Abstract
The crude methanolic extract and subsequent fractions of Teucrium royleanum (Labiatea) were screened for antibacterial and antifungal activities. Against tested pathogens, crude extract and subsequent fractions demonstrated moderate to excellent antibacterial activities. Highest antibacterial activity was displayed by the ethyl acetate fraction against S. typhi (100%), against E.coli (76.7%) and against P. aerugenosa (70.8%) followed by the chloroform fraction against S. typhi (85.7%). Similarly, the crude extract and its subsequent fractions showed mild to excellent activities in the antifungal bioassay with maximum antifungal activity against M. canis (87%) by the chloroform fraction followed by the ethyl acetate (71%) and n-butanol (70%) fractions.
Introduction
The recognition of verocytotoxin (VT)-producing Escherichia coli (VTEC) as etiological agents of diarrhoea represents one of the most important and exciting recent advances in the field of enteric infections. Not only do VTEC rival non-typhoidal Salmonellae and Ampylobacter species as the most frequent causes of diarrhoea in some geographical settings [Citation1,Citation2], but a significant risk of two life-threatening complications, hemorrhagic colitis and the hemolytic uremic syndrome (HUS), makes VTEC infection a public health problem of serious concern.
Pseudomonas aeruginosa is an opportunistic pathogen. It rarely causes disease in healthy persons. In most cases of infection, the integrity of a physical barrier to infection (e.g., skin, mucous membrane) is lost or an underlying immune deficiency (e.g., neutropenia, immunosuppression) is present. Adding to its pathogenicity, this bacterium has minimal nutritional requirements and can tolerate a wide variety of physical conditions. The pathogenesis of pseudomonal infections is multifactorial and complex. Pseudomonas is both invasive and toxigenic and causes respiratory tract, CNS, otitis media, eye infections, bone and joints infections, GIT, UTI, endocarditis and skin infections.
Salmonella typhi is a flagellated, gram-negative bacillus belonging to the family Enterobacteriaceae responsible for typhoid fever, which is a prolonged bacteraemic, systemic illness with minimal, at least initially, diarrhoea [Citation7]. Bacteria of the genus of Salmonella can produce a wide range of infections in humans, including gastroenteritis, typhoid fever, bacteraemia and localized infections, other than an asymptomatic carrier state. [Citation8,Citation9]. Multidrug-resistant (MDR) S. typhi (resistant to chloramphenicol, ampicillin, and trimethoprim-sulphamethoxazole) and isolates with reduced susceptibility to fluoroquinolones (indicated by resistance to nalidixic acid) are known. Alternative synthetic drugs are reasonably effective but quite expensive and have many side effects [Citation10,Citation11].
Plant background
Species of the Teucrium genus are bitter, astringent, antirheumatic herbs that reduce inflammation, stimulate the digestion and have been used as herbal medicines for coughs and asthma since ancient times. Several studies concerning bacteriostatic, spasmolytic, antioxidant, antinflammatory and antifungal activity of Teucrium species have been reported in the literature Citation12-15. As a folk medicine, Teucrium royleanum is used as an antispasmodic, astringent, antipyretic, and for skin rashes [Citation16]. The constituents of Teucrium montanum are montanins A, B, C and D, teucrins A and E from Teucrium chamaedrys, 6-ketoteuscordin and teuscordinin from Teucrium scordium [Citation17] and from Teucrium fruticans, neo-clerodanes, namely 11-hydroxyfruticolone, deacetylfruticolone and 6- acetyl-10- hydroxyteucjaponin B [Citation18].
Interest in the phytochemical exploration of Teucrium royleanum began in 1992 when Mashooda Hassan isolated triterpenoids, aliphatic, steroidal and acidic compounds, a mixture of triaocontane and hentriacontane, lupeol and β-amyrin, hexacosianic acid and stigmastatrien-3-ol [Citation19]. The plant extract displayed outstanding inhibition on acetylcholinesterase and butyrylcholinesterase [Citation21]. Keeping in mind its uses as folk medicine, though deserved, this plant has not been extensively subjected to phytochemical and pharmacological investigation. The present study is the continuation of our previous work [Citation22,Citation23].
Methods and materials
Plant material
Teucrium royleanum (Labiatea) as the whole plant was collected from Dir district, N.W.F.P (Pakistan) in June-August 2004 and identified by Professor Dr. Jehandar Shah, Plant Taxonomist, Vice Chancellor University of Malakand, Chakdara Dir. A voucher specimen (CA-013) has been deposited in the herbarium of the University of Malakand.
Extraction
The shade dried plant material was chopped into small pieces and finally pulverized into a fine powder. The plant material (12 kg) was soaked in methanol with occasional shaking, at room temperature. After 15 days, the methanol-soluble materials were filtered off. The filtrate was concentrated under vacuum at low temperature (40°C) using a rotary evaporator when a blackish crude extract (350 g) was obtained.
Fractionation
The crude methanolic extract (350 g) was suspended in distilled water (500 mL) and sequentially partitioned with n-hexane (3 × 500 mL), chloroform (3 × 500 mL), ethyl acetate (3 × 500 mL) and n-butanol (3 × 500 mL) to yield n- hexane (41 g), chloroform (83 g), ethyl acetate (88 g), n-butanol (54 g) and aqueous (67 g) fractions, respectively.
Antibacterial activity
The crude extract along with fractions were screened against various human pathogens including Escherichia coli, Bacillus subtilis, Klebsiella pneumonae, Shigella flexenari, Staphylococcus aureous, P. aeruginosa and S. typhi by the agar well diffusion method [Citation20]. In this method, 10 mL aliquots of nutrients broth (Sigma-Aldrich, Germany) were inoculated with the test organism and incubated at 37°C for 24 h. Using a sterile pipette, 0.6 mL of the broth culture of the test organism was added to 60 mL of molten agar, which had been cooled to 45°C, mixed well and poured into a sterile Petri dish (for the 9 cm Petri dish, 0.2 mL of the culture was added to 20 mL of agar). Duplicate plates of each organism were prepared. The agar was allowed to set and harden and the required number of wells were dug in the medium with the help of a sterile metallic cork borer ensuring proper distribution of the wells in the periphery and one in the center. Agar plugs were removed. Stock solutions of the test samples at a concentration of 1 mg/mL were prepared in sterile dimethyl sulfoxide (DMSO) and 100 μL and 200 μL of each dilution was added in their respective wells. The control well received only 100 μL and 200 μL of DMSO. Imipinem was used as standard drug. The plates were left at room temperature for 2hr to allow diffusion of samples then incubated face upwards at 37°C for 24 h. The diameter of the zones of inhibition was measured to the nearest mm (the well size also being noted).
Antifungal activity
Similarly the antifungal activity was evaluated by the agar tube dilution method [Citation20]. The samples at a concentration of 24 mg/mL were dissolved in sterile (autoclaved) dimethyl sulfoxide (DMSO, Merck), which served as stock solution. Sabouraud dextrose agar (SDA, Sigma-Aldrich, Germany) was prepared by mixing 32.5 g sabouraud, 4% glucose agar and 4.0 g of agar-agar in 500 mL distilled water and the mixture was mixed thoroughly with a magnetic stirrer. Then 4 ml aliquots were dispensed into screw cap tubes, which were autoclaved at 120°C for 15 min and then cooled to 15°C. The non-solidified SDA media was mixed with stock solution (66.6 μL) giving a final concentration of 400 μg of the extract per ml of SDA. Tubes were then allowed to solidify in the slanted position at room temperature. Each tube was inoculated with a piece (4 mm diameter) of inoculums removed from a seven days old culture of fungi for non-mycelial growth; an agar surface streak was employed. Other media supplemented with dimethyl sulfoxide (DMSO) and reference anti-fungal drugs served as negative and positive control respectively. Inhibition of fungal growth was observed visually after 7-days of incubation at 28 ± 1°C. Humidity (40-50%) was controlled by placing an open pan of water in the incubator.
Results and discusion
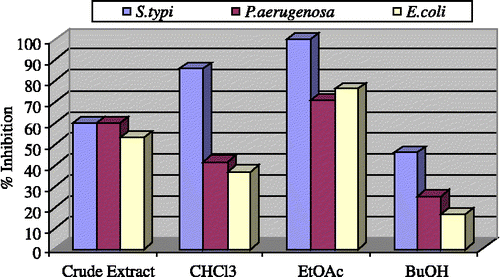
As shown in the crude extract displayed moderate activity against E.coli (53%), both P. aeruginosa and S. typhi showed good activity (60%), S. aureous (27.2%) and B. subtilis, (39.3%). No activity was displayed against S. flexenari.
Table I. Anti bacterial activities of crude extract & various fractions of Teucrium Royleanum.
The chloroform fraction showed excellent activity against S. typhi (85.7%) and low activity against P. aeruginosa (41.6%), E. coli (36.6%) and B. subtilis (12.1%). No activity was shown against S. aureous and S. flexenari. The ethyl acetate fraction showed outstanding activity against S. typhi (100%) and excellent activity against P. aeruginosa (70.8%), E. coli (76.6%) and low activity against B. subtilis (42.4%) No activity was displayed against S. aureous and S. flexenari. While the n-butanol fraction showed low activity against P. aeruginosa (25%), E. coli (16.6%) and S. typhi (48.8%), against S. aurous, B. subtilis, S. flexenari it exhibited no activity.
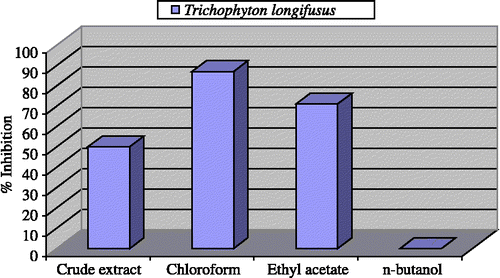
Anti-fungal activities of the crude extract and various fractions of the Teucrium royleanum were evaluated for activity against Trichophyton longifusus, Candida albicans, Aspergilusflavus, Microsporum canis, Fusarium solani, and Candida glaberata, in comparison with miconazole and amphotericin-B, The results of the antifungal activities are presented in .
Table II. Antifungal activities of crude extract & various fractions of teucrium royleanum.
The crude extract displayed moderate antifungal activity against T. longifusus (50%) and low activity against M. canis (40%). While the crude extract showed no activity against, F. solani, C. albicans, A. flavus and C. glaberata.
The chloroform fraction showed an excellent result against T. longifusus (87%), while against M. canis it showed moderate activity (53%) but no activity against C. albicans, F. solani, A. flavus, C. albicans, A. flavus and C. glaberata ().
Figure 1 Antibacterial activity of the crude extract and fractions of Teucrium royleanum at 3 mg/mL.
The ethyl acetate fraction also showed excellent antifungal activity against T. longifusus (71%) and low activity against M. canis (40%), but no activity against A. flavus, C. glabera, C. albicans and F. solani. The n-butanol fraction was found inactive against T. longifusus, M. canis, A. flavus, C. glabera, C. albicans and F. solani.
As presented in , the chloroform and ethyl acetate fractions of Teucrium royleanum showed an outstanding activity against S. typhi (100%), which is a facultative intracellular pathogen that causes diseases ranging from self-limiting enteritis to typhoid fever. Development of new effective and safe products for the treatment of typhoid fever urgently needed. Therefore, this plant species could be an excellent natural source for the treatment of typhoid fever and a potential target for the activity-guided isolation of active constituents in order to explore the mechanism of action and relevant uses in the indigenous system of medicine.
References
- Jiang ZD, Okhuysen PC, Guo DC, He R, King TM, DuPont HL, Milewicz DM. Genetic susceptibility to enteroaggregative Escherichia coli diarrhea: Polymorphism in the interleukin-8 promotor region. J Infect Dis 2003; 188: 506–511
- Moyenuddin M, Rahman KM. Enteropathogenic Escherichia coli diarrhea in hospitalized children in Bangladesh. J Clin Microbiol 1985; 22: 838–840
- Robson WL, Leung AK, Miller-Hughes DJ. Recurrent hemorrhagic colitis caused by Escherichia coli O157:H7. Pediatr Infect Dis J 1993; 12: 699–701
- Abuqaddom AI, Darwish RM, Muti H. The effects of some formulation factors used in ophthalmic preparations on thiomersal activity against Pseudomonas aeruginosa and Staphylococcus aureus. J Appl Microbiol 2003; 95: 250–255
- Chamot E, Boffi El, Amari E, Rohner P, Van Delden C. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 2003; 47: 2756–2764
- Klibanov OM, Raasch RH, Rublein JC:. Single versus combined antibiotic therapy for gram-negative infections. Ann Pharmacother 2004; 38: 332–337
- Marzano AV, Mercogliano M, Borghi A, Facchetti M, Caputo R. Cutaneous infection caused by Salmonella typhi. European Academy of Dermatology and Venereology 2003; 17: 575–577
- Vellodi C. Subcutaneous salmonella abscesses, an unusual manifestation of salmonellosis. J Roy Soc Med 1990; 83: 190
- Molina M, Ortega G, Pérez Garcia A, Pretel L. Cutaneous abscesses caused by Salmonella. Enferm Infecc Microbiol Clinic 1990; 8: 259–260
- Parry CM. The treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever in viet nam. Trans Roy Soc Trop Med and Hyg 2004; 98: 413–422
- Cooke FJ, Wain J. The emergence of antibiotic resistance in typhoid fever. Travel Med and Infect Dis 2004; 2: 67–74
- Yang HO, Suh D, Han B, H. Isolation and characterization of platelet-activating factor receptor binding antagonists from Biota orientalis. Planta Medica 1995; 61: 37
- Harborne JB, Valdes B. Identification of scutellarein 4′-methyl ether in Linaria aeruginea. Phytochemistry 1971; 10: 2850
- Youssef D, Frahm A, W. Constituents of the egyptian Centaurea scoparia; III. Phenolic constituents of the aerial parts. Planta Medica 1995; 61: 570
- Tourandokht Baluchnejadmojarada. Tourandokht baluchnejadmojarada, mehrdad roghanib, farshad roghani- dehkordic. Journal of Ethnopharmacology 2005; 97: 207–210
- Kirtikar, Basu. Indian Medicinal Plant, Vol. 8 2000. p 2696
- Eszter Gacs, Kajtar Marton. Hetrocycles 1982; 19: 3
- Coll Josep, Tandron Yudelsy. Isolation and structure elucidation of three neo-clerodane diterpenes from Teucrium Fruticans L. (Labiatae). Phytochemistry 2005; 66: 2298–2303
- Mashooda Hasan M, Ramzan MariaC, Alvarez Garcia, Uddin Ahmad Viqar. Constituents of Teucrium royleanum. Fitoterapia 1993; 64: 555
- Atta-ur-Rehman ChoudharyMI, Thomsen. Manual of bioassay techniques for natural product research. Harward Academic Press, Amsterdam 1991; 82–84
- Ahmad B, Hassan Shah SM, Bashir Shumaila, Khan Haroon, Shah Jehandar. Enzyme inhibition activities of Andrachine cordifolia. J Enz Inhib Med Chem 2007; 22(2)235–238
- Ahmad B, Khan H, Bashir S. Inhibition activities of Colchicum luteum Baker on lipoxygenase and other enzymes. J Enz Inhib Med Chem 2006; 21(4)449–452
- Ahmad B, Khan H, Bashir S, Ali M. Antimicrobial bioassay of Colchicum luteum. J Enz Inhib Med Chem 2006; 21(6)765–769