Abstract
The anticancer activity of rhinacanthins and related naphthoquinone esters is quantitatively analyzed through Fujita-Ban and Hansch approaches. The analyses have helped to ascertain the role of different substituents in explaining the observed inhibitory actions of these compounds. From both approaches it appeared that naphthalene ring instead of benzene ring, dimethyl substitution at R1 and R2, and hydrogen-bond acceptor substituents at R3 (Figure ) are advantageous to improve the activity of a compound against KB cell lines. This in turn leads to the suggestion that the rhinacanthin-N scaffold is the structural entity that needs exploration for new potential compounds. Further, in the Fujita-Ban analysis, it is observed that the compounds bearing a OMe substitution, relative to H, at R4 have a slight positive contribution to pIC50 (KB) whereas the substituents H or OMe at R5, relative to OH, have negative contributions. In conformity with these findings, the Hansch approach revealed that a more hydrophobic group at R4 and a more hydrophilic group at R5 positions are beneficial in raising the activity. The two quantitative structure-activity relationship (QSAR) analyses, differing in parametric approach, therefore, provided the grounds for rationalizing the substituent selection to design more potent compounds of the series.
Introduction
Rhinacanthins Citation1-3 are naphthoquinone ester derivatives isolated from the methanolic extract of the roots of the medicinal plant Rhinacanthus nasutus (Acanthaceae). In Thailand, the roots and leaves of this plant are used for the treatment of cancer [Citation4]. A few extracted compounds have also been reported [Citation4] to reveal cytotoxicity against P388, A-549, HT-29 and HL-60 cell lines. There was no reported synthesis of rhinacanthins till the recent work carried out by Kongkathip et al. [Citation5]. These authors have reported the synthesis of rhinacanthin-M, -N, -Q and 39 related naphthoquinone esters together with their cytotoxicities against human carcinoma cell lines, KB (oral human epidermoid carcinoma), HeLa (human cervical carcinoma), and HepG2 (human hepatocellular carcinoma). Apart from a preliminary emphasis on the mode of action of some of these derivatives, the study was mainly concerned with the alteration of substituents at different positions of the rhinacanthin moiety and provided no rationale to reduce the trial-and-error factors. To the best of our knowledge no quantitative relationship study, explaining the cytotoxicity against the above cell lines pertaining to any family of rhinacanthins, has been reported so far. The present quantitative structure-activity relationship (QSAR) study on the reported analogues, therefore, represents the novelty of the work. It also provides grounds for rationalization of substituent selection and helps in exploring the possible mechanism of their action.
Material and methods
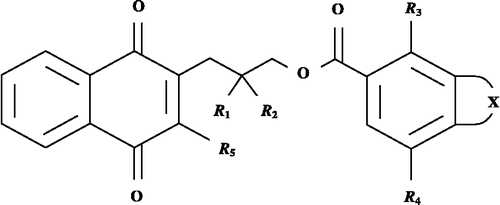
The reported compounds, reproduced in , consists of the analogues of both rhinacanthin-M (1) and rhinacanthin-N (14)/–Q (15) and are represented by the general structure, shown in . The biological effects, against KB, HeLa and HepG2 cell lines, of these compounds are included in while appropriate quantifying parameters of the substituents, present at different positions of the parent structure, are given in . The biological effect, measured as IC50, represents the concentration of a compound required to exhibit 50% cytotoxicitiy against KB, HeLa and HepG2 cell lines. For a given compound, these are expressed as –logIC50 or simply pIC50 on a molar basis in the present study.
Table I. Observed, calculated and predicted cytotoxicities of rhinacanthins and naphthoquinone esters (Figure 1) against human carcinoma cell lines (KB, HeLa and HepG2).
Table II. QSAR parameters for the substituents of varying positions of the title compounds.
In order to obtain important QSAR on these congeners, both, the parametric and the non-parametric approaches employing the method of multiple regression analysis (MRA), were carried out. The parametric approach is analogues to the Hansch type of analysis Citation6-8, which employs physicochemical, theoretical and structural parameters to explain the activity induced in the biological system. The physicochemical model of the biological activity assumes that the activity of a compound is a function of three separable factors, viz, electronic effects, steric effects and solvent-partitioning or hydrophobic effects, with provision to account for the effect exhibited by certain structural binary variations. This method is more formally expressed as in Equation (1)
where the descriptors
are the physicochemical parameters. Step-wise regression was used to develop the best QSAR from the relevant descriptors. Once the significant equation is established, it may be used to increase the understanding of the mechanisms of actions of sets of congeners and to direct drug-design in a congeneric series, as well as to attempt to quantitatively predict biological activities of untested compounds. This methodology has also been called the extra-thermodynamic, linear free energy, multiple parameter and physicochemical structure-activity relations (PSAR) approach. In addition to physicochemical parameters the indicator variables, representing the presence or absence of certain structural characteristics, are sometimes also used in this approach. The derived QSAR equations were also validated by the leave-one-out (LOO) method [Citation10].
For the present work the most appropriate parameters were found to be the hydrophobic constant, π, the hydrogen-bond acceptor parameter, HA, and the molecular weight, MW, for the substituents at different positions. The physicochemical parameters were taken from the literature [Citation9] and the MWs were calculated by adding the atomic weights of different atoms in a substituent. For the present work, the MW parameter is scaled to 0.01 to make it comparable to other quantifying parameters. In addition, the indicator variable, accounting for certain structural attributes, is also employed to derive parametric QSAR equations.
The Fujita-Ban methodology [Citation11], being a non-parametric approach, is based on an additvity principle, wherein the biological activity is expressed as
The slope ai and the intercept μ are, respectively, the contribution of the ith substituent and the theoretical biological activity of the reference compound of the series. For a given position, the variable Xi takes a value of 1 if the ith substituent is present otherwise the value is 0. The linear equations generated using Equation (2) were solved by the MRA [Citation12] employing the method of least squares [Citation13] for the unknowns ai and μ. The computer programs for both of the above methods, have been developed in our laboratory and validated from a number of previously reported results, dealing with QSAR studies.
The importance of the non-parametric method along with the parametric, for ligands active at the adenosine receptor [Citation14,Citation15], inhibitors of cyclooxygenase-2 Citation16-18, epidermal growth factor receptor protein tyrosine kinase [Citation19], adenosine kinase [Citation20], cytokine release [Citation21], non-nucleoside reverse transcriptase inhibitors of HIV-1 [Citation22] and antiallergic agents [Citation23] has already been established.
Results and discussion
All the compounds in Table I were used in the construction of the Fujita-Ban matrix with compound 7 as the reference congener. Tabulation of the resulting matrix of 42 linear equations in 10 unknowns including the contribution of the parent compound is avoided here for the sake of brevity. These equations were solved by the MRA for the unknowns ai and μ. The contributions of different substituents obtained thereby are summarized in the third column of and the ± data within parentheses, associated with them, are the 90% confidence intervals. The resulting statistical parameters of the study are:
where n, r, s and F are respectively the number of data points, multiple regression coefficient, standard deviation and F-ratio between the variances of calculated and observed activities. Two compounds, 10 and 36 in Table I, appear to follow a different trend from the remaining compounds of the series as their calculated pIC50 values were largely deviating from the observed ones. Compound 36 with R1 = R2 = R3 = R4 = R5 = H may not able to interact properly with receptor sites whereas compound 10 having R1 = Me, R2 = H, R3 = R4 = OMe, R5 = OH seems to undergo hydrolysis prior to reaching the active sites of the receptor. These two compounds were, therefore, ignored in the subsequent study. In doing so, the corresponding rows were removed from the Fujita-Ban matrix and MRA of resulting matrix lead to the results summarized in the last column of Table III. The much improved statistical parameters of the study are:
Table III. Fujita-Ban contribution of substituents and parent compound (7) to the cytotoxicity against human carcinoma KB cell lines of the title compounds.
The r2-value now accounts for 86% in the variance and the F-value obtained is significant at 99% level [F10,29(0.01) = 3.034]. The calculated values of pIC50, listed in Table I, are also in close agreement with the observed ones. The substituents to be incorporated at various positions of the parent moiety, that make positive contributions to activity may only be used to design more active compounds of the series in future. From Table III, the possible combinations of various substituents, relative to parent compound 7, have the following pattern:
The positive contribution obtained for –(CH = CH)2– shows that compounds possessing a naphthalene ring make a higher contribution to activity against KB cell lines compared to those having simply a benzene ring. Thus, the congeners derived from rhinacanthin-N/-Q are more active than the derivatives of rhinacanthin-M. In the rhinacanthin-N/-Q derivatives, the favorable conditions persist when both R1 and R2 positions have Me substituents there. In addition, the R3 position may have either OH or OMe substituent while R4 position better remains unsubstituted or may have only OMe.
It is to be noted that the Fujita-Ban approach cannot extrapolate beyond the substituents used in the training set whereas the parametric approach namely the Hansch approach, given below, can do so. Initially, the pIC50 values pertaining to HeLa and HepG2 cell lines were correlated to pIC50 values corresponding to KB cell lines for all 42 congeners to confer the diversity amongst these cancerous cell lines. The derived correlations are given in Equations (3) and (4)
Both these equations have divulged highly significant statistical parameters. This ensures us that the activity of compounds against KB cell lines is dependent upon those of either HeLa or HepG2 cell lines. We have, therefore, considered only the pIC50(KB) as the dependent variable in the subsequent parametric analysis. A number of physicochemical and structural parameters accounting for hydrophobic, electronic and steric interactions were examined for varying positions of the molecules in various possible ways. A large number of regression equations were derived and subjected to various statistical tests. The most significant correlation that was appeared is shown in Equation (5)
The subscripted numerals associated to independent descriptors indicate the varying positions of the molecules. The indicator variable, IAr stands to differentiate the rhinacanthin analogues possessing either a benzene ring or a naphthalene ring. Thus, an arbitrary value of 1 assigned to this variable indicates the derivatives of rhinacanthin-N/ -Q while the value of 0 designates the derivatives of rhinacanthin-M. The derived parameters, r, s, F, and q2 obtained above for Equation (5) denote statistically significant results and the equation as such reflects upon the parametric requirement of various substitutions at different positions in the novel rhinacanthins and related naththoquinone esters having cytotoxicities against KB cell lines. Removal of previously identified ‘outliers’, 10 and 36, the MRA revealed the much significant correlation shown in Equation (6)
Now both the r2- and the F-values are increased to account respectively for 84% of variance in the observed activities and 99% level of significance [F5,34(0.01) = 3.625]. The improved q2-index, over that of Equation (5), conveys a satisfactory sound model in statistical language. Further, the independent variables used in deriving the above equation showed poor intercorrelations among themselves (). The equation was, therefore, used to calculate the activity values of all 40 compounds of the test data set. These values, listed in Table I, were found to be in close agreement with the observed values. The predicated pIC50(KB) values of all the compounds, obtained through the LOO approach were also listed in Table I for the sake of comparison.
Table IV. Intercorrelation matrixa amongst the predictor variables of Equation (6).
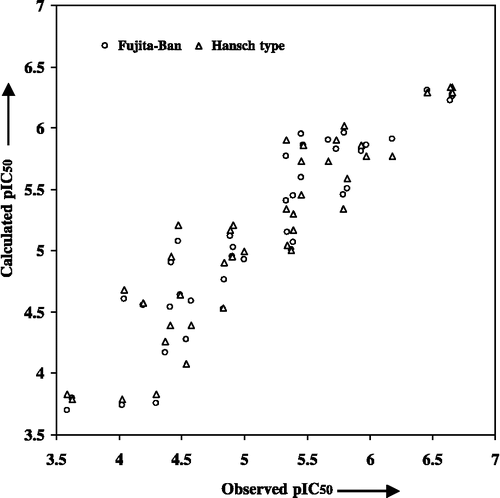
From Equation (6), it appears that the compounds derived only from rhinacanthin-N/–Q may lead to better results. In addition, the R3-substituents that are hydrogen-bond acceptors, the R4-substituents that are more hydrophobic and the R1- and R2-substituents that have a higher molecular bulk (weights) are favorable in increasing the potency of a compound. Similarly, the R5-substituents that are more hydrophilic rather than hydrophobic are also helpful. This strategy may, therefore, be followed for designing more potent compounds for future synthesis. The plot showing the variation of observed versus calculated activities, obtained through the Fujita-Ban and the Hansch type approaches for the compounds in Table I is shown in . Such a demonstration may help to understand the goodness of fit and to identify systematic variation of observed versus calculated activities by the two models for the compounds under the present study.
In conclusion, Equation (6) suggested that the naphthalene ring (in compounds 14 – 42) instead of the benzene ring (1 – 13) is advantageous to improve the activity of a compound against KB cell lines. The Fujita-Ban study, in conformity with this, assigned a positive contribution to Ar. Thus, the scaffold of rhinacanthin-N, instead of rhinacanthin-M, is important for further exploration to derive new potent congeners. The dimethyl substitution, instead of one methyl or no methyl group, at R1 and R2 conferred (Equation 6) more potent cytotoxicity against the cancer cell line. The positive contribution made by Me, Me substitution (Table III) at these positions supports the same. The R3-substituents having a hydrogen-bond acceptor property, are predicted to be more potent. The same was supported by the Fujita-Ban analysis in which higher positive contributions were obtained for the substituents such as OH or OMe. Only the OMe substituent relative to H at R4 and none of the substituents relative to OH at R5 is found, in the Fujita-Ban analysis, to have the positive substituent contribution that may additively improve the activity of a compound. The parametric QSAR study, on the other hand, favors a more hydrophobic group at the R4 position and a more hydrophilic group at the R5 position. Thus the two studies, corroborating each other, provide the ground for rationalizing substituent selection to design more potential compounds of the series.
Acknowledgements
We are thankful to the Department of Chemistry, S. K. Government College and Department of Engineering Chemistry, Sobhasaria Engineering College, Sikar for providing the facilities to complete this work.
References
- Wu TS, Hsu HC, Wu PL, Leu YL, Chan YY, Chern CY, Yeh MY, Tien HJ. Naphthoquinone esters from the root of rhinacanthus nasutus. Chem Pharm Bull 1998; 46: 413–418
- Wu TS, Hsu HC, Wu PL, Teng CM, Wu YC. Rhinacanthin-Q a naphthoquinone from rhinacanthus nasutus and its biological activity. Phytochemistry 1998; 49: 2001–2003
- Kuwahara S, Awai N, Kodama O. A revised structure for rhinacanthone. J Nat Prod 1995; 58: 1455–1458
- Rojanapo W, Tepsuwan A, Siripong P. Mutagenicity and antimutagenicity of thai medicinal plants. Basic Life Sci 1991; 52: 447–452
- Kongkathip N, Luangkamin S, Kongkathip B, Sangma C, Grigg R, Kongsaeree P, Prabpai S, Pradidphol N, Piyaviriyagul S, Siripong P. Synthesis of novel rhinacanthins and related anticancer naphthoquinone esters. J Med Chem 2004; 47: 4427–4438
- Hansch C, Fujita T. p-σ-π-Analysis: A method for the correlation of biological activity and chemical structure. J Am Chem Soc 1964; 86: 1616–1626
- Hansch C. A quantitative approach to biochemical structure-activity relationships. Acc Chem Res 1969; 2: 232–239
- Hansch C. Drug design. EJ Ariens. Academic Press, New York, N Y 1971; Vol. I: 271
- Hansch C, Leo A. Substituents constants for correlation analysis in chemistry and biology. John Wiley, New York 1979
- Wold S. Validation of QSAR's. Quant Struct-Act Relat 1991; 10: 191–193
- Fujita T, Ban T. Structure-activity study of phenethylamines as substrates of biosynthetic enzymes of sympathetic transmitters. J Med Chem 1971; 14: 148–152
- Snedecor GW, Cochran WG. Statistical methods. Iowa State University Press, Ames 1967
- Draper NR, Smith H. Applied regression analysis. Wiley, New York 1966
- Sharma RC, Singh P, Ojha TN, (Km) Tiwari S. A quantitative analysis of binding affinities of ligands active at adenosine receptors. Drug Des and Disc 1994; 12: 169–177
- Singh P, Ojha TN, (Km) Tiwari S, Sharma RC. Fujita-Ban and Hansch analyses of A1- and A2-adenosine receptor binding affinities of some 4-amino[1,2,4]triazolo [4,3-a]quinoxalines. Ind J Chem 1996; 35B: 929–934
- Kumar R, Singh P. Diarylspiro[2.4]heptenes as selective cyclooxygenase-2 inhibitors: A quantitative structure-activity relationship analysis. Ind J Chem 1997; 36B: 1164–1168
- Singh P, Kumar R. Novel inhibitors of cyclooxygenase-2: The sulfones and sulfonamides of 1,2-diaryl-4,5-difluorobenzene. Analysis of quantitative structure-activity relationship. J Enz Inhib 1998; 13: 409–417
- Singh P, Kumar R. 1,2-Diarylimidazoles as inhibitors of cyclooxygenase-2: A quantitative structure-activity relationship study. J Enz Inhib 1999; 14: 277–288
- Singh P, Kumar R. Inhibitors of the epidermal growth factor receptor protein tyrosine kinase: A quantitative structure-activity relationship analysis. J Enz Inhib 1998; 13: 125–134
- Singh P, Kumar Rajesh, Sharma BK. Quantitative structure-activity relationship study of 5-iodo- and diaryl-analogues of tubercidin: Inhibitors of adenosine kinase. J Enz Inhib Med Chem 2003; 18: 395–402
- Singh P, Sharma BK. Quantitative structure-activity relationship study of benzylsulfanyl imidazoles as cytokine release inhibitors. J Enz Inhib Med Chem 2007; 22: 15–21
- Sharma BK, Kumar Rajendra, Singh P. Quantitative structure-activity relationship study of 1-arylsulfonyl-6-substituted benzonitriles as non-nucleoside reverse transcriptase inhibitors of HIV-1. J Enz Inhib Med Chem 2002; 17: 219–225
- Singh P, Sharma BK, Kumar R. Quantitative structure-activity relationship study on N-(pyridine-4-yl)-(indol-3-yl)alkylamides as antiallergic agents. Indian J Biochem Biophys 2002; 39: 351–355