Abstract
The increasing clinical importance of drug resistant microbial pathogens has lent additional urgency to microbiological research and new antimicrobial compound development. For this purpose, a new series of 3-[phenyldiazenyl] benzaldehyde N-phenylthiosemicarbazones were synthesized and evaluated for antifungal and antibacterial activity. The reaction of 2-hydroxy-5-[phenyldiazenyl] benzaldehyde (I) with N-phenylhydrazinecarbothioamide (II) were carried out in DMF. The antimicrobial activity of the synthesized target compounds (III) were evaluated by screening on different human pathogens using the disc diffusion assay. All the compounds exhibited considerable inhibition against the bacteria and fungi tested.
Keywords::
Introduction
Infections caused by microorganisms pose a serious challenge to the medical community and there is a need for a reliably effective therapy for such infectious diseases that had previously caused extensive mortality, morbidity and fear. The resurgence of infections in industrialized countries as well as the appearance of multidrug resistant (MDR) strains of bacterial and fungal pathogens have prompted the quest for new drugs acting both as antibacterial and antifungal agents, without cross- resistance with known antimicrobials. Development of MDR [Citation1,Citation2] to existing drugs is a constant growing phenomenon that has concerned researchers world-wide, and now has reached an alarming level for certain infectious diseases lacking ready treatment regimens Citation3-5.
There are two basic approaches to develop a new drug for microbial infections: (i) Synthesis of analogues, modifications or derivatives of existing compounds for improving microbial treatment, (ii) Searching for novel structures, that the concerned organisms has never encountered, for the treatment of MDR microbial infections, which may act either by the same or a new mechanism [Citation6].
To pursue this goal, our research efforts are directed to finding new chemical classes of antimicrobial agents. The methods of investigation enabled us to find some new pharmacophores of the above mentioned activities. Many studies have been conducted on compounds bearing –N = N-, -N-C = S, -CH = N- and electron withdrawing moieties as a pharmacophore Citation7-10. Various substituted aromatic rings were utilised as a basis to constitute a large series of N-and S heteroatomic compounds. Special attention was paid to correlate their biological activity with their structure which proved that, activity is enhanced by electron withdrawing groups attached to aryl ring Citation11-16. Furthermore, interest in the chemistry, synthesis and biology of these pharmacophores continues to be fuelled by their wide range of biological properties viz. antifungal, anticancer, antibacterial, antiviral, antiamoebic, antiproliferative, antitubercular, antitumor, anticonvulsant, antimalarial and trypanocidal activities Citation17-37.
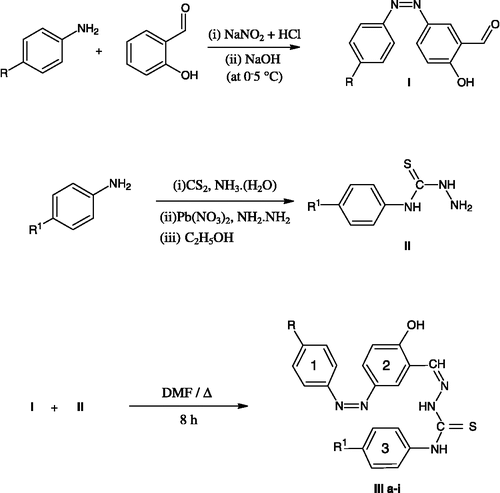
In view of conclusions drawn from our previous work Citation38-40and considering the antimicrobial efficacy of –OCH3, Cl and F groups attached to an aryl ring it seems logical and attractive to combine all these moieties together in a molecular framework. In the present work we have selected substituted amines containing these substituents for the synthesis of azosalicylaldehydes(I) and thiosemicarbazides(II) and finally synthesized some new 3-[phenyldiazenyl] benzaldehyde N-phenylthiosemicarbazones(III) by condensing (I) & (II). which were evaluated for their antimicrobial efficacy.
Experimental
Chemistry
All melting points were determined in open capillaries and are uncorrected. The purity of the compounds was routinely checked by thin layer chromatography (TLC) using silica gel 60G (Merck) with acetone/hexane (1:3). Spectroscopic data were recorded using the following instruments. IR: Perkin Elmer RxI (FT IR) spectrophotometer;1NMR: Bruker DRX 300 (300MHz, FT NMR); MS –FAB: Jeol –SX 102 Mass spectrometer.
The synthetic route to the required compounds is outlined in Scheme . For the synthesis of the titled compounds, 2-hydroxy-5-[phenyldiazenyl] benzaldehyde (I) required as a starting material was prepared by the diazotisation and coupling method. N-phenylhydrazinecarbothioamide (II) was prepared by reacting p-substituted phenyl isothiocyanate with hydrazine hydrate in the presence of ethanol. The reaction of equimolar quantities of (I) with (II) in the presence of DMF resulted in the formation of the titled compounds (III a-i) .
Table I. Some characteristics of the compounds.
General procedure for synthesis of compounds
2-hydroxy-5-[phenyldiazenyl] benzaldehyde (I)
Aniline (3.72mL) was dissolved in aqueous hydrochloric acid (28mL,6N) and mechanically stirred at 0–5°C. A cold solution of sodium nitrite (5gm/10 mL water) was added dropwise to the constantly stirred reaction mixture. The diazotized solution was immediately added in small portions to salicylaldehyde (5mL dissolved in 40mL 6N NaOH), with constant stirring at 0–5°C. The stirring was continued for 4h. The solid obtained was filtered under suction washed with cold water and recrystallised from glacial acetic acid.
N-phenylhydrazinecarbothioamide (II)
Carbon disulphide(12mL) was added dropwise to a mixture of an ethanolic(20mL) solution of aniline(10 mL) and liquor ammonia (15 mL) and stirred at 10–15°C for 2h. The reaction mixture was transferred to another flask containing lead nitrate (75gm in 200mL distilled water) and stirred further until the precipitation of lead sulphide was complete. The reaction mixture was steam distilled and isothiocyanatobenzene was collected, dissolved in ethanol (10mL) and refluxed with an ethanolic solution of hydrazine hydrate(25%, 0.6mL in 10 mL ethanol) for 5h. The product obtained was collected by filtration, washed with cold water and recrystallised from ethanol.
5-[(4- chlorophenyl) diazenyl]- 2- hydroxybenzaldehyde N -(4- methylphenyl) thiosemicarbazones (III a-i)
A mixture of the appropriate, 2-hydroxy-5-[phenyldiazenyl] benzaldehyde (I)(2.5 gm) and the N-phenylhydrazinecarbothioamide (II) (1.5 gm) were refluxed for 8h in DMF (30 mL). The mixture was then cooled in an ice bath and the product separated was repeatedly washed with water followed by ethanol and recrystallised from diethyl ether.
III a-i
IR (KBr)ν max (cm− 1): 3460-3215(-OH & -NH), 1670-1655 (-CH = N), 1610-1595(-N = N-), 1270-1240(-C = S)
III a
1H NMR (DMSO-d6) δ (ppm): 6.59(1H,s,-OH), 9.2(1H,s,-CH = N),9.60 (1H,s, = N-NH), 7.05-7.50 (12H,m,ArH), 8.41(1H,s,-NH-Ar). MS (FAB): m/z 389.
IIId
1HNMR (DMSO-d6) δ (ppm):6.54 (1H,s,-OH), 8.9 (1H,s,-CH = N), 9.58(1H,s, = N-NH), 7.45-7.66 (12H,m,ArH), 8.20(1H,s,-NH-Ar).MS(FAB): m/z 305,[M + 2]:m/z 307.
III g
1H NMR (DMSO-d6) δ (ppm):6.49(1H,s,-OH)9.02(1H,s,-CH = N), 9.54(1H, s, = N-NH), 6.95-7.40 (12H,m,Ar H),8.3(1H,s,-NH-Ar) 3.5(3H,s,-OCH3).MS(FAB): m/z 301.
Microbiology
All the compounds were screened for their in vitro antimicrobial activity at the Birla institute of Medical Research and College of Life Sciences, Gwalior, against 24h old cultures of bacterial and fungal pathogens. Antimicrobial activity was determined against Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Shigella flexinerri and Klebisiella pneumoniae bacterial strains and Candida albicans, Aspergillus fumigatus, Candida neoformans, Aspergillus niger and Penicillium ittalecum fungal strains using the Disc Diffusion assay. For this, a sterile filter paper disc (6mm) impregnated with fixed doses (1000 μg/mL) and (800 μg/mL) of the synthesized compounds under investigation were placed upon the seeded petri dishes. Similar discs were prepared for the standard drugs, chloramphenicol, fluconazole and the solvent control, dimethyl formamide. The plates were incubated for 24h at 37°C for the bacterial strains and 48 h at 37°C for the fungal strains. The zone of inhibition, observed around the disc after incubation was measured. The results are presented in .
Table II. In vitro antimicrobial activity of compounds III a-i.
Results and discussion
The structure of the nine new compounds synthesized were elucidated by spectral data. In the IR spectra, some significant stretching bands due to –OH and -NH, -N = N- and –C = S were observed at 3460-3215, 1610-1595and 1270-1240 cm− 1 respectively. The specific band for thiosemicarbazone (-CH = N-) was observed at 1670-1655 cm− 1. In the 1NMR spectra, the signal due to –CH = N protons, present in all compounds, appeared at 8.9-9.2 ppm as a singlet. The, = N-NH and NH-Ar, –OH protons were observed at 9.54-9.60, 8.20-8.41 and 6.49-6.59 ppm as a singlet, respectively. All the aromatic protons were observed in the expected regions. Mass spectra[MS(FAB)] of compounds showed a (M + 1) and (M + 2) peaks, in agreement with their molecular formula.
The antimicrobial activities of the synthesized compounds were screened in vitro using the Disc Diffusion technique against different human pathogens at 1000 μg/mL and 800 μg/mL. All of the compounds tested, showed moderate activity (). The major findings gathered from the antimicrobial study of the compounds are highlighted as follows:
a p-Chloro group of ring 1 and/or 3, in the new thiosemicarbazones enhanced the antimicrobial profile.
a p- methoxy substituent in aryl ring 1 showed poor antimicrobial activity.
Substitution of p-chloro and p-methoxy moieties of aryl rings 1 & 3 by a p-fluoro group markedly reduced antimicrobial activity.
The incorporation of halogen moieties in an unsubstituted ring gradually increased activity.
Acknowledgements
Sincere thanks are due to the Dean, Birla Institute of Medical Research and College of Life Sciences, Gwalior, for providing screening facilities (including pathogens, media and instruments) and to the UGC, New Delhi, for financial assistance.
References
- Harbart S, Albrich W, Goldman DA, Huebner. Control of multiply resistant cocci: Do international comparisons help?. J Lancet Infect Dis 2001; 1: 251–261
- Barber I, Cokmus C, Atalan E. Comparison of Staphylococcus spp. cellular and extracellular proteins by SDS-PAGE. Microbiol 2003; 72: 54–59
- WHO website., http: //www.who.org and NIAID Website, http:// www. niaid.nih.goe/factsheets/tb.htm.
- Snider DE, Castro KGN. The Global Threat of Drug-Resistant Tuberculosis. New Engl J Med 1998; 338: 1689–1690
- Sbardella G, Mai A, Artico M, Loddo R, Setzu MG, La Colla P. Synthesis and in vitro antimycobacterial activity of novel 3-(1H-pyrrol-1-yl)-2-oxazolidinone analogues of PNU-100480. Bioorg Med Chem Lett 2004; 14: 1537–1541
- Weber JT, Courvalin P. An emptying quiver: Antimicrobial drugs and resistance. Emerg Infect Dis 2005; 11: 791–793, www.cdc.goe/eid
- Banik I, Becker FF, Banik BK. Stereoselective synthesis of beta-lactams with polyaromatic imines: Entry to new and novel anticancer agents. J Med Chem 2003; 46: 12–15
- Bindu P, Kurup MRP, Satya Keeuty TR. Cyclic Voltametric and Biological Activities of Cu(II) Complexes of Salicyladehyde N(4)-Substituted Thiosemicarbazone and Heterocyclic Bases. Polyhedron 1999; 18: 321–331
- Fujii N, et al. Discovery of potent thiosemicarbazone inhibitors of rhodesain and cruzain. Bioorg Med Chem Lett 2005; 15: 121–123
- Satoshkar RS, Bhandarkar SD, Ainapuri SS. Pharmacology Pharmacotherapeutics18th edn. 2003; p. 625
- Jacob N, Gutty GN. Scientific and Research Publication from Indian Drug Manufacturers Association. 2004; 41: 76–79
- Wujec M, Pitucha M, Dabosz M, Malam A. Synthesis and potential antimycotic activity of 4-substituted-3-(thiophene-2-yl-methyl)-_2-1,2,4-triazoline-5-thiones. Acta Pharm 2004; 54: 251–254
- Sakiyan I, Logoglu E, Arslan S, Sari N, Sakiyan N. Antimicrobial activities of N-(2-hydroxy-1-naphthalidene)-amino acid (glycine, alanine, phenylalanine, histidine, tryptophane) Schiff bases and their manganese(III) complexes. Biometals 2004; 17: 115–119
- Aytemir MD, Colis U, Ozalp M. Synthesis and evaluation of anticonvulsant and antimicrobial activities of 3-hydroxy-6-methyl-2-substituted 4h-pyran-4-one derivatives. Arch Pharma 2004; 337: 281–285
- Willard J. Medical MycologyEdn 2nd. 2002, 73–73.
- Menozzi G, Merello L, Fossa P, Schenone S, Ranise A, Mosti L, Bondavalli F, Loddo R, Murgioni C, Mascia V, La Colla P, Tamburini E. Synthesis, antimicrobial activity and molecular modeling studies of halogenated4-[1H-imidazol-1-yl(phenyl)methyl]-1,5-diphenyl-1H-pyraz oles. Bioorg Med Chem 2004; 12: 5465–5483
- Chen J, Huang Y, Liu G, Afrasiaki Z, Sinn E, Padhye S, Ma Y. The cytotoxicity and mechanisms of 1, 2-naphthoquinone thiosemicarbazone and its metal derivatives against MCF-7 human breast cancer cells. Toxicol Appl Pharmacol 2004; 197: 40–48
- Quiroga AG, Ranninger CN. Contribution to the SAR field of metallated and coordination complexes: Studies of the palladium and platinum derivatives with selected thiosemicarbazones as antitumoral drugs. Coord Chem Rev 2004; 248: 119–133
- Afriaski Z, Sinn E, Padhye S, Dutta S, Newton C, Anson CE, Powell AK. Transition metal complexes of phenanthrenequinone thiosemicarbazone as potential anticancer agents: Synthesis structure, spectroscopy, electrochemistry and in vitro anticancer activity against human brest cancer cell-line, T47D. J Inorg Biochem 2003; 95: 306–314
- Casas JS, Castano MV, Cifuentes MC, Sanchez A, Sordo J. Synthesis and structures of acetylferrocene thiosemicarbazones and their dimethylthallium(III) complexes, which have four- or five-membered chelate rings. Polyhedron 2002; 21: 1651–1660
- Blanco MA, Toress EL, Mendiola MA, Brunet E, Sevilla MT. Macrocyclization of cyclic thiosemicarbazones with mercury salts. Tetrahedron 2002; 58: 1525–1531
- Perez Rebolledo A, Ayala JD, de Lima GM, Marchini M, Bombieri G, Zani CL, Souza-Fagundes EM, Beraldo H. Structural studies and cytotoxic activity of N(4)-phenyl-2-benzoylpyridine thiosemicarbazone Sn(IV) complexes. Eur J Med Chem 2005; 40: 467–472
- Karatas F, Koca M, Kara H. Servisuleyman. Synthesis and oxidant properties of novel (5-bromobenzofuran-2-yl)(3-methyl-3-mesityl cyclobutyl) ketonethiosemicarbazone. Eur J Med Chem 2006; 41: 664–669
- Ramesh Kumar N, Veena A, Ilavarasan R, Adiraj M, Shaimugapandyan P, Shridhar SK. Synthesis and anticancer activity of thiosemicarbazones. Biol Pharm Bull 2003; 26: 188–193
- Hu W, Jhou W, Xia C, Wen X. Synthesis and anticancer activity of thiosemicarbazones. Bioorg Med Chem Lett 2006; 16: 2213–2218
- Kolocouris A, Dimas K, Pannecouque C, Witvrouw M, Foscolos GB, Stamatiou G, Fytas G, Zoidis G, Kolocouris N, Andrei G, Snoeck R, Clercq ED. New 2-(1-adamantylcarbonyl) pyridine and 1-acetyladamantane thiosemicarbazones-thiocarbonohydrazones: Cell growth inhibitory, antiviral and antimicrobial activity evaluation. Bioorg Med Chem Lett 2002; 120: 723–727
- Terzioglu N, Karah N, Gursoy A, Pannecouque C, Leyson P, Paeshuyse J, Neyts J, De Clerq E. Synthesis and primary antiviral activity evaluation of 3-hydrazono-5-nitro-2-indolinone derivatives. Arkivoc 2006; i: 109–118
- Chohan ZH, Pervez H, Khan KM, Supuran CT. Organometallic-based Antibacterial and Antifungal Compounds Transition Metal: Complexes of 1, 1-Diacetylferrocene-derived Thiocarbohydrazone, Carbohydrazone, Thiosemicarbazone and Semicarbazone. J Enz Inhib Med Chem 2005; 20: 81–88
- Sharma S, Athar F, Maurya MR, Azam A. Copper (II) complexes with substituted thiosemicarbazones of thiophene-2-carboxaldehyde: Synthesis, characterization and antiamoebic activity against E. histolytica. Eur J Med Chem 2005; 40: 1414–1419
- Singh S, Athar F, Maurya MR, Azam A. Cyclooctadiene Ru(II) complexes of thiophene-2-carboxaldehyde-derived thiosemicarbazones: Synthesis, characterization and antiamoebic activity. Eur J Med Chem 2006; 41: 592–598
- Patole J, Padhey S, Moodbidri NS, Shirsat N. Antiproliferative activities of iron and platinum conjugates of salicylaldehyde semi-/thiosemicarbazones against C6 glioma cells. Eur J Med Chem 2005; 40: 1052–1055
- Shriram D, Yogeeswari P, Thirumurugan R, Pavana RK. Discovery of new antitubercular oxazolyl thiosemicarbazones. J Med Chem 2006; 49: 3448–3450
- Easman J, Purstinger G, Heinisch G, Roth T, Fiebig HH, Holzer W, Jager W, Jenny M, Hofmann J. Synthesis, cytotoxicity, and antitumor activity of Copper (II) and Iron (II) complexes of N- azabicyclo [3, 2, 2] nonane thiosemicarbazones derived from acyl diazines. J Med Chem 2001; 44: 2164–2171
- Seebacher W, Brun R, Weis R. New 4-aminobicyclo [2.2.2] octane derivatives and their activities against Plasmodium falciparum and Trypanosoma b. rhodesiense. Eur J Pharm Sci 2004; 21: 225–233
- Yogeeswari P, Shriram D, Ramamoorthy L, Jit JS, Kumar SS, Stables JP. Anticonvulsant and neurotoxicity evaluation of some 6-chlorobenzothiazolyl-2-thiosemicarbazones. Eur J Med Chem 2002; 37: 231–236
- Du X, Guo C, Hansell E, Doyle PS, Caffrey CR, Holler TP, Mckerrow JH, Cohen EE. Synthesis and Structure-Activity Relationship Study of Potent TrypanocidalThio Semicarbazone Inhibitors of the Trypanosomal Cysteine Protease Cruzain. J Med Chem 2002; 45: 2695–2707
- Aguirre G, Boiani L, Cerecetto H, Fernandez N, Gonzalez M, Denicola A, Oteroe L, Ceambino D, Rigol C, Olea Azar C, Faundez M. In vitro activity and mechanism of action against the protozoan parasite Trypanosoma cruzi of 5-nitrofuryl containing thiosemicarbazones. Biorg Med Chem 2004; 12: 4885–4893
- Halve AK, Dubey R, Bhadauria D. N/C-4 substituted azetidin-2-ones: Synthesis and preliminary evaluation of new class of Antimicrobial agents. Bioorg Med Chem Lett. 2007, 17: 341–345
- Halve AK, Dubey R, Bhadauria D, Bhaskar B, Bhadauria R. Synthesis, antimicrobial screening and structure activity relationship of some novel 2-hydroxy-5-(nitro substituted phenyl azo) benzylidene anilines. Ind J Pharm Sci July-Aug 2006; 68(4)510
- Halve AK, Gaur P, Dubey R, Bhadauria D, Bhaskar B. Synthesis and antimicrobial screening of 2′- hydroxy-5′-(phenylazo)- N-(1″,3″-diketophenylamine)-3-chloro azetidine-2-ones. Ind J Chem Oct 2005; 44B: 2163