Abstract
A novel method has been developed for the synthesis of 6-aryl-1,2,4,5-tetrazinan-3-ones through a one-pot reaction of urea, various substituted aromatic benzaldehyde having electron donating and electron withdrawing groups and ammonium acetate in the presence of reusable NaHSO4.SiO2 heterogeneous catalyst in dry media under microwave irradiation. FT-IR, 1H NMR, D2O Exchange, 13C NMR, Heteronuclear Single Quantum Correlation (HSQC) spectra, MS and elemental analysis characterized all the synthesized compounds. In vitro antibacterial/fungal activities were evaluated for six new compounds. The antibacterial studies revealed that compounds 1–6 had better activity against tested Gram-positive and Gram-negative organisms. Compounds 1 and 5 were more active against β-Heamolytic streptococcus, a Gram-positive bacteria and Pseudomonas, a Gram-negative bacteria, respectively, than the standard drug ciprofloxacin. Besides, of all the compounds tested, compound 5 was more effective against Aspergillus flavus, a fungal strain than the standard drug fluconazole.
Introduction
The use of environmentally benign reaction media is very important in view of today's environmentally conscious attitude. As there is a need for “clean technology revolution”Citation1-5, there has been considerable interest in the microwave irradiation protocol for rapid synthesis of a variety of organic compounds due to the selective absorption of microwave energy by polar molecules. The use of heterogeneous catalysts, in particular NaHSO4.SiO2 Citation6-8, have made a landmark in different areas of organic synthesis due to their environmental compatibility combined with the good yield and selectivities that can be achieved.
A literature survey shows that 1,2,4,5-tetrazines represent an important class of heterocyclic compounds that find many practical and synthetic applications[Citation9], and are now under investigation for their effectiveness against cancer by the National Cancer Institute-USA [Citation10]. Numerous biological activities were reported for tetrazoloheterocycles, such as being useful with antiallergic [Citation11], antiulcer [Citation11], anti-inflammatory [Citation12], analgesic [Citation12], bronchodilating [Citation12], bactericidal [Citation12], hypotensive [Citation13] and pesticidal [Citation14] activities. In addition, some tetrazole derivatives, which have been introduced in the design of non-peptide ligands for GHS receptor [Citation15], show competitive inhibition potency for the carbapenem and cephamyci-resistant dinuclear zinc metallo-ß-lactamase from Bacteroides fragilis [Citation16], and treatment of type 2 diabetes [Citation17]. Such interesting results encouraged us to examine the following reaction. Since the formation of the N-N bond is relatively difficult, 1,2,4,5-tetrazines were generally prepared form hydrazine derivatives or from nitrilimines. A number of mono hydrazones of simple aldehydes and ketones with thiocarbohydrazide and 6-alkylhexahydro-1,2,4,5-tetrazine-3-thiones have been reported [Citation18]. In this report, only aliphatic aldehydes gave 1,2,4,5-tetrazines. But benzaldehyde gave only true monohydrazone with thiocarbohydrazide. In this sense, the present work describes ‘one-pot’ synthesis of novel 1,2,4,5-tetrazines for potential pharmacological activities, where NaHSO4.SiO2 heterogeneous catalyst plays an important role.
Results and discussion
Chemistry
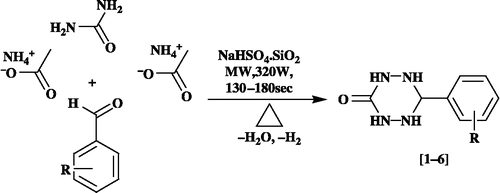
‘One-pot’ synthesis of 6-aryl-1,2,4,5-tetrazinan-3-ones (1–6) is obtained by the multicomponent cyclocondensation reaction of one mole of urea, one mole of substituted benzaldehyde and two moles of ammonium acetate in the presence of NaHSO4.SiO2 heterogeneous catalyst in dry media under microwave irradiation and thermal conditions (Scheme ).
Experimental
Chemistry
TLC was used to assess the reactions and the purity of the products. All the reported melting points were taken in open capillaries and were uncorrected. IR spectra were recorded in KBr (pellet forms) on a Nicolet-Avatar–360 FT-IR spectrophotometer and noteworthy absorption values (cm− 1) alone are listed. 1H and 13C NMR spectra were recorded at 400 MHz and 100 MHz respectively on a Bruker AMX 400 NMR spectrometer using DMSO as solvent. Heteronuclear Single Quantum Correlation (HSQC) Spectrum was recorded on a Bruker DRX 500 NMR spectrometer using standard parameters. The ESI + ve MS spectra were recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis were obtained on Carlo Erba 1106 CHN analyzer. A conventional (unmodified) domestic microwave oven equipped with a turntable (LG, MG-395 WA, 230V ∼ 50 Hz, 760 W) was used for the irradiation.
General procedure for the synthesis of 6-aryl-1,2,4,5-tetrazinan-3-ones catalyzed by NaHSO4.SiO2 under microwave irradiation (entries 1–6)
A mixture containing urea (10 mmol), substituted benzaldehyde (10 mmol), ammonium acetate (20 mmol) and NaHSO4.SiO2 (100 mg) was added to an alumina bath, mixed with the aid of a glass rod (10s) and then irradiated in a microwave oven for the appropriate period of time recorded in at 320 W (monitored by TLC). After completion of the reaction, the reaction mixture was extracted with ethyl acetate (3 × 5 mL). The catalyst was removed by filtration and re-used. The combined organic layer was washed with water three times and then dried over anhydrous MgSO4. The organic layer was concentrated in vacuo to furnish the products, which were purified by column chromatography using petroleum ether (40:60): ethyl acetate [3:7] as eluent.
Table I. Physical and analytical properties of 6-aryl-1,2,4,5-tetrazinan-3-ones (1–6).
6-phenyl-1,2,4,5-tetrazinan-3-one
IR (KBr) (cm− 1): 3444, 3212, 3061,2920, 1684, 1493, 1451, 697; 1H NMR (δ ppm): 2.77 [3.68] (t, 2H, H1&5), 6.82 [7.01] (s, 2H, H2&4), 5.39 ( J = 11.22 Hz) [4.99, ( J = 8.26 Hz)] (d, 1H, H6), 7.31–7.52 (m, 5H, Harom). 13C NMR (δ ppm): 68.7 [64.5] –C6, 156.2 [155.1] –C = O, 140.4 –ipso C, 127.4-128.3 –Carom. (The values in parentheses [], represent the minor isomer).
6-(4-chlorophenyl)-1,2,4,5-tetrazinan-3-one
IR (KBr) (cm− 1): 3386, 3220, 3068, 2965, 1596, 1486, 1450, 820; 1H NMR (δ ppm): 2.90 [3.12] (t, 2H, H1&5), 6.39 [6.57] (s, 2H, H2&4), 5.52 ( J = 11.21 Hz), [4.94 ( J = 8.42 Hz)] (d, 1H, H6), 7.60–7.76 (m, 4H, Harom). 13C NMR (δ ppm): 64.0 [67.3] –C6, 155.3 [156.1] –C–O, 132.4, 138.2 –ipso C, 128.2–129.3 –Carom.
6-(4-fluorophenyl)-1,2,4,5-tetrazinan-3-one
IR (KBr) (cm− 1): 3392, 3216, 3026, 2960, 1586, 1484, 1451, 780; 1H NMR (δ ppm): 2.98 [3.74] (t, 2H, H1&5), 6.85 [6.88] (s, 2H, H2&4), 5.36 ( J = 11.21 Hz), [4.95, ( J = 8.41 Hz)] (d, 1H, H6), 7.12–7.51 (m, 4H, Harom). 13C NMR (δ ppm): 67.8 [63.8] –C6, 156.1 [154.9] –C = O, 136.6, 162.8 –ipso C, 128.7–129.2 –Carom.
6-(4-methylphenyl)-1,2,4,5-tetrazinan-3-one
IR (KBr) (cm− 1): 3328, 3209, 3057, 2920, 1679, 1512, 1446, 813; 1H NMR (δ ppm): 2.28 (s, 3H, CH3), 2.49 [3.52] (t, 2H, H1&5), 6.88 [6.83] (s, 2H, H2&4), 4.92 ( J = 12.30 Hz), [5.40, ( J = 8.33 Hz)] (d, 1H, H6), 7.11–7.35 (m, 4H, Harom). 13C NMR (δ ppm): 20.5 –CH3, 64.2 [63.9] –C6, 155.0 [154.9] –C = O, 136.6, 138.6 –ipso C, 126.5–128.5 –Carom.
6-(4-methoxyphenyl)-1,2,4,5-tetrazinan-3-one
IR (KBr) (cm− 1): 3443, 3216, 3000, 2918, 1662, 1512, 1459, 834; 1H NMR (δ ppm): 3.87 (s, 3H, OCH3), 2.70 [3.87] (t, 2H, H1&5), 6.92 [6.86] (s, 2H, H2&4), 4.95 ( J = 12.02 Hz), [5.35, ( J = 8.35 Hz)] (d, 1H, H6), 7.12–7.33 (m, 4H, Harom). 13C NMR (δ ppm): 55.0 –OCH3, 63.9 [66.8] –C6, 157.9 [158.7] –C = O, 130.8, 160.3 –ipso C, 128.5–130.8 –Carom.
6-(3-nitrophenyl)-1,2,4,5-tetrazinan-3-one
IR (KBr) (cm− 1): 3445, 3213, 3061, 2916, 1689, 1493, 1453, 754; 1H NMR (δ ppm): 3.81 [4.18] (t, 2H, H1&5), 7.19 [7.31] (s, 2H, H2&4), 5.53 ( J = 10.05 Hz), [5.12 ( J = 8.47 Hz)] (d, 1H, H6), 7.53–7.89 (m, 9H, Harom). 13C NMR (δ ppm): 67.15 [66.85] –C6, 155.6 [154.6] –C = O, 134.1, 147.3 –ipso C, 121.8–129.8 –Carom.
Microbiology
Materials
All the bacterial strains namely Staphylococcus aureus, β-Haemolytic streptococcus, Vibreo cholerae, Salmonella typhii, Shigella felxneri, Escherichia coli, Klebsiella pneumonia, Pseudomonas and fungal strains namely Aspergillus flavus, Mucor, Rhizopus and Microsporum gypsuem were from the Faculty of Medicine, Annamalai University, Annamalainagar-608 002, Tamil Nadu, India.
In vitro antibacterial and antifungal activity
The in vitro activities of the compounds were tested in Sabourauds dextrose broth (SDB) (Hi-media, Mumbai) for fungi and nutrient broth (NB) (Hi-media, Mumbai) for bacteria by the Disc Diffusion method[Citation21]. The respective hydrochlorides of the test compounds 1–6 were dissolved in water to obtain 1 mg ml− 1 stock solution and different concentrations (100, 200, 500 ppm) were prepared from the stock solution. Seeded broth (broth containing microbial spores) was prepared in NB from 24 h old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C while fungal spores from 1 to 7 days old Sabourauds agar (Hi-media, Mumbai) slant cultures were suspended in SDB. A sterile paper disc of 5 mm diameter was saturated with three different concentrations of the compounds and placed in each seeded agar plate. The petri plates were incubated in a BOD incubator at 37°C for bacteria and at 28°C for fungi. The zone of inhibition was recorded by visual observations after 24 h of inhibition for bacteria and after 72–96 h of inhibition for fungi. The zone of inhibition was measured by excluding the diameter of the paper disc. Ciprofloxacin was used as standard for bacteria and fluconazole as standard for fungi under analogous conditions.
The reactions were performed at 320 W of MW power for 130–180 s (75°C, conventional heating using oil bath, 35–43 min.). After completion of the reaction as indicated by TLC, the reaction mixture was extracted with ethyl acetate, concentrated in vacuo and purified by column chromatography using petroleum ether (40:60): ethyl acetate [3:7] as eluent. The stoichiometry (1:2:1 urea: NH4OAc: benzaldehyde) of the reaction is also supported by the formation of novel 6-aryl-1,2,4,5-tetrazinan-3-ones (, entries 1–6).
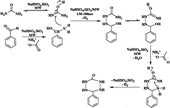
The structure of the 6-aryl-1,2,4,5-tetrazinan-3-ones was elucidated on the basis of their melting points, elemental analysis, MS, IR, 1H NMR, D2O exchange, 13C NMR and two dimensional NMR spectral study including Heteronuclear Single Quantum Correlation (HSQC) spectra. The probable mechanistic pathway is given in Scheme .
With the aim of achieving striking reduction in reaction times, better yields and cleaner reactions compared to the purely thermal processes, reactions were carried out under microwave irradiation at 320 W. This behaviour is consistent with the mechanistic considerations of the reactionCitation19-20. As they are connected to polarity of the medium, specific MW effects are expected if the transition state (TS) is more polar than the ground state (GS) of the reaction. Due to the dipole-dipole electrostatic interactions being more developed in the TS, the stabilization of the TS is superior to the GS, leading thus to a decrease in the activation energy. In the TS, the polarity of the system is enhanced as looser ion pairs are involved when compared to the GS.
The NaHSO4.SiO2 heterogeneous catalyst served as a good dehydrating agent to facilitate the removal of water under both microwave irradiation and thermal conditions for the formation of product. Washing easily regenerated the NaHSO4.SiO2 catalyst with ethyl acetate, followed by drying at 110°C for 1 h. It was shown that NaHSO4.SiO2 remained catalytic active for 6 runs and the yields were 75, 70, 60, 50, 40 and 30% respectively for 6-phenyl-1,2,4,5-tetrazinan-3-one (, entry-1). On the other hand, there was a significant decrease in its activity was observed after four uses since recycling led to loss of catalytic efficiency owing to absorption of organics. Organics on the solid surface result in the reduction of the number of active centers. Also NaHSO4 on the SiO2 was lost during washing. Organic species could be removed at higher temperature, so reactivating the catalyst.
Spectral analysis of 6-phenyl-1,2,4,5-tetrazinan-3-one (, entry 1)
The IR spectrum of 6-phenyl-1,2,4,5-tetrazinan-3-ones exhibits characteristic NH bands at 3444 cm− 1, in addition to amide (C = O) stretching at 1684 cm− 1. The band for the carbonyl group of aldehyde disappeared in the IR spectra of all compounds and the presence of weak bands at 1451 cm− 1 (N–N) are more evident for the formation of 6-phenyl-1,2,4,5-tetrazinan-3-ones.
The 1H NMR spectrum of 6-phenyl-1,2,4,5-tetrazinan-3-one shows the presence of two isomers, one major and one minor. For the major isomer a doublet at 5.39 ppm is due to benzylic proton. A triplet like signal observed at 2.77 ppm is due to NH protons adjacent to a benzylic carbon. A singlet at 6.82 ppm is due to imido protons. Aromatic protons appeared in the range of 7.31-7.58 ppm. The minor isomer signals are observed at 4.99, 3.68 and 7.01 ppm. The pattern of the spectrum is almost similar to that of the major isomer. Hence the signals are assigned to benzylic, imino and imido protons. The D2O exchange spectrum was also recorded to confirm the –NH proton signals. The signals 2.77, 3.68, 6.82, 7.01 ppm are exchanged with D2O.
The 13C NMR spectrum of 6-phenyl-1,2,4,5-tetrazinan-3-ones shows the presence of two isomers, one major and one minor. For the major isomer the signals observed at 68.7 ppm is due to benzylic carbon. The signal which appeared at 156.2 ppm is due to the presence of are amide carbonyl carbon. The ipso carbon appeared at 140.47 ppm. The aromatic carbons appeared in the region of 127.4–128.3 ppm. The minor isomer signals are observed at 64.54, 155.1 ppm. The pattern of the spectrum is almost similar to that of the major isomer. Hence the signals are assigned to benzylic and amide carbonyl carbons ().
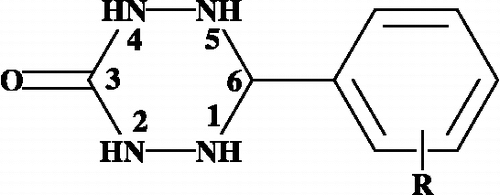
In order to confirm the assignment of signals, HSQC spectrum is also recorded for the 6-phenyl-1,2,4,5-tetrazinan-3-ones. The signals at 5.39 and 4.99 ppm show cross peaks with signals at 68.7 and 64.5 ppm. The above spectral analysis reveals that existences of two isomers for the 6-phenyl-1,2,4,5-tetrazinan-3-ones. Between the two isomers, one isomer with a phenyl ring is equatorial-like in the major isomer and a phenyl ring in an axial-like orientation is the minor isomer.
The existence of two isomeric structures of 6-phenyl-1,2,4,5-tetrazinan-3-ones is due to two different conformations in solution. Due to the nitrogen quadrapole effect, it is difficult to calculate the coupling constant of a proton attached to nitrogen atoms. The coupling constant of a proton attached to nitrogens and H6 is only measurable. One should expect a triplet or double-doublet for the H6 protons not the two separate doubles at 5.39 and 4.99 with coupling constants 11.22 and 8.26 Hz were seen. This clearly indicates that the proton at position-1 or-5 is coupled with the H6 proton. In , the phenyl group occupies an equatorial-like orientation, having a proton at position-5 coupled with the H6 proton, but the proton at position-1 does not couple with the H6 proton. In , the phenyl group occupies an axial-like orientation and the proton at position-5 does not couple but the proton at position-1 couples with the H6 proton. The existences of two conformations are also supported by the 13C NMR spectra.
Structure-activity relationship results
Antibacterial activity
All the synthesized novel target molecule 6-aryl-1,2,4,5-tetrazinan-3-ones (1–6) were tested for their antibacterial activity in vitro ( ) against aureus, β-H. streptococcus, V. cholerae, S. typhii, S. felxneri, E. coli, K. pneumonia and Pseudomonas. Ciprofloxacin was used as standard drug; whose zone of inhibition (mm) values for the bacteria were 25, 28, 23, 22, 23, 24, 26 and 23, respectively. In general all the synthesized novel 6-aryl-1,2,4,5-tetrazinan-3-ones (1–6) exerted a wide range of modest antibacterial activity in vitro against the tested organisms. But their activity decreased upon dilution. All the compounds 1–6 were active against S. aureus, V. cholerae, S. typhii, S. felxneri, E. coli and K. pneumonia. Compounds 1 and 5 wee more active against β-Haemolytic streptococcus and Pseudomonas respectively than the standard drug ciprofloxacin.
Table II. In vitro screening of compounds 1–3 against test bacteria and fungi.
Table III. In vitro screening of compounds 4–6 against test bacteria and fungi.
Antifungal activity
The in vitro antifungal activity ( ) of the synthesized novel 6-aryl-1,2,4,5-tetrazinan-3-ones (1–6) was studied against the fungal strains Aspergillus flavus, Mucor, Rhizopus and Microsporum gypsuem. Fluconazole was used as a standard drug whose zone of inhibition (mm) values for these fungi was 20 ± 0.5 mm. Generally all the compounds exerted a wide range of modest in vitro antifungal activity against all the tested organisms. But their activity decreased upon dilution. Moreover, of all the compounds tested, compound 5 was more effective against Aspergillus flavus, than the standard drug fluconazole.
Conclusion
This study shows that the nature of the substituent at position-4 on the phenyl ring viz., chloro, fluoro, methyl and methoxy, in 6-aryl-1,2,4,5-tetrazinan-3-ones is determinant for the nature and extent of the activity of the synthesized compounds. The method of action of these compounds is unknown. Compounds 1 and 5 are more active against β-Heamolytic streptococcus and Pseudomonas respectively than the standard drug, ciprofloxacin. Compound 5 is more effective against Aspergillus flavus, than the standard drug, fluconazole. The strong antibacterial and antifungal activity of these compounds against tested bacteria and fungi suggested further investigation for the design of better drugs to combat bacterial and fungal infection.
References
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V, Govindaraju R, Jayasri G. J Enzyme Inhibition Med Chem 2007;, (in press)
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J, Govindaraju R. Arkivoc 2006; 13: 130
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J, Govindaraju R, Ezhilarasi MR. Lett Org Chem 2006; 3: 484
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Catalysis Commun 2005; 6: 753
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Lett Org Chem 2005; 2: 444
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Prabhu C, Kanagarajan V. J Chem Res 2007, (in press). Gopalakrishnan, M.; Sureshkumar, P.; Kanagarajan, V.; Thanusu, J.; Govindaraju, R.J. Chem. Res. 2005, 5, 299
- Das NB, Ravindranath N, Venkataiah, Madhusudhan P. J Chem Res 2000; 482
- Das NB, Banerjee J. Chem Lett 2004; 8: 960
- Neunhoeffer H. Tetrazines- in comprehensive heterocyclic chemistry. AR Katritzky, CW Rees. Pergamon Press, London 1984; 3: 531
- Ferwanah ARS, Awadallah AM. Private Communication 2003.
- Bindhar JS. US Patent 4, 085 213. Chem Abstr 1978, 89, 197852
- Kottke K, Kuehmstedt H, Landmann, Wehlan H. Chem Abstr 1984; 100, 103388
- Liu KC, Hsu SW, Hu MK. Taiwan Yao Hseuh Tsa Chin 1986; 38: 242, (Chem. Abstr. 1988, 108, 94490)
- Bowie R, Cox JM, Farrel GM, Shephard MC. Offen Ger Patent 1976, 2, 539 396. (Chem. Abstr. 1976, 85, 5681)
- Lin P. Bioorg Med Chem Lett 1999; 9: 3237
- Toney J. Chem Biol 1998; 185
- Xu J. Bioorg Med Chem Lett 2005; 15: 2533
- Lamon RWJ. Org Chem 1969; 34: 756
- Perreux L, Loupy A, Delmotte M. Tetrahedron 2003; 59: 2185
- Perreux L, Loupy A. Tetrahedron 2001; 57, 9199
- Maruzella JC, Percival AH. J Am Pharm Assoc 1958; 47: 471