Abstract
The inhibitory activity of glucose-induced insulin secretion on isolated rat pancreatic islets and the contractile activity of KCl-depolarized rat aorta rings of the derivatives of 3-alkylamino-4H-1,2,4-benzothiadiazine 1,1-dioxide are quantitatively analyzed using multiple regression analysis. The study has helped to ascertain the role of different substituents in explaining these observed inhibitory activities. From a derived most significant correlation equation, it was concluded that a less hydrophobic 3-substituent and a less bulky 7-substituent in addition to a 3-aminoisopropyl and a 6-chloro substituent are advantageous to enhance the inhibitory action of a compound towards rat pancreatic islets. On the other hand, the more hydrophobic 6- and 7-substituents augment the contractile activity. The analysis, in this way, provided the grounds for rationalizing the substituent selection in designing the improved potency compounds in the series.
Introduction
ATP-sensitive potassium channels (KATP channels) regulate the flow of potassium ions through the cell membrane. These were identified in a wide range of cell types and are found to link the metabolic state to the electric state of the cell Citation1-8. KATP channels are composed of two different protein subunits in a 4 + 4 stoichiometry [Citation9]. The KATP channel pore belongs to the inwardly rectifying potassium channel family, which is known as Kir6.x [Citation10]. The second subunit, the sulfonylurea receptor (SUR) subunit, contains the regulatory sites for most drugs [Citation10]. Four variants of SUR, namely SUR1, SUR2A, SUR2B and SUR2C have been reported [Citation11]. KATP channels are composed of different subunits according to their tissue localization. For example, SUR1 combined with Kir6.2 forms the pancreatic KATP channels [Citation12]. The combination of SUR2A and Kir6.2 subunits is found in cardiac and skeletal muscle whereas the smooth muscle KATP channel is composed of SUR2B and Kir6.1 or Kir6.2 subunits [Citation13]. The pancreatic KATP channels are well-known to be involved in the insulin-releasing process [Citation14,Citation15] and smooth muscle KATP channels in the control of muscle tone [Citation16,Citation17], the physiological roles of the different channel subtypes have not yet been thoroughly assessed [Citation18,Citation19].
Several drugs, named as potassium channel openers (PCOs), have been found to activate KATP channels [Citation20,Citation21], leading to plasma membrane hyperpolarization and reduction in cell excitability. This, in turn, may provoke the relaxation of smooth muscles and/or the inhibition of endocrine release [Citation22,Citation23]. Due to their broad therapeutic potential, a large variety of KATP channel agonists has been developed [Citation24,Citation25] including chromane derivatives such as cromakalim [Citation26], cyanoguanidine compounds such as pinacidil [Citation27] and 1,2,4-benzothiadiazine derivatives such as diazoxide [Citation28]. Selective activation of pancreatic KATP channels has been demonstrated to be of clinical value in the treatment of several metabolic disorders, including type I and type II diabetes, obesity and hyperinsulinemia Citation29-32. Until recently, diazoxide was the only reported compound to activate pancreatic KATP channels, but as a consequence of lack of tissue selectivity, it induces many side effects such as hypertrichosis, edema, headache and hypertension [Citation33].
In the search for new pancreatic- selective PCOs, a series comprising of 3-alkylamino-4H-pyrido- and − 1,2,4-benzothiadiazine 1,1-dioxides has been developed; among them BPDZ 44 [Citation34], BPDZ 73 [Citation35], BPDZ 138 [Citation36] and BPDZ 216 [Citation37] were identified as the first potent and selective pancreatic KATP channel openers.
Recently, a series of 3-alkylamino-4H-1,2,4-benzothiadiazine 1,1-dioxides was reported [Citation38] and were tested as putative KATP channel openers on a vascular and a pancreatic pharmacological model in order to evaluate their potency and tissue selectivity. The initial structure-activity relationship (SAR) study on these compounds was, however, directed only to alteration of the substituents at different positions of the structure but no rationale was provided to reduce the trial-and-error factors. Hence, a quantitative SAR (QSAR), on these analogues was conducted since QSAR not only provides the rationale for drug design but also illuminates their possible mechanism of action at the molecular level.
Materials and methods
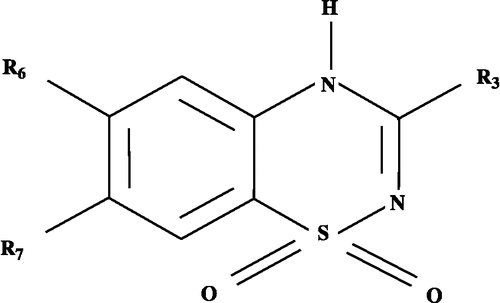
The reported series [Citation38] consists of substituted 4H-1,2,4-benzothiadiazine 1,1-dioxides bearing in most cases, a short alkylamino side chain in the 3-position ().These compounds along with their activity values for rat pancreatic islets and rat aorta rings are compiled in . The activity, IC50, of a compound represents its ability to inhibit glucose-induced insulin secretion and was evaluated on isolated rat pancreatic islets. The EC50, on the other hand, represents the myorelaxant effect on the contractile activity of KCl-depolarized rat aorta rings. For the present work, these are expressed as pIC50 and pEC50 on a molar basis. The most appropriate quantifying parameters are also listed in this Table. The physicochemical parameter, the hydrophobicity, π, is taken from the literature [Citation39] and the van der Waals volume for a given substituent was calculated according to the method discussed in one of our earlier publications [Citation40]. The ClogP values for the substituents at R3 were calculated from Chemdraw software, following the default description of substituents. For the present work the same is, therefore, designated as ClogP(R3) to represent the descriptor as a substituent property. Additionally, indicator variables were also employed to reflect upon some special structural features of a compound. The subscripted numerals following these variables are indicative of the varying positions in the title compounds. The multiple regression analysis (MRA), employing the method of least squares, was used to derive significant correlations for further discussion. In addition to this, the final QSAR equations were subjected to a validation test [Citation42] by the leave-one-out (LOO) method to derive the cross-validation index, q2. For a statistical robust QSAR model, the internally validated q2 index should have a value between 0.6 and 0.9 [Citation43].
Table I. QSAR parameters and inhibitory and contractile activity of substituted 4H-1,2,4-benzothiadiazine 1,1-dioxides (See Figure1 for structure).
Results and discussion
lists the compounds where the alteration in substituents occurred at different positions of the diazoxide scaffold. To account for the effects produced by such substituents, a large number of descriptors related to hydrophobic, electronic and steric interactions were initially examined for the varying positions in various possible permutations. The selected parameters for each of these positions were hydrophobicity, π or ClogP, hydrogen-bond acceptor, HA, molar refraction, MR, electronic (para and meta), σ, field, F, resonance, R, Taft's steric, Es, molecular weight, MW and van der Waals volume, Vw. The step-wise regression analysis was followed to derive QSAR equations. A large number of equations so obtained were then subjected to different statistical tests. The correlation equations, which returned the highest correlation coefficient, r and F-statistic and lowest standard deviation, s, were finally retained for further discussion. The significant correlation, derived in the most appropriate quantifying parameters is shown in Equation (1)
As given above, n is the number of data points, F-statistic is the F-ratio between the variances of calculated and observed activities, and the ± data within the parentheses are the 90% confidence intervals. The arbitrarily chosen indicator variables, I3 and I6, stand to account, respectively for an aminoisopropyl substituent at the 3-position and a chloro substituent at the 6-position. Thus a value either 1 or 0 for I3, in that order, indicates the presence or absence of an aminoisopropyl substituent at the 3-position of diazoxide scaffold. Likewise, I6 = 1 or 0 indicates, respectively, the presence or absence of a 6-chloro substituent.
From Equation (1), it appears that the 3-substituents are engaged in a hydrophobic interaction while the 7-substituents are involved in a steric/polar interaction. In addition, the presence of the 3-aminoisopropyl and 6-chloro substituents are prerequisite for inhibitory action. The statistical parameters of the above equation, however, do not represent a sound model as the r2 value accounts for 65% of the variance and q2 is below the specified level of significance, though the F-value remained significant at 99% [F4,19(0.01) = 4.500] level. These observations merely reflect upon the parametric requirements of the substituents in a compound that may lead to agonistic activity for KATP channel. In order to improve upon the significance levels of Equation (1), all data points in , were further analyzed for their deviation from a regular trend. The lone compound 5 (), having a 3-NH-cPr substituent, showed unusual behavior. At present, no plausible explanation could be assigned for such an abnormality. After removal of this compound the QSAR analysis, through successive steps (), has revealed correlation Equation (2)
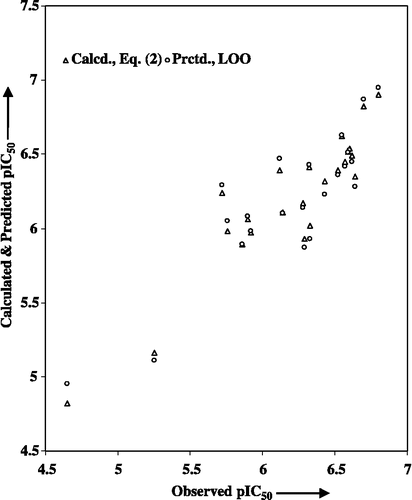
Now both the r- and F-values were increased to account for 85% (r2 = 0.846) of variance in the observed activities and 99% level of significance [F4,18(0.01) = 4.579], respectively. Also, the s-value and 90% confidence intervals ( ± data within parentheses) associated with regression coefficients were significantly lowered. Additionally, the higher value obtained for q2 expressed a reasonable QSAR model. That the variables used in deriving Equation (2) had no mutual correlation is shown in . The calculated activity values, using this equation and listed in , are in close agreement with the observed ones. The predicted activity values, using Equation (2), are also listed in this Table for the sake of comparison. The plot of observed versus calculated and predicted pIC50 values, is shown in . Such a plot is useful to understand the goodness of fit and to identify the systematic trend. From Equation (2), it appeared that a less hydrophobic 3-substituent and a less bulky 7-substituent are advantageous to improve the pIC50 value. In addition, the presence of 3-aminoisopropyl and 6-chloro substituents are favorable to enhance the activity.
Table II. Stepwise development of Equation (2) pIC50(pancreas) =a0 +a1ClogP(R3)+a2Vw7 +a3 I3 +a4 I6
Table III. Intercorrelation matrixa amongst independent variables of Equation (2).
The myorelaxant effects, reported in terms of the contractile activity of KCl-depolarized rat aorta rings, of these diazoxides were also correlated with quantifying parameters. The derived correlation for the same, is shown in Equation (3)
This equation analyzes the importance of 6- and 7-substituents while the 3-substituents remained silent. Compound 6 and 19 could not fit into the model and are ignored to derive an improved QSAR Equation (4) compared to Equation (3)
All the statistical parameters, including 90% confidence intervals, of this equation have significantly improved over that of Equation (3). The r-value now accounts for 81% of the variance and the s-value is lowered. In addition, the F value remained significant at 99% level, and the q2 index, explaining a satisfactory statistical model, are both increased. The calculated pEC50 values using Equation (4), and predicted pEC50 values, using the LOO method, listed in , are in close agreement with the observed ones. The independent variables of this equation fulfill the mutual orthogonality condition (π6 vs π7 = 0.071). From Equation (4), it appears that more hydrophobic substituents present at 6- and 7-positions augment activity.
The conclusions deduced from Equations (2) and (4) may be used as guidelines to obtain more potent compounds in the further synthesis of similar compounds.
Acknowledgements
We are grateful to the Department of Chemistry, S. K. Government College and Department of Engineering Chemistry, Sobhasaria Engineering College for providing the facilities to complete this work.
References
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 1983; 305: 147–148
- Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B- cells. Nature 1984; 311: 271–273
- Bernardi H, Fosset AM, Lazdunski M. Characterization, purification and affinity labeling of the brain[3H]glibendamide-binding protein, a putative neuronal ATP- regulated K+ channel. Proc Natl Acad Sci USA 1988; 85: 9816–9820
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodialaters activate ATP-sensitive K+ channels in arterial smooth muscle. Science 1989; 245: 177–180
- Allard B, Lazdunski M. Pharmacological properties of ATP-sensitive K+ channels in mammalian skeletal muscle cells. Eur J Pharmacol 1993; 236: 419–426
- Quayle JM, Nelson MT, Standen NB. ATP sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 1997; 77: 1165–1232
- Bryan J, Aguilar-Bryan L. The ABCs of ATP-sensitive potassium channels: More pieces of the puzzle. Curr Opin Cell Biol 1997; 9: 553–559
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 2003; 81: 133–176
- Babenko AP, Aguilar-Bryan JA. A view of SUR/Kir6.X KATP channels. Annu Rev Physiol 1998; 60: 667–687
- D'hahan N, Jacquet H, Moreau C, Catty P, Vivaudou M. A transmembrane domain of the sulfonylurea receptor mediates activation of ATP-sensitive K+ channels by K+ channel openers. Mol Pharmacol 1999; 56: 308–315
- Seino S. ATP-sensitive potassium channels: A model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol 1999; 61: 337–362
- Inagaki N, Gonio T, Clement JP. Reconstitution of IKATP: An inward rectifier subunit plus a sulfonylurea receptor. Science 1995; 270: 1166–1170
- Hambrock A, Löfter-Walz C, Delabar U, Horio Y, Kurachi Y, Quast U. ATP- sensitive K+ channel modulator binding to sulfonylurea receptor SUR2A and SUR2B: Opposite effects of MgADP. Mol Pharmacol 1999; 55: 832–840
- Petersen OH, Dunne MJ. Regulation of K+ channels plays a crucial role in the control of insulin secretion. Pflueger's Arch 1989; 414: S115–S120
- Lebrun P. Cationic flux in B-cells from pancreatic islets and pharmacological investigations. Rev Fr Endocrinol Clin Nutr Metab 1993; 34: 241–254
- Kolb HA. Potassium channels in excitable and non-excitable cells. Rev Physiol Biochem Pharmacol 1990; 15: 51–79
- Brayden JE. Functional roles of KATP channels in vascular smooth muscles. Clin Exp Pharmacol Physiol 2002; 29: 312–316
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 2003; 81: 133–176
- Cotzee WA. ATP-sensitive potassium channels and myocardial ischemia: Why do they open?. Cardiovasc 1992; 6: 201–208
- Mannhold R. KATP channel openers: Structure-activity relationships and therapeutic potential. Med Res Rev 2004; 24: 213–266
- Coghlan MJ, Carroll WA, Gopalakrishnan M. Recent development in the biology and medicinal chemistry of potassium channel modulators: Update from a decade progress. J Med Chem 2001; 44: 1627–1653
- Lebrun P, Devreux V, Hermann M, Herchuelz A. Similarities between the effects of pinacidil and diazoxide on ionic and secretory events in rat pancreatic islets. J Pharmacol Exp Ther 1989; 250: 1011–1018
- Quast U. Do the K+ channel openers relax smooth muscle by opening K+ channels?. Trends Pharmacol Sci 1993; 14: 332–337
- Atwal KS. Advances in the structure-activity relationships, mechanism of action, and therapeutic utilities of ATP-sensitive potassium channel openers. Drugs Dev Res 1994; 33: 250–262
- Gribble FM, Reimann F. Pharmacological modulation of K(ATP) channels. Biochem Soc Trans 2002; 30: 333–339
- Sebille S, de Tullio P, Boverie S, Antoine M-H, Lebrun P, Pirotte B. Recent development in the chemistry of potassium channel activators: The cromakalim analogues. Curr Med Chem 2004; 11: 1213–1222
- Manley PW, Quast U. Structure-activity studies of potassium channel opening in pinacidil-types cyanoguanidines, nitroethenediamines, thioureas, and ureas. J Med Chem 1992; 35: 2327–2340
- Pirotte B, Fontaine J, Lebrun P. Recent advances in the chemistry of potassium channel openers. Curr Med Chem 1995; 2: 537–582
- Björk E, Berne C, Kämpe O, Wibell P, Oskarsson P, Karlsson FA. Diazoxide treatment at onset preserves residual insulin secretion in adults with autoimmune diabetes. Diabetes 1996; 45: 1427–1430
- Alemzadeh R, Langley G, Upchurch L, Smith P, Slonim AE. Beneficial effect of diazoxide in obese hyperinsulinemic adults. J Clin Endocrinol Metab 1998; 83: 1911–1915
- Rasmussen SB, Sorensen TS, Hansen JB, Mandrup-Poulsen T, Hornum L, Markholst H. Functional rest through intensive treatment with insulin and potassium channel openers preserves residual beta-cells function and mass in acutely diabetic BB rats. Horm Metab Res 2000; 32: 294–300
- Cosgrove K, Antoine M-H, Lee A, Barnes P, de Tullio P, Clayton P, McCloy P, De Lonlay P, Nihoul-Féekéeté C, Robert J, Saudubray J-M, Rahier J, Lindley K, Hussain K, Aynsley-Green A, Pirotte B, Lebrun P, Dunne M. BPDZ 154 activates adenosine 5’-triphosphate-sensitive potassium channels: in vitro studies using rodent insulin-secreting cells and islets isolated from patients with hyperinsulinism. J Clin Endocrinol Metab 2002; 87: 4860–4868
- Kumar GK, Dastoor FC, Robayo JR, Razzaque MA. Side effects of diazoxide. J Am Med Assoc 1976; 235: 275–276
- Pirotte B, Antoine M-H, de Tullio P, Hermann M, Herchuelz A, Delange J, Lebrun P. A pyridothiadiazine(BPDZ 44) as a new and potent activator of ATP-sensitive K+ channels. Biochem Pharmacol 1994; 47: 1381–1386
- Lebrun P, Arkhammar P, Antoine M-H, Nguyen Q-A, Bondo Hansen J, Pirotte B. A potent diazoxide analogue activating ATP-sensitive K+ channels and inhibiting insulin release. Diabetologia 2000; 43: 723–732
- de Tullio P, Becker B, Boverie S, Dabrowski M, Wahl P, Antoine M-H, Somers F, Sebille S, Ouedraogo R, Bondo Hansen J, Lebrun P, Pirotte P. Toward tissue- selective pancreatic B-cells KATP channel openers belonging to 3-alkylamino-7-halo-4H-1,2,4-benzothiadiazine 1,1-dioxides. J Med Chem 2003; 46: 3342–3353
- Dabrowski M, Ashcroft FM, Ashfield R, Lebrun P, Pirotte B, Egebjerk J, Hansen JB, Wahl P. The novel diazoxide analog 3-isopropylamino-7-methoxy-4H-1,2,4-benzothiadiazine 1,1-dioxide is a selective Kir6.2/SUR1 channel opener. Diabetes 2002; 51: 1896–1906
- de Tullio P, Boverie S, Becker B, Antoine M-H, Nguyen Q-A, Francotte P, Counerotte S, Sebille S, Pirotte B, Lebrun P. 3-Alkylamino-4H-1,2,4-benzothiadiazine 1,1-dioxides as ATP-sensitive potassium channel openers: Effect of 6,7-disubstitution on potency and tissue selectivity. J Med Chem 2005; 48: 4990–5000
- Hansch C, Leo A. Substituents constants for correlation analysis in chemistry and biology. John Wiley, New York 1979
- ChemDraw Ultra 6.0 and Chem3D Ultra., Cambridge Soft Corporation, Cambridge,USA.
- Gupta SP, Bhatanagar RP, Singh P, Bindal MC. The relationship of cellular respiration inhibition activity of 7-substituted-4-hydroxyquinoline-3-carboxylic acids with van der Waals volume. Res Commun Chem Pathol Pharmacol 1979; 25: 441–451
- Wold S. Validation of QSAR's. Quant Struct-Act Relat 1991; 10: 191–193
- Eriksson L, Jaworska J, Worth AP, Cronin Mark TD, Mc Dowell RM, Gramatica P. Methods for reliability and uncertainty assessment and for applicability evaluation of classification- and regression-based QSARs. Environ Health Persp 2003; 111: 1361–1375