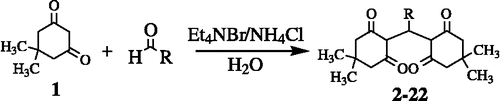Abstract
A mild and efficient route to tetraketones (2–22) has been developed by way of tetraethyl ammonium bromide (Et4N+Br− ) mediated condensation of dimedone (5,5-dimethylcyclohexane-1,3-dione, 1) with a variety of aldehydes. All these compounds showed significant lipoxygenase inhibitory activity and moderate to strong antioxidant potential. Compounds 19 (IC50 = 7.8 μM), 22 (IC50 = 12.5 μM), 3 (IC50 = 16.3 μM), 11 (IC50 = 17.5 μM) and 8 (IC50 = 21.3 μM) showed significant inhibitory potential against lipoxygenase (baicalein, IC50 = 22.4 μM). On the other hand compound 19 (IC50 = 33.6 μM) also showed strong antioxidant activity compared to the standard (IC50 = 44.7 μM). This study is likely to lead to the discovery of therapeutically efficient agents against very important disorders including inflammation, asthma, cancer and autoimmune diseases.
Introduction
Tetraketones are an important class of compounds which are extensively used as important precursors for the synthesis of acridinediones as laser dyes of various heterocyclic compounds [Citation1]. Previously these target compounds have been prepared by several methods but most of these involve either forcing reaction conditions, tedious reaction workup and involvement of heat to afford the products [Citation2,Citation3]. Herein we report a mild and high yielding access to these potential compounds involving tetraethyl ammonium bromide-mediated condensation of dimedone (1) with a variety of aldehydes in water at room temperature. The resulting tetraketones (2–22) were obtained in a yield ranging from 90%–98%. This study is likely to lead to the discovery of therapeutically efficient agents against disorders like inflammation, asthma, cancer and autoimmune diseases.
Lipoxygenases (LOX; EC 1.13.11.12) constitute a family of non-heme iron containing dioxygenases that are widely distributed in animals and plants. In mammalian cells these are key enzymes in the biosynthesis of a variety of bioregulatory compounds such as hydroxyeicosatetraenoic acids (HETEs), leukotrienes, lipoxins and hepoxylines [Citation4]. It has been found that these lipoxygenase products play a role in a variety of disorders such as inflammation [Citation5], tumor angiogenesis [Citation6] and bronchial asthma [Citation7]. LOXs are therefore a potential target for the rational drug design and discovery of mechanism-based inhibitors for the treatment of inflammation, bronchial asthma, cancer and autoimmune diseases.
Antioxidants are the substances which, when present at low concentrations, in relation to oxidizable substrates, significantly inhibit or delay oxidative processes, while often being oxidized themselves. The applications of antioxidants are widespread in different industries including cosmetics, food, beverages [Citation8] etc. In recent years there has been an increased interest in the application of antioxidants to medical treatment as information is constantly gathered linking the development of human diseases to oxidative stress. In any biological system, an important balance must be maintained between the formation of reactive oxygen and nitrogen species (ROS and RNS). ROS and RNS are formed regularly as a result of normal organ functions or as a result of excess oxidative stress. The reactive species superoxide () hydrogen peroxide (H2O2), hydroxyl radical (HO·) nitrogenoxide (NO·), peroxynitrite (
) and hypochlorous acid (HOCl), are all products of normal metabolic pathways of human organs, but under certain conditions, they can exert harmful effects and can cause tissue injury when in excess [Citation9]. To maintain an oxido/redox balance, organs protect themselves from the toxicity of excess ROS/RNS in different ways, including the use of endogenous and exogenous antioxidants [Citation10]. This natural antioxidant mechanism can be inefficient and hence dietary intake of antioxidant compounds is important [Citation11,Citation12]. There are some synthetic antioxidant compounds such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), commonly used in processed foods. However, it has been suggested that these compounds have some side effects [Citation9,Citation13]. Therefore development and utilization of more effective antioxidants are desperately needed.
Materials and methods
Chemistry
Melting points were determined by a Buchi 434 melting point apparatus. NMR spectroscopy was performed on a Bruker 400 MHz. Infrared spectra (IR) were recorded on JASCO IR-A-302 Spectrophotometer. EIMS were recorded on a FINNIGAN MAT-311A (Germany). Thin layer chromatography (TLC) was performed on pre-coated TLC plates (Kieselgel 60, 254, E. Merck, Germany). Chromatograms were visualized by iodine vapours, ultraviolet light (UV) at 254 and 365 nm or ceric sulphate solution followed by heating.
General procedure for the preparation of compounds 2–22
To a mixture of 5,5-dimethylcyclohexnane-1,3-dione (1) (10 mM), ammonium chloride (10 mM), tetraethyl ammonium bromide (0.5 mM) dissolved in water, the aldehyde (5 mM) was added dropwise and the reaction mixture was stirred at room temperature for 30 min (TLC monitoring). Then cold water (15 mL) was added to the reaction mixture and the resultant precipitates were filtered and recrystallized from aqueous ethanol.
Phenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (2)
Yield: 98%; mp: 194–195°C; Rf: 0.61 (CH2Cl2/EtOAc, 8:2); UV (CHCl3): λmax 261 (log ε = 3.37) nm; IR (KBr): νmax 3123, 2958, 1610, 1601, 1512, 1461, 1334, 849 cm− 1; 1H–NMR (400 MHz, CDCl3): δ 7.06-7.26 (5H, m, Ar-H), 5.52-5.54 (1H, m, H-7), 3.69-3.80 (2H, m, H-2/H-2′), 2.23-2.46 (8H, m, H-4/H-6/H-4′/H-6′), 1.21 (6H, s, 2 × CH3), 1.08 (6H, s, 2 × CH3); EIMS: m/z 368 (31), 253 (4), 227 (100), 171(28), 140 (23), 102 (27), 82 (3), 55 (62%); Anal. Calcd. for C23H28O4. C, 74.97; H, 7.66. Found: C, 74.95; H, 7.65%.
4-Methylphenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (3)
Yield: 95%; mp: 158–160°C; Rf: 0.46 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 261 (log ε = 4.1) nm; IR (KBr): νmax 3111, 1616, 1603, 1513, 1475, 843 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.01 (2H, d, J = 8.0 Hz, H-2″/H-6″), 6.92 (2H, d, J = 8.0 Hz, H-3″/H-5″), 5.55-5.67 (1H, m, H-7), 2.48-2.54 (2H, m, H-2/H-2′), 2.32-2.41 (8H, m, H-4/H-6/H-4′/H-6′), 2.26 (3H, s, CH3), 1.11 (12H, s, 4 × CH3); EIMS: m/z 382 (24), 242 (24), 227 (100), 170 (19), 114 (75), 83 (25), 55 (30); Anal. Calcd. for C24H30O4. C, 75.36; H, 7.91. Found: C, 75.36; H, 7.91%.
2-Hydroxyphenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (4)
Yield: 92%; mp: 222–224°C; Rf: 0.48 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 288 (log ε = 4.0) nm; IR (KBr): νmax 3101, 1611, 1602, 1518, 1462, 1340, 848 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 6.81-7.14 (4H, m, Ar-H), 5.15-5.26 (1H, m, H-7), 2.44-2.54 (2H, m, H-2/H-2′), 2.15-2.27 (8H, m, H-4/H-6/H-4′/H-6′), 1.06 (6H, s, 2 × CH3), 1.01 (6H, s, 2 × CH3); EIMS: 384 (15), 367 (17), 292 (9), 253 (10), 227 (40), 171(5), 140 (12), 102 (11); Anal. Calcd. for C23H28O5. C, 71.85; H, 7.34. Found: C, 71.82; H, 7.33%.
3-Hydroxyphenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (5)
Yield: 95%; mp: 248–250°C; Rf: 0.49 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 289 (log ε = 3.1) nm; IR (KBr): νmax 3109, 1610, 1605, 1515, 1470, 1340, 845 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.20 (1H, t, J = 8.0 Hz, H-5″), 7.05 (1H, d, J = 8.0 Hz, H-4″), 6.71 (1H, dd, J = 8.0 Hz, J = 2.2 Hz, H-6″), 6.21 (1H, d, J = 2.2 Hz, H-2″), 5.06-5.10 (1H, m, H-7), 2.44-2.56 (2H, m, H-2/H-2′), 2.14-2.25 (8H, m, H-4/H-6/H-4′/H-6′), 1.06 (6H, s, 2 × CH3), 1.02 (6H, s, 2 × CH3); EIMS: m/z 384 (15), 367 (17), 292 (9), 253 (10), 227 (40), 171(5), 140 (12), 102 (11); Anal. Calcd. for C23H28O5. C, 71.85; H, 7.34. Found: C, 71.83; H, 7.32%.
4-Hydroxyphenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (6)
Yield: 93%; mp: 190–192°C; Rf: 0.52 (CH2Cl2/EtOAc, 8:2); UV (CHCl3): λmax 289 (log ε = 3.95) nm; IR (KBr): νmax 3100, 1606, 1600, 1512, 1465, 1344, 848 cm− 1; 1H–NMR (400 MHz, CDCl3): δ 7.32 (2H, d, J = 8.4 Hz, H-2″/H-6″), 6.72 (2H, d, J = 8.4 Hz, H-3″/H-5″), 5.06-5.10 (1H, m, H-7), 3.68-3.80 (2H, m, H-2/H-2′), 2.15-2.27 (8H, m, H-4/H-6/H-4′/H-6′), 1.04 (6H, s, 2 × CH3), 0.99 (6H, s, 2 × CH3); EIMS: m/z 384 (22), 367 (12), 292 (4), 253 (11), 227 (45), 171(7), 140 (19), 102 (17), 82 (3), 55 (100); Anal. Calcd. for C23H28O5. C, 71.85; H, 7.34. Found: C, 71.81; H, 7.33%.
2-Methoxyphenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (7)
Yield: 96%; mp: 181–183°C; Rf: 0.55 (CH2Cl2/EtOAc, 8:2); UV (CHCl3): λmax 274 (log ε = 3.9) nm; IR (KBr): νmax 3123, 1613, 1601, 1517, 1461, 1339, 841 cm− 1; 1H–NMR (400 MHz, CDCl3): δ 7.15-7.24 (2H, m, H-5″/H-4″), 6.87 (1H, td, J = 7.9 Hz, J = 1.8 Hz, H-4″), 6.77 (1H, d, J = 7.9 Hz, H-6″), 5.33-5.50 (1H, m, H-7), 3.70 (3H, s, OCH3), 3.72-3.80 (2H, m, H-2/H-2′), 2.13-2.45 (8H, m, H-4/H-6/H-4′/H-6′), 1.14 (6H, s, 2 × CH3), 1.08 (s, 6H, 2 × CH3); EIMS: m/z 398 (77), 366 (9), 282 (70), 271 (83), 227 (100), 199 (31), 171 (36), 131 (52), 83 (8), 55 (83); Anal. Calcd. for C24H30O5. C, 72.34; H, 7.59. Found: C, 72.38; H, 7.59%.
4-Methoxyphenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (8)
Yield: 96%; mp: 185–186°C; Rf: 0.56 (CH2Cl2/EtOAc, 8:2); UV (CHCl3): λmax 301 (log ε = 3.45) nm; IR (KBr): υmax 3123, 1614, 1605, 1512, 1470, 1343, 839 cm− 1; 1H–NMR (400 MHz, CDCl3): δ 6.97 (2H, d, J = 8.4 Hz, H-2″/H-6″), 6.79 (2H, d, J = 8.4 Hz, H-3″/H-5″), 5.22-5.40 (1H, m, H-7), 3.77 (3H, s, OCH3), 3.70-3.75 (2H, m, H-2/H-2′), 2.26-2.45 (8H, m, H-4/H-6/H-4′/H-6′), 1.20 (6H, s, 2 × CH3), 1.07 (6H, s, 2 × CH3); EIMS: m/z 398 (13), 349 (4), 273 (7), 257 (78), 227 (66), 146 (24), 117 (19), 83 (100), 55 (92); Anal. Calcd. for C24H30O5. C, 72.34; H, 7.59. Found: C, 72.31; H, 7.60%.
3,4-Dimethoxyphenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (9)
Yield: 90%; mp: 173–175°C; Rf: 0.56 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 308 (log ε = 3.5) nm; IR (KBr): νmax 3111, 1616, 1609, 1510, 1475, 841 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 6.81 (1H, d, J = 8.4, H-5″), 6.64 (1H, d, J = 1.3 Hz, H-2″), 6.60 (1H, dd, J = 8.4 Hz, J = 1.3 Hz, H-6″), 5.57-5.66 (1H, m, H-7), 3.78 (3H, s, OCH3), 3.71 (3H, s, OCH3), 2.47-2.55 (2H, m, H-2/H-2′), 2.33-2.42 (8H, m, H-4/H-6/H-4′/H-6′), 1.13 (12H, s, 4 × CH3); EIMS: m/z 428 (34), 289 (43), 257 (38), 201 (17), 140 (13), 83 (100), 55 (66); Anal. calcd. for C25H32O6. C, 70.07; H, 7.53. Found: C, 70.08; H, 7.53%.
4-Ethoxyphenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (10)
Yield: 90%; mp: 164–166°C; Rf: 0.54 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 301 (log ε = 3.65) nm; IR (KBr): νmax 3102, 1614, 1601, 1510, 1470, 1340, 845 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.00 (2H, d, J = 8.1 Hz, H-2″/H-6″), 6.61 (2H, d, J = 8.1 Hz, H-3″/H-5″), 5.12-5.25 (1H, m, H-7), 3.77 (2H, q, J = 6.1 Hz, OCH2), 2.48-2.56 (2H, m, H-2/H-2′), 2.12-2.22 (8H, m, H-4/H-6/H-4′/H-6′), 1.20 (3H, t, J = 6.1 Hz, CH3), 1.12 (6H, s, 2 × CH3), 1.01 (6H, s, 2 × CH3); EIMS: m/z 412 (33), 396 (13), 286 (11), 255 (36), 225 (30), 161 (23), 84 (59); Anal. Calcd. for C25H32O5. C, 72.79; H, 7.82. Found: C, 72.78; H, 7.83%.
4-N,n-dimethylaminophenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (11)
Yield: 93%; mp: 194–195°C; Rf: 0.54 (CH2Cl2/EtOAc, 8:2); UV (CHCl3): λmax 261 (log ε = 3.372) nm; IR (KBr): νmax 3129, 1628, 1611, 1506, 1311, 850 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 6.93 (2H, d, J = 8.4 Hz, H-3″/H-5″), 6.70 (2H, d, J = 8.4 Hz, H-2″/H-6″), 4.99-5.24 (1H, m, H-7), 3.59-3.67 (2H, m, H-2/H-2′), 2.89 (6H, s, N(CH3)2), 2.17-2.39 (m, 8H, H-4/H-6/H-4′/H-6′), 1.11 (6H, s, 2 × CH3), 1.01 (6H, s, 2 × CH3); EIMS: m/z 411 (56), 327 (3), 284 (12), 272 (100), 214(11), 174 (24), 169 (3), 144 (47), 121 (73), 83 (69), 55 (66); Anal. Calcd. for C23H33NO4. C, 72.96; H, 8.08; N, 3.40. Found: C, 72.90; H, 8.09; N, 3.41%.
2-Chlorophenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (12)
Yield: 93%; mp: 201–203°C; Rf: 0.48 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 295 (log ε = 3.9) nm; IR (KBr): νmax 3120, 1614, 1610, 855 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.26 (1H, d, J = 7.5 Hz, H-3″), 7.12 (1H, dd, J = 7.5, J = 2.0 Hz, H-5″), 7.04 (1H, t, J = 7.5 Hz, H-4″), 6.95 (1H, d, J = 7.5 Hz, H-6″), 4.70-4.85 (1H, m, H-7), 2.43-2.54 (2H, m, H-2/H-2′), 2.12-2.41 (8H, m, H-4/H-6/H-4′/H-6′), 1.15 (6H, s, 2 × CH3), 1.09 (6H, s, 2 × CH3); EIMS: m/z 402 (87), 367 (59), 227 (100), 171 (96), 138 (28), 83 (70), 55 (40); Anal. Calcd. for C23H27ClO4. C, 68.56; H, 6.75. Found: C, 68.57; H, 6.75%.
3-Chlorophenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (13)
Yield: 92%; mp: 222–224°C; Rf: 0.56 (CH2Cl2/EtOAc, 8:2); UV (CHCl3): λmax 301 (log ε = 4.00) nm; IR (KBr): νmax 3122, 1621, 1619, 1520, 1318, 846, 612 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.27 (1H, dd, J = 1.5 Hz, J = 7.7 Hz, H-4″), 7.24 (1H, d, J = 1.5 Hz, H-2″), 7.16 (1H, td, J = 1.5 Hz, J = 7.7 Hz, H-5″), 7.12 (1H, dd, J = 1.5 Hz, J = 7.7 Hz, H-6″), 5.03-5.14 (1H, m, H-7), 3.59-3.67 (2H, m, H-2/H-2′), 2.17-2.39 (8H, m, H-4/H-6/H-4′/H-6′), 1.11 (6H, s, 2 × CH3), 1.01 (6H, s, 2 × CH3); EIMS: m/z 404 (5), 402 (12), 367 (18), 275 (20), 255 (26), 227 (100), 199 (14), 171 (22), 115 (22), 83 (22), 55 (27); Anal. Calcd. for C23H27ClO4. C, 68.56; H, 6.75. Found: C, 68.52; H, 6.74%.
3-Bromophenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (14)
Yield: 92%; mp: 229–231°C; Rf: 0.56 (CH2Cl2/EtOAc, 8:2); UV (CHCl3): λmax 2.93 (log ε = 3.88) nm; IR (KBr): νmax 3129, 1628, 1611, 1506, 1311, 850, 608 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.47 (1H, dd, J = 1.7 Hz, J = 7.9 Hz, H-4″), 7.42 (1H, d, J = 1.7 Hz, H-2″), 7.23 (1H, td, J = 1.7 Hz, J = 7.9 Hz, H-5″), 7.17 (1H, dd, J = 1.7 Hz, J = 7.9 Hz, H-6″), 5.12-5.24 (1H, m, H-7), 3.58-3.69 (2H, m, H-2/H-2′), 2.17-2.39 (8H, m, H-4/H-6/H-4′/H-6′), 1.16 (6H, s, 2 × CH3), 0.98 (6H, s, 2 × CH3); EIMS: m/z 449 (4), 447(5), 349 (12), 320 (4), 255 (5), 227 (100), 171 (23), 115 (8), 83 (21), 55 (35); Anal. Calcd. for C23H27BrO4. C, 61.75; H, 6.08. Found: C, 661.74; H, 6.08%.
3-Bromo-4-hydroxyphenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (15)
Yield: 94%; mp: 155–157°C; Rf: 0.54 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 299 (log ε = 3.85) nm; IR (KBr): νmax 3112, 1618, 1614, 1515, 1475, 840 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.10 (1H, d, J = 1.5, H-2″), 6.82 (1H, dd, J = 8.4 Hz, J = 8.4 Hz, H-6″), 6.74 (1H, d, J = 8.4 Hz, H-5″), 5.59-5.67 (1H, m, H-7), 2.49-2.55 (2H, m, H-2/H-2′), 2.32-2.42 (8H, m, H-4/H-6/H-4′/H-6′), 1.11 (12H, s, 4 × CH3): EIMS: m/z 464 (36), 322 (77), 243 (59), 211 (100), 187 (39), 83 (100), 55 (74); Anal. Calcd. for C23H27BrO5. C, 59.62; H, 5.78. Found: C, 59.62; H, 5.78%.
2-Nitrophenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (16)
Yield: 94%; mp: 188–190°C; Rf: 0.52 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 302 (log ε = 3.7) nm; IR (KBr): νmax 3121, 1650, 1617, 1596, 845 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.89 (1H, dd, J = 8.1 Hz, J = 1.2 Hz, H-5″), 7.46 (1H, d, J = 8.1 Hz, H-3″), 7.39 (1H, d, J = 8.1 Hz, H-4″), 7.32 (1H, dd, J = 8.1 Hz, J = 1.2 Hz, H-6″), 4.90-5.08 (1H, m, H-7), 2.52-2.60 (2H, m, H-2/H-2′), 2.13-2.33 (8H, m, H-4/H-6/H-4′/H-6′), 1.13 (6H, s, 2 × CH3), 1.03 (6H, s, 2 × CH3); EIMS: m/z 413 (25), 395 (10), 258 (15), 227(66), 171 (38), 83 (100), 55 (49); Anal. Calcd. for C23H27NO6. C, 66.81; H, 6.58; N, 3.39. Found: C, 66.82; H, 6.59; N, 3.39%.
3-Nitrophenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (17)
Yield: 91%; mp: 196–198°C; Rf: 0.65 (CH2Cl2/EtOAc, 8:2); UV (CHCl3): λmax 307 (log ε = 4.07) nm; IR (KBr): νmax 3123, 1655, 1616, 1595, 1366, 841 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 8.12 (1H, d, J = 8.4 Hz, H-4″), 7.27 (1H, d, J = 1.8 Hz, H-2″), 7.22 (1H, td, J = 1.8 Hz, J = 8.4 Hz, H-5″), 6.87 (1H, dd, J = 1.8 Hz, J = 8.4 Hz, H-4″), 4.91-5.01 (1H, m, H-7), 3.71-3.78 (2H, m, H-2/H-2′), 2.05-2.40 (8H, m, H-4/H-6/H-4′/H-6′), 1.10 (6H, s, 2 × CH3), 1.00 (6H, s, 2 × CH3); EIMS: m/z 413 (33), 396 (13), 286 (11), 255 (36), 225(30), 161 (23), 84 (59), 55 (100); Anal. Calcd. for C23H27NO6. C, 66.81; H, 6.58; N, 3.39. Found: C, 66.79; H, 6.58; N, 3.38%.
4-Nitrophenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (18)
Yield: 93%; mp: 188–190°C; Rf: 0.43 (CH2Cl2/EtOAc, 8:2); UV (CHCl3): λmax 310 (log ε = 3.91) nm; IR (KBr): νmax 3123, 1675, 1626, 1512, 1366, 1344, 1465, 848 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 8.09 (2H, d, J = 8.7 Hz, H-3″/H-5″), 7.26 (2H, d, J = 8.7 Hz, H-2″/H-6″), 5.10-5.19 (1H, m, H-7), 3.71-3.78 (2H, m, H-2/H-2′), 2.05-2.40 (8H, m, H-4/H-6/H-4′/H-6′), 1.10 (6H, s, 2 × CH3), 1.00 (6H, s, 2 × CH3); EIMS: m/z 413 (33), 396 (13), 286 (11), 255 (36), 225(30), 161 (23), 84 (59), 55 (100); Anal. Calcd. for C23H27NO6. C, 66.81; H, 6.58; N, 3.39. Found: C, 66.85; H, 6.57; N, 3.39%.
Styrenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (19)
Yield: 92%; mp: 215–217°C; Rf: 0.53 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 285 (log ε = 3.7) nm; IR (KBr): νmax 3110, 1615, 1605, 1510, 1470, 840 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.24 (2H, t, J = 7.1 Hz, H-3″/H-5″), 7.14 (3H, d, J = 7.1 Hz, H-2″/H-4″/H-6″), 5.75 (1H, d, J = 11.5 Hz, H-8″), 5.60 (1H, d, J = 11.5 Hz, H-7″), 5.10-5.22 (1H, m, H-7), 2.42-2.48 (2H, m, H-2/H-2′), 2.19-2.32 (8H, m, H-4/H-6/H-4′/H-6′), 1.02 (12H, s, 4 × CH3); EIMS: m/z 394 (33), 376 (38), 254 (61), 241 (83), 229 (38), 170 (43), 83 (100); Anal. Calcd. for C25H30O4. C, 76.11; H, 7.66. Found: C, 76.12; H, 7.65%.
α-Bromostyrenyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (20)
Yield: 97%; mp: 171–173°C; Rf: 0.51 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 315 (log ε = 3.7) nm; IR (KBr): νmax 3112, 1614, 1544, 1512, 1475, 845 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 6.81 (2H, d, J = 8.3 Hz, H-2″/ H-6″), 6.75 (1H, t, J = 8.3 Hz, H-4″), 6.64 (1H, s, H-7″), 6.50 (2H, t, J = 8.3 Hz, H-3″/ H-5″), 5.58-5.60 (1H, m, H-7), 2.47-2.53 (2H, m, H-2/H-2′), 2.32-2.42 (8H, m, H-4/H-6/H-4′/H-6′), 1.13 (12H, s, 4 × CH3); EIMS: m/z; 473 (44), 455 (34), 394 (38), 292 (41), 244 (25), 229 (38), 170 (43), 83 (100); Anal. Calcd. for C25H29BrO4. C, 63.43; H, 6.17. Found: C, 63.44; H, 6.17%.
Furanyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (21)
Yield: 95%; mp: 168–170°C; Rf: 0.48 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 214 (log ε = 3.2) nm; IR (KBr): νmax 3112, 1612, 1615, 1312, 841 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 7.26 (1H, d, J = 1.9 Hz, H-3″), 6.26 (1H, dd, J = 3.1, J = 1.9 Hz, H-4″), 5.87 (1H, d, J = 3.1 Hz, H-5″), 5.43-5.59 (1H, m, H-7), 2.55-2.61 (2H, m, H-2/H-2′), 2.29-2.38 (8H, m, H-4/H-6/H-4″/H-6′), 1.09 (12H, s, 4 × CH3); EIMS: m/z 358 (100), 274 (18), 218 (49), 162 (36), 106 (73), 83 (80), 55 (70); Anal. Calcd. for C21H26O5. C, 70.37; H, 7.31. Found: C, 70.36; H, 7.30%.
5-Methylfuranyl-2,2′-methylenebis-(5,5-dimethylcyclohexane-1,3-dione) (22)
Yield: 96%; mp: 157–159°C; Rf: 0.49 (CH2Cl2/MeOH, 9:1); UV (CH3OH): λmax 214 (log ε = 3.5) nm; IR (KBr): νmax 3112, 1615, 1603, 1512, 1475, 845 cm− 1; 1H–NMR (400 MHz, CD3OD): δ 5.82 (1H, d, J = 2.4 Hz, H-3″), 5.71 (1H, d, J = 2.4 Hz, H-4″), 5.35-5.58 (1H, m, H-7), 2.48-2.58 (2H, m, H-2/H-2′), 2.33-2.42 (8H, m, H-4/H-6/H-4′/H-6′), 1.09 (12H, s, 4 × CH3); EIMS: m/z 372 (97), 329 (100), 245 (26), 217 (82), 176 (75), 120 (36), 55 (53); Anal. Calcd. for C22H28O5. C, 70.94; H, 7.58. Found: C, 70.93; H, 7.58%.
Biology
In vitro lipoxygenase inhibition assay
Lipoxygenase inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel [Citation14]. Lipoxygenase enzyme solution was prepared so that the enzyme concentration in the reaction mixture was adjusted to give rates of 0.05 absorbance/min. The test compounds were prepared in methanol at various concentrations (50, 25, 12.5, 6.25 and 3.125 μM). The reaction mixture contained 160 μL (100 mM) sodium phosphate buffer (pH 8.0), 10μL of test-compound solution and 20μL of LOX solution. The contents were mixed and incubated for 10 min at 25°C. The reaction was then initiated by the addition of 10μL substrate solution (linoleic acid, 0.5 mM, 0.12% w/v tween 20 in the ratio of 1:2), with the formation of (9Z,11E)-(13S)-13-hydroperoxyoctadeca-9,11-dienoate and the change in absorbance at 234 nm was followed for 6 min. The concentration of the test compound that inhibited lipoxygenase activity by 50% (IC50) was determined by monitoring the effect of increasing concentrations of these compounds in the assays on the degree of inhibition. The IC50 values were calculated by means of the EZ-Fit Enzyme-Kinetics Program (Perrella Scientific Inc., Amherst, USA).
Determination of DPPH radical scavenging activity
The free radical scavenging activity was measured by 1,1-diphenyl-2-picrylhydrazil (DPPH) using the method described by Gulcin et al [Citation15]. The solution of DPPH (0.1 mM) was prepared in ethanol. Five microlitres of each compound at a different concentration was dissolved in DMSO and mixed with 95 μL of DPPH solution in ethanol. The mixture was dispersed in 96 well plate and incubated at 37°C for 30 min. The absorbance at 515 nm was measured by a microtitre plate reader (Spectramax plus 384 Molecular Device, USA) and percent radical scavenging activity was determined in comparison with the DMSO-treated control.
Where; Ac = absorbance of control (DMSO treated); As = absorbance of sample
Results and discussion
Chemistry
In our continuing research on biologically active metabolites for their potential use in medicinal chemistry, we herein report an alternate reagent Et4NBr/NH4Cl for the synthesis of tetraketones. It serves as a phase transfer reagent which not only increases the surface area of the reaction mixture but also enhances the water solubility of starting materials, resulting in mild reaction conditions and a significant increase in the percentage yields of the target compounds compared to the previously reported protocols. Polyoxygenated compounds have previously been reported as lipoxygenase inhibitors Citation16-18 and antioxidants [Citation19,Citation20]. Here we designed and synthesized polyphenolics, polyoxygenated and other tetraketones.
Compounds 2–22 (Scheme ) were screened for their lipoxygenase and DPPH free radical scavenging potential. The tetraketones reported in the present work were invariably expected to possess lipoxygenase inhibiting potential which was subsequently supported by the results of our experiments. The scope and structure-activity relationship has been demonstrated using a variety of aldehydes.
Twenty one tetraketones (2–22) were synthesized by condensing different aryl aldehydes with 5,5-dimethylcyclohexane-1,3-dione (1), in the presence of tetraethyl ammonium bromide (Et4NBr) and NH4Cl using water as solvent at room temperature, in high yields. The structures of the synthesized compounds were determined spectroscopically by 1H-NMR, EIMS, UV, IR and their purity was confirmed by CHN analysis.
Biology
All the synthesized compounds were subjected for their lipoxygenase inhibition and DPPH free radical scavenging assay, ability according to the developed methods described by Tappel [Citation14] and Gulcin et al. [Citation15], respectively, the results and shown in .
Table I. In vitro inhibition of lipoxygenase and antioxidant activities.
The product derived from benzaldehyde [2 (IC50 = 33.0 μM)] showed significant lipoxygenase inhibitory activity but the corresponding product derived from cinnamaldehyde [19 (IC50 = 7.8 μM)] showed the most pronounced activity which was even higher than baicalein (IC50 = 22.4 μM) used as positive control; thus the presence of a double bond in conjugation with an aromatic ring has a strong pharmacophoric effect. Among the substituted aromatic aldehydes, the target compounds derived from the electron donating alkyl, hydroxyl, methoxyl and amino groups at para-positions showed very promising lipoxygenase inhibitory potential [3 (IC50 = 16.3 μM), 6 (IC50 = 26.4 μM), 8 (IC50 = 21.3 μM), 9 (IC50 = 33.1 μM), 10 (IC50 = 30.8 μM) and 11 (IC50 = 17.5 μM)], while no or little effect was observed when these substituents were present at the ortho- and meta-positions. On the other hand, the electron withdrawing groups when attached to the aromatic ring, lowered the inhibitory activity [16–18 (IC50 = 191.1 μM, 00, 00)]. Replacement of the aromatic ring by a furan moiety decreased the inhibitory activity [21 (IC50 = 51.5 μM)], while the presence of an alkyl group at position-2 of the furan ring markedly increase the inhibitory activity [22 (IC50 = 12.5 μM)].
No clear trend was observed in the case of antioxidant activity. However, 19 was again found to be the most potent with an IC50 value of 33.6 μM, and was more potent than n-butyl-4-hydroxyanisole (IC50 = 44.7 μM) used as a positive control. Compounds 3 (IC50 = 52.5 μM), 6 (IC50 = 53.7 μM), 9 (IC50 = 57.2 μM), 14 (IC50 = 52.4 μM), 20 (IC50 = 58.4 μM) and 21 (IC50 = 49.4 μM) nevertheless showed significant antioxidant potential.
References
- Ren Z, Cao W, Tong W, Jing X. Knoevenagel condensation of aldehydes with cyclic active methylene compounds in water. Syn Commun 2002; 32: 1947–1952
- Horning EC, Horning MG. Methone derivatives of aldehydes. J Org Chem 1946; 11: 95–99
- Shanmugasundram P, Murugan P, Ramakrishnan VT, Srividya N, Ramamurthy P. Synthesis of acridinedione derivatives as laser dyes. Heteroatom Chem 1996; 7: 17–22
- Steinhilber D. 5-Lipoxygenase: A target for antiinflammatory drugs revisited. Curr Med Chem 1999; 6: 71–85
- Nie D, Honn KV. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell Mol Life Sci 2002; 59: 799–807
- Nikam SS, Kornberg BE. AMPA receptor antagonists. Curr Med Chem 2001; 8: 155–170
- Schneider I, Bucar F. Lipoxygenase inhibitors from natural plant sources. Phytother Res 2005; 19: 263–272, Part 2: Medicinal plants with inhibitory activity on arachidonate 12-lipoxygenase, 15-lipoxygenase and leukotriene receptor antagonists
- Vaya J, Aviram M. Nutritional antioxidants: Mechanism of action, analyses of activities and medical applications. Curr Med Chem-Imm Endoc and Metab Agents 2001; 1: 99–117
- Yildirim A, Oktay M, Bilaloglu V. The antioxidant activity of leaves of Cydonia vugaris. Turk J Med Sci 2001; 31: 23–27
- Mushtaq M, Sheikh AS, Jafari SA, Sheikh AS, Ahmad S, Mushtaq A. Reactive oxygen species in health and disease: A review. Pak J Biochem Mol Biol 2005; 38: 1–11
- Halliwel B. Free radicals, antioxidants and human disease: Curiosity, cause or consequence. The Lancet 1994; 344: 721–724
- Terao J, Piskula M, Yao O. Protective effect of epicatchin, epicatechin gallate and quercetin on lipid peroxidation in phospholipids bilayers. Arch Biochem Biophys 1994; 308: 278–284
- Fukushima IN, Hassegawa S, Shibata A, Ogiso T. Carcinogenity of butylated hydroxyanisole in F 344 rats. J Natl Cancer Inst 1983; 70: 343–347
- Tappel AL. Methods of enzymology. Academic Press, NewYork 1962; Vol. 5: 539–542
- Gulcin I, Alici HA, Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull 2005; 53: 281–285
- Riaz N, Malik A, Rehman AU, Ahmed Z, Muhammad P, Nawaz SA, Choudhary MI. Lipoxygenase inhibiting and antioxidant oligostilbene and monoterpene galactoside from Paeonia emodi. Phytochem 2004; 65: 1129–1135
- Rehman AU, Malik A, Riaz N, Ahmed Z, Ahmad H, Nawaz SA, Choudhary MI. Lipoxygenase inhibiting flavonoids from Indegofera hetrantha. Heterocycles 2004; 63: 359–366
- Rehman AU, Malik A, Riaz N, Nawaz HR, Ahmad H, Nawaz SA, Choudhary MI. Lipoxygenase inhibitory constituents from Periploca aphylla. J Nat Prod 2004; 67: 1450–1454
- Khan KM, Maharvi GM, Abbaskhan A, Hayat S, Khan MTH, Makhmoor T, Choudhary MI, Shaheen F, Atta-ur-Rahman. Three tyrosinase inhibitors and antioxidant compounds from Salsola foetida. Helv Chim Acta 2003; 86: 457–464
- Khan KM, Saifi ZS, Hayat S, Khan MZ, Noor F, Mukhmoor T, Choudhary MI, Ullah Z, Perveen S. Synthesis, antioxidant and insecticidal activities of coumarin derivatives. J Chem Soc Pak 2002; 24: 226–231