyramidal geometry coordinating with N-N/N-S of neutral bidentate ligands, two oxygens of cip.(L = 7-Chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid) and the nitrogen of the piperazine ring. The copper complexes are dimeric in nature. The [Cu2(Cip.)2(bpy)2(pip)]•·6H2O synthesis and its crystal structure has been reported by Wang et.al., who also proposed a possible reaction scheme for formation of the dimeric complex with liberation of piperazine ring from ciprofloxacin[Citation29]. In continuation of earlier work [Citation26] we have synthesized eight dimeric complexes which also follow the proposed reaction scheme (Scheme ) for dimeric complexes. The synthesized dimeric compounds were characterized using IR spectra, electronic spectra, magnetic measurements and thermogravimetric analyses. The thermal decomposition suggests five water molecule of crystallization and stepwise decomposition of the complexes. The elemental analyses were in good agreement with the proposed 1:1:1; Cu(II):Cip:An formulation for all complexes.
1H NMR and 13C NMR spectra of Schiff bases
The 1H NMR spectra and 13C NMR spectra of the ligands in DMSO-d6 are reported along with the possible assignment. The 1H NMR spectra of ligands exhibited peaks at about 7.0–8.0 ppm assigned to the aromatic protons. The singlet peak which appeared at 7.8–9.1 ppm was assigned to the azomethine proton (–CH = N–). In the 13C NMR spectra, the peaks observed at about 114.5–136.4, 113.5–144.5, and 128.5–143.0 ppm were assigned to aromatic, pyridine, and thiophene carbons respectively. Peaks observed at about 123.5–155.3, 155.3–161.2, 157.9–180.5 ppm were assigned to C–N, CH = N, and C = N carbons respectively.
IR spectra
The IR spectra of the complexes are shown in . The ν(C = O) stretching vibration band appears at 1708 cm− 1 in the spectra of ciprofloxacin, and in the complexes at 1626–1633 cm− 1; this shift towards lower energy suggests that coordination occurs through the carbonyl oxygen atom [Citation30]. The sharp band in ciprofloxacin at 3520 cm− 1 [Citation31] is due to hydrogen bonding; which is contributed to ionic resonance structure and peak observed because of free hydroxyl stretching vibration. This band completely vanished in the spectra of the metal complexes indicating deprotonation of the carboxylic proton. The data is further supported by ν(M–O) [Citation32] band which appeared at 502 ∼ 525 cm− 1. The strong absorption band obtained at 1624 cm− 1 and 1340 cm− 1 in ciprofloxacin is assigned to ν(COO)asy and ν(COO)sym respectively, while in the metal complexes these bands were observed at ∼ 1580 and ∼ 1370 cm− 1, respectively. The frequency separation (Δν) in the investigated complexes is greater than 200 cm− 1, suggesting a unidentate bonding nature for the carboxyl group Citation30Citation33-37. In the investigated compound the ν (C = N) band of 2,2′ bipyridylamine appeared at 1580 cm− 1. which shifted to higher frequency at 1612 cm− 1 [Citation38,Citation39] in the complexes indicating the bidentate N–N coordination of the ligand. Similarly for benzylidene-2-aminopyridine two strong bands were assigned at 1615 and 1593 cm− 1 for the ν (C = N) stretching vibration of the azomethine and pyridine ring respectively which on complexation are shifted to 1666 cm− 1 and 1620 cm− 1, respectively, suggesting the bidentate N–N participation in coordination [Citation40]. The ν (C = N) peak for the Schiff bases A3, A4, A5, A6, A7, and A8 was observed at 1601 cm− 1–1629 cm− 1 which on complexation were shifted to 1570 cm− 1–1605 cm− 1, indicating the N–S and N–N bidentate coordination of the ligand Citation41-44. This data was further supported by ν(M–N) [Citation45] which appeared at 520 ∼ 545 cm− 1. In the case of [Cu2(Cip)2(tca)2(pip)]·5H2O, the ν(C–S) band of tca observed at 765 cm− 1 is shifted lower to 750 cm− 1 in the spectra of the complex indicating the participation of the sulfur atom of the thiophene ring. This data was further supported by a new band observed at 420 cm− 1 which can be assigned to ν (M–S) Citation46-48 mode.
Table II. Infrared spectral data of complexes.
Electronic spectra and magnetic properties
The electronic spectral data and magnetic moments are summarized in . The diffuse reflectance spectra of dimeric copper(II) complexes [Cu2(L)2(An)2(pip)] ·5H2O were taken in solid as well as liquid (DMSO) states; both states showed quite similar results. The spectra of Cu(II) complexes exhibited a broad band in the 589–783 nm region Citation49-51Citation17. These bands are characteristic of a Cu(II) d–d transition in a tetragonal field in which the Cu(II) atom is in a distorted square pyramidal coordination environment. The magnetic moments of all compounds was obtained between 1.76–2.10 B.M and is good agreement for a five-coordinated dimeric copper(II) mixed-ligand system and consistent with the presence of a single unpaired electron [Citation52,Citation53].
Table III. Electronic spectral data of complexes.
TGA and DSC
The thermogravimetric (TGA) and differential scanning calorimetric (DSC) data for the synthesized complexes is given in . The dehydration processes are interpreted by endothermic peaks in the DSC curves. The DSC curves showed the melting process for I to VIII complexes at 260, 293, 349, 273, 273, 284, 288, and 251, respectively, decomposition is followed by an exothermic continuous plot. The initial weight loss which occurred in the 24–110°C temperature range for all complexes is attributed to a loss of five water molecules of crystallization. In the second step weight loss during 140–220°C corresponding to the (pip) molecule, followed by liberation of (L) in between 220–490°C. Finally, decomposition of An occurs in the temperature range 510–710°C, and the remaining weight is in good agreement with two copper atoms. The TGA of [Cu2(Cip)2(bipym)2(pip)]·5H2O is shown in .
Table IV. Thermal data of complexes.
Biocidal activity
Comparative analysis showed higher antibacterial activity for the complexes than the free ligands, metal salt, fluconazole (fungicide) and control (DMSO) against S. aureus etc., while good activities were noted as compared to the standard drugs ofloxacin and levofloxacin. In the case of fungi no significant antifungal activity was observed against Aspergillus niger etc. The increase in biocidal activity of the complexes over that of the ligand may be due to the effect of the metal ion on the normal cell process. A possible mode for increase in biocidal activity may be considered in the light of Overton's concept [Citation54] and the chelation theory [Citation55]. According to Overton's concept of cell permeability, the lipid membrane that surrounds the cell favors the passage of only lipid-soluble materials so that liposolubility is an important factor which controls biocidal activity. On chelation, the polarity of the metal ion will be reduced to a great extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups. Further, it increases the delocalization of π-electrons over the whole chelate ring and enhances the lipophilicity of the complexes.
SOD mimic activity
The superoxide dismutase-like activity of the dimeric complexes was determined (). The IC50 values of the complexes are lower than that of free Cu(II) (IC50 = 0.450 μM)[Citation17], suggesting that the improvement in activity is not due to dissociation of the complexes. The superoxide scavenging data suggest that all the complexes exhibit higher activity than the previously reported activity of monomeric and dimeric copper complexes Citation56-60. It is noted that the activity exhibited by complexes I–VIII is only 8.0–15.0 fold respectively, less than that of the native enzyme (IC50 = 0.006 μM). It has been reported that a more distorted geometry leads to higher SOD activity [Citation17,Citation61]. An important point observed here is that all eight complexes have almost identical IC50 values. The similar activity of complexes can be explained on the basis of almost identical geometry, limited steric hindrance, and vacant coordination sites that permit the access and the successful coordination of the O2• − radical. In addition to these, a favorable response of electrons of the C = N bond and aromatic ligand could be to stabilize the Cu–O2– interaction.
Table V. IC50 values of copper complexes.
DNA interaction
Absorption spectroscopy is widely well known to determine the binding of complexes with DNA. Complexes bound to DNA through interaction result in hypochromism (blue shift) and bathochromism (red shift) due to interaction between chromophores and the base pairs of DNA. The extent of hypochromism is commonly consistent with the strength of the intercalative interaction Citation62-64. The DNA interaction data with the complexes are shown in . The absorption spectra of the complex [Cu2(Cip)2(bipym)2(pip)].5H2O is shown in A maxima at about 275 nm is observed in the spectrum of complex without DNA, which was decreased as the amount of DNA increased and observed at about 258 nm in presence of 7 μgm of DNA. Thus, the red shift (bathochromism) is due to interaction between chromophores and the base pair of DNA, suggesting that the complexes were bound to DNA through intercalation Citation65-67.
Table VI. DNA interaction data with the complexes.
Electrophoretic behavior of complex-DNA systems
The effect of the binding of complexes on supercoiled (SC) pBR322 was determined by its ability to make it bulky by binding with reactive sites of pBR322 DNA and produce changes in its conformation from supercoiled(SC) to nicked open circular(OC) form. When pBR322 is subjected to electrophoresis, the fastest migration is observed for SC. If one strand is cleaved due to binding with a reactive species, the SC form is converted into the OC form. shows the electrophoresis of all eight complexes after incubation for one hour by comparison with the same experiments carried out with Cu(II) and ligands. Complexes exhibit higher activity nuclease than that of Cu(II).
Figure 3 Electropheresis of pBR 322 after incubation with different agents. (A) Lane 1: pBR322 (control), lane 2: pBR322 + I, lane 3: pBR322+ II, lane 4: pBR322+ III, lane 5: pBR322+ IV, lane 6: pBR322 + V, lane 7: pBR322+ VI, lane 8: pBR322+ VII, lane 9: pBR322+ VIII (B) Lane 1: pBR322+ Cu(II), lane 2: pBR322 + A1, lane 3: pBR322 + A2, lane 4: pBR322 + A3, lane 5: pBR322 + A4, lane 6: pBR322 + A5, lane 7: pBR322 + A6, lane 8: pBR322 + A7, lane 9: pBR322 + A8.
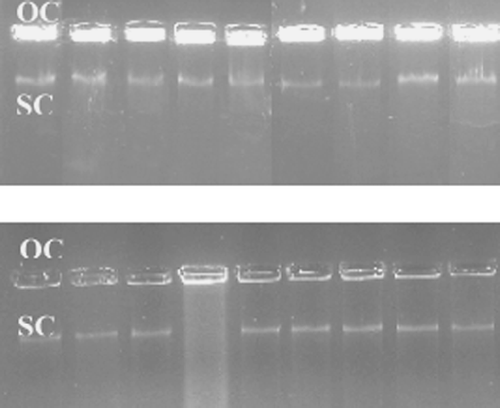
The greater cleavage activity of the complexes with respect to the ligands is clearly seen from and data (). It can be seen that SC migrated faster than OC. SC is a smear on the gel while OC remains in the well. There may be two reasons for this, (I) OC become bulky having a higher molecular weight due to intercalation of compounds. (II) OC requires more time to run on the gel than SC. From the experiment we can conclude that the conversion of SC to OC is higher in the presence of complexes than in the presence of free ligands or Cu(II).
Table ViI. cleavage Of Pbr322 Dna.
Conclusion
All the copper complexes are dimeric in nature having a distorted square pyramidal geometry. The complexes exhibit good biocidal activities as when compared to the standard drugs ofloxacin and levofloxacin. In the case of fungi no significant antifungal activity was observed. All eight compounds possess good SOD activity and results lead to the indication that the dimeric nature increases the ability of the compounds to dismutate the superoxide radical. Absorption titration and gel electrophoresis analyses suggest that the complexes have good DNA binding properties.
Acknowledgements
We thank the Prof. J. S. Parmar, Head, Department of Chemistry, and Prof. D. Madammvar, Head, Department of Biosciences, for providing the laboratory facilities. The authors wish to acknowledge the help of Dr. V. Thakkar and Mr. Pinakin Dhandhukia of the Department of Biochemistry of Sardar Patel University.
References
- Turel I. The interactions of metal ions with quinolone antibacterial agents. Coord Chem Rev 2002; 232: 27–47
- Chen S, Ma H, Zhao H, Feng R, Jin L. Terbium-sensitized flourescence method for the determination of pazufloxacin mesilate and its application. Analytical Sciences 2004; 20: 1075–1078
- Merás ID, Peña AL, López FS, Cáceres IP. Complexation of antibacterial quinolonic acid and cinolonic derivatives with Zn(II) and Al(III): Application to their determination in human urine. The Analyst 2000; 125: 1471–1476
- El_Breshy AM, Metwally ME, El_Sepai FA. Spectrophotometric determination of some flouroquinolone antibacterials by ion-pair complex formation with Cobalt(II) tetrathiocyanate. J Chin Chem Soc 2005; 52: 77–84
- Williuns DR, Reinhold VN. The metal of life. London 1971
- Sorenson JRJ. Copper chelates as possible active forms of the antiarthritic agents. J Med Chem 1976; 19: 135–148
- Sorenson JRJ, Nraign JO. (Ed). Copper in the environment. Wiley-Interscience, New York. Part 2, 1981, Chapter 5.
- Brown DH, Smith WE, Teape JW. Antiinflammatory effects of some copper complexes. J Med Chem 1980; 23: 729
- Sharma S, Ather A, Maurya MR, Azam A. Copper(II) complexes with subsittuted thiosemicarbozones of thiophene-2-carboxaldehyde: Synthesis, Copper(II) characterization and antiamoebic acitvity against E Histolytica. Euro J Med Chem 2005; 40: 1414–1419
- Vančo J, Švajlenová O, Račanská E, Muselík J, Valentová J. Antiradical activity of different copper(II) schiff base complexes and their effect on alloxan-induced diabetes. J Trac Elem Med biol 2004; 18: 155–161
- Torre MH, Gambino D, Cerecetto J, Gambino D, González M, Lavaggi ML, Azqueta A, Cerain AL, Vega AM, Abrum V, Costa-Filho AS. Novel Cu(II) quinoxaline N1, N4 –dioxide complexes as selective hypoxic cytotoxins. Euro J Med Chem 2005; 40: 473–480
- Navarro M, Cisneroj-Fajardo FJ, Sanchezd elgada, Lehmann T, Sànchez-Delgada RA, Silva AR, Liva R, Urbina JA. toward a novel metal-based chemotherapy against tropical diseases. 6. Synthesis and characterization of new copper(II) and gold(I) clotrimazole and ketoconazole complexes and evaluation of their activity against trypanosoma cruzi. Inorg Chem 2001; 20: 6879–6884
- Turel I, Golič L, Bukovec P, Gubina M. Antibacterial tests of bismuth(III)-quinolone (ciprofloxacin,cf) compounds against Helicobacter pyroli and some other bacteria. Crystal structure of (cfH2)2[Bi2 Cl10].4H2O. J Inorg Biochem 1998; 71: 53–60
- Panchal PK, Pansuriya PB, Patel MN. Bactericidal activity of oxovanadium(IV) complexes with schiff bases and application of chelation theory. J Enz Inhib Med Chem 2006; 21(2)203–209
- Ruiz M, Perelló L, Ortiz R, Castiñeiras A, Maichle-Mössmer C, Cantón E. Synthesis, characterization, and cyrstal structure of [Cu(cinoxacinate)2].2H2O complex: A square-planar CuO4 chromofore. Antibacterial studies. J Inorg Biochem 1995; 59: 801–810
- Turel I, Golobič A, Klavžar A, Pihlar B, Buglyó P, Tolis E, Rehder D, Sepčić K. Interactions of oxovanadium(IV) and the quinolone faminly member ciprofloxacin. J Inorg Biochem 2003; 95: 199–207
- González-Alvarez M, Borrás J, Alzuet G, Agudo LC, Garciá-Grunda S, Montejo-Benardo JM. Comparison of protective effects against reactive oxygen species of mononuclear and dinuclear Cu(II) complexes with N-substituted benzothiozolesulfonamides. Inorg Chem 2005; 44: 9424–9433
- McCord JM, Fridovich I. Superoxide dismutase. An enzymatic function for erythrocuprein(Hemocuprin). J Biol Chem 1969; 244: 6049–6055
- Batinic-Haberte I, Spasujeviê I, Stevens RD, Hambright P, Pedatsur N. New class of potent catalysts of O2– dismutation. Mn(III)ortho-methoxyethylpyridyl- and di-ortho- methoxyethylimidazolylporphyrins. Dalton Trans 2004; 1696–1702
- Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR. Vogel's textbook of practical organic chemistry, 2004, 5th ed.
- Vogel AI. A textbook of quantitative inorganic analysis. ELBS and Longman, London 1989
- A Weiss H. Witte, Magnetochemie. Verlag Chemie, Weinheim 1973
- Panchal PK, Pansuriya PB, Patel MN. In-vitro biological evaluation of some ONS and NS donor Schiff bases and their metal complexes. J Enz Inhib Med Chem 2006; 21(4)453–458
- Parekh HM, Pansuriya PB, Patel MN. characterization and antifungal activity of genuine oxovanadium(IV) mixed-ligand complexes with Schiff bases. Polish J Chem 2005; 79: 1843–1851
- Parekh HM, Mehta SR, Patel MN. Synthesis, structural characterization and antifungal activity of the schiff bases and their transition metal mixed-ligand complexes. Russ J Inorg Chem 2006; 51(1)67–72
- Pansuriya PB, Dhandhukia P, Thakkar V, Patel MN. Synthesis, spectroscopic and biological aspects of iron(II) complxes. J Enz Inhib Med Chem, In Press
- Pelczar MJ, Chan ECS, Krieg NR, Microbiology, TATA McGRAW-HILL Edition, 5th Ed. 1993; 23: 488.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometic assay of superoxide dismutase. Indian J Biochem Biophys 1984; 21: 130–132
- Wu G, Wang G, Fu X, Zhu L. Synthesis, crystal structure, stracking effect and antibacterial studies of novel quaternary copper(II) complex with quinolone. Molecules 2003; 8: 287–296
- Leban I, Turel I, Bukovec N. Crystal structure and characterization of the bismuth(III) compound with quinolones family member (ciprofloxacin). Antibacterial study. J Inorg Biochem 1999; 241–245
- Silverstein RM, Webster FX. Spectrometric identification of organic compounds6th Ed. John Wiley & Sons, Inc. 2004
- Yan C, Li Y, Lou J, Zhu C. Synthesis, characterization, and magnetic properties of tetracarboxylato-bridged binuclear iron(II) complexes. Synt React Inorg Met-Org Chem 2004; 34(5)979–991
- Nakamoto K. Infrared and raman spectra of inorganic and coordination compounds4th ed. A Wiley Interscience Publication. 1986
- Anacona JR, Rodriguez I. Synthesis and antibacterial activity of cephalexin metal complexes. J Coord Chem 2004; 57: 1263–1269
- Deacon GB, Philips RJ. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord Chem Rev 1980; 23: 227–250
- Chohan ZH, Suparan CT, Scozzafava A. Metal binding and antibacterial activity of ciprofloxacin complexes. J Enz Inhib Med Chem 2005; 20(3)303–307
- Patel NH, Panchal PK, Pansuriya PB, Patel MN. Synthesis and spectral investigation of La(III), Ce(III), Pr(III), Nd(III) and Sm(III) coordination chain polymeric assemblies. J Macro Sci Part-A Pure Appl Chem 2006; 43: 1083–1090
- Panchal PK, Patel MN. synthesis, structural characterization, and antibacterial studies of some mixed-ligand first row-d-transition metal complexes. Synth React Inorg Met-Org Chem 2004; 34(7)1277–1289
- Freedman HH. Intramolecular H-bonds. I. A spectroscopic study of the hydrogen bond between hydroxyl and nitrogen. J Am Chem Soc 1961; 83: 2900–2905
- Gudasi KB, Goudar TR. Synthesis and charcterization of Lanthenide(III) complexes with salicylidene-2-aminopyridine. Synt React Inorg Met-Org Chem 2000; 30(10)1859–1869
- Panchal PK, Pansuriya PB, Patel MN. Study on increase in toxicity of schiff bases on microorganism on chelation with metal. Toxicol and Env Chem 2005; 88(1)57–64
- Parekh HM, Panchal PK, Patel MN. Transition metal(II) ions with dinegative tetradentate schiff base: Synthetic, thermal, spectroscopic and coordination aspects. J Thermal Anal and Cal 2006; 86(3)803–807
- Raman N, Kulandaisamy A, Jayasubramananian K. Synthesis, structural characterization, redox and antibacterial studies of schiff base copper(II), nickel(II), cobalt(II), manganese(II), zinc(II) and oxovanadium(II) complexes derived from benzil and 2-aminobenzyl alcohol. Polish J Chem 2002; 76: 1085–1094
- Parekh HM, Panchal PK, Pansuriya PB, Patel MN. Synthesis and physicochemical study of 3d metal coordination polymers with dinegative tetradentate [NSNS] Schiff bas. Polish J Chem 2006; 80: 989–992
- Chandra S, Gupta N, Gupta LK. Synthesis and EPR spectral studies of mono and binuclear cobalt(II) and nickel(II) complexes with 20-member ring dithiatetraazamacrocyclic[N4S2] ligand. Synth React Inorg Met-Org Chem 2004; 34(5)919–927
- Chattopadhayay SK, Benerjee T, Roychoudhury P, Ghosh S, Mak TCW. Synthesis, characterization and crystal structure analysis of bis (pyridine-2-carbaldehyde thiosemicarbazonato) cobalt(III) thiocyanate monohydrate. Trans Met Chem 1997; 22: 216
- Zenglu L. Synthesis of 1-(1-H-benzotriazolyl-1-acetyl)-5-hydroxy-3-trifluoromethyl-5-(2-thienyl)-2-pyrazoline and its transition metal complexes. Synth React Inorg Met-Org Chem 2004; 34: 469
- Sargent AL, Titus EP, Riordan CG, Rheingold AL, Ge P. Poly(2-thienyl) borates: An investigation into the coordination of thiophene and its derivatives. Inorg Chem 1996; 35(21)7095
- Mendoza-Diaz G, Martineza-Auguilera LMR, Perez-Alonso R, Solans X, Moreno-Esparza R. Synthesis and characterization of mixed ligand complexes of copper with nalidixic acid and (N—N) donors. Crystal structure of [Cu(Phen)(Nal)–(H2O)]NO3·3H2O. Inorg Chim Acta 1987; 138: 41–47
- Melnik M. Mono-, bi-, tetra- and polynuclear copper(II) halogenocarboxylates. Coord Chem Rev 1981; 36(1)1–44
- Iskander MF, El-Sayed L, Salem NMH, Werner R, Haase W. Synthesis, characterization and magnetochemical studies of dicopper(II) complexes derived from bis(N-salycylidine) dicarboxylic and dihydrazides. J Coord Chem 2005; 58(2)125–139
- Figgis BN, Lewis J. Modern Coordination chemistry: Principles and methods, interscience, J Lewis, RG Wilkins, New York 1960; 400
- Carbello R, Castiñeiras A, Covelo B, Garcí-Martínez E, Niclós J, Váquez-López EM. Solid state coordination chemistry of mononuclear mixed-ligand complexes of Ni(II), Cu(II) and Zn(II) with α-hydrixycarboxylic acids and imidozole. Polyhedron 2004; 23: 1505–1518
- Anjaneyula Y, Rao RP. Preparation, characterization and antimicrobial activity studies on some ternary complexes of Cu(II) with acetyl acetone and various salicylic acid. Synth React Inorg Met-Org Chem 1986; 16: 257
- Taylor DM, William DR. Trace element medicine and chelation therapy. Royal Society of Chemistry, London 1998
- González-Alvarez M, Alzuet G, Borrás J, Agudo LC, Montejo-Benardo JM, Garciá-Grunda S. Development of novel copper(II) complexes of benzothiazole-N-sulfonamides as protective agents against superoxide anion. Crystal structures of [Cu(N-2-(4-methylbenzothiazole)benzenesulfonamidate)2(py)2] and [Cu(N-2-(6-nitrobenzothiazole)naphthalenesulfonamidate)2(py)2]. J Biol Inorg Chem 2003; 8: 112
- González-Alvarez M, Alzuet G, Borrás J, Agudo LC, Garciá-Grunda S, Montejo-Benardo JM. Strong protective action of copper(II) N-substituted sulfonamide complexes against reactive oxygen species. J Inorg Biochem 2004; 98: 189–198
- Casanova J, Alzuet G, Borrás J, LaTorre J, Sanau M, Garciá-Grunda S. Coordination behavior of sulfathiazole. Crystal structure of [Cu (sulfathiazole) (py)3Cl] superoxide dismutase activity. J Inorg Biochem 1995; 60: 219–230
- Casanova J, Alzuet G, Borrás J, Carugo O. Crystal structures and superoxide dismutase mimetic activity of [CuL2(Him)2]·MeOH and [CuL2(mim)2]·H2O [HL = 4-amino-N-(thiazol-2-yl)benzenesulfonamide, Him = imidazole, mim = N-methylimidazole]. J Chem Soc Dalton Trans 1996; 2239
- Casanova J, Borrás J, Ferrer S, Alzuet G, LaTorre JA, Ramírez J. Superoxide dismutase activity of ternary copper complexes of sulfathiazole and imidazole derivatives. Synthesis and properties of [CuL2(R-Him)2] [HL = 4-amino-N-(thiazol-2-yl)benzenesulfonamide, R-Him = 4-methylimidazole, 4,4-dimethylimidazoline or 1,2-dimethylimidazole]. Crystal structure of [CuL2(4,4-dimethylimidazoline)2]. Inorg Chim Acta 2000; 304: 170–177
- Bonomo RP, Conte E, Impellizzeri G, Pappalardo G, Purrello R, Rizzarelli E. Copper(II) complexes with cyclo(L-aspartyl-L-aspartyl) and cyclo(L-glutamyl-L-glutamyl) derivatives and their antioxidant properties. J Chem Soc Dalton Trans 1996; 3093
- Barton JK, Danishefsky AT, Goldberg JM. Tris(phenanthroline)ruthenium(II): Stereoselectivity in binding to DNA. J Am Chem Soc 1984; 106: 2172–2176
- Tysoe SA, Margan RJ, Baker AD, Strekas TC. Spectroscopic investigation of differential binding modes of.DELTA.- and.LAMBDA.-Ru(bpy)2(ppz)2+ with calf thymus DNA. J Phy Chem 1993; 97: 1707–1711
- Kelly TM, Tossi AB, McConnell DJ, OhUigin C. A study of the interactions of some polypyridylruthenium (II) complexes with DNA using fluorescence spectroscopy, topoisomerisation and thermal denaturation. Nucl Acid Res 1985; 13: 6017–6034
- Kang J, Dong S, Lu X, Su B, Wu H, Sun K. Study on DNA cleavage by the hexaaza macrocyclic copper(II) complex. J Macro Mol Sci Pure Appl Chem 2006; 43: 279–288
- Efthimiadou EK, Sanakis Y, Raptopoulou CP, Karaliota A, Katsaros N, Psomas G. Crystal structure, specroscopic, and biological study of the copper(II) complex with third-generation quinolone antibiotic sparfloxacin. Bioorg Med Chem Lett 2006, In press
- Santra BK, Reddy PAN, Neelakanta G, Mahadevan S, Nethaji M, Chakravarty AR. Oxidative cleavage of DNA by a dipyridoquinoxaline copper(II) complex in the presence of ascorbic acid. J Inorg Biochem 2002; 89: 191

![Figure 1 TGA of [Cu2(Cip)2(bipym)2(pip)]·5H2O.](/cms/asset/9d0f3c73-867b-4220-ac0f-ca3eb49fe440/ienz_a_238292_f0003_b.gif)
![Figure 2 Absorption spectra of complex [Cu2(L)2(A1)2(pip)]·5H2O in absence and presence of [DNA].](/cms/asset/317373f1-8474-4304-8bda-f6e7da7d63b8/ienz_a_238292_f0004_b.gif)