Abstract
In the present study we have evaluated the antigenotoxic effects of Farnesol (FL) a 15-carbon isoprenoid alcohol against benzo (a) pyrene [B(a)P] (125 mg kg− 1.b.wt oral) induced toxicity. B(a)P administration lead to significant induction in Cytochrome P450 (CYP) content and aryl hydrocarbon hydrolase (AHH) activity (p < 0.001), DNA strand breaks and DNA adducts (p < 0.001) formation. FL was shown to suppress the activities of both CYP and AHH (p < 0.005) in modulator groups. FL pretreatment significantly (p < 0.001) restored depleted levels of reduced glutathione (GSH), quinone reductase (QR) and glutathione –S-transferase (GST). A simultaneous significant and at both the doses reduction was seen in DNA strand breaks and in in-vivo DNA adducts formation (p < 0.005), which gives some insight on restoration of DNA integrity. The results support the protective nature of FL. Hence present data supports FL as a future drug to preclude B (a) P induced toxicity.
Introduction:
Experimental data correlates human cancer with environmental polyaromatic hydrocarbons (PAH) [Citation1]. Benzo(a)pyrene (B(a)P) and its metabolites have been shown to induce tumors in mice [Citation2] and there is enough data to support its correlation with cancer [Citation3]. Previous studies have shown that it binds to DNA both in-vitro and in-vivo [Citation4]. Metabolic activation of B(a)P to the 7, 8- dihydrodiol- 9, 10- epoxide i.e. the ultimate carcinogen, is considered essential for induction of its genotoxic and carcinogenic effects. The cytochrome p450 (CYP) system is the major enzyme system involved in activation and detoxification of carcinogens; natural products can modulate its role and hence that of carcinogens [Citation5]. Inhibitory effects of plant phenols in PAH induced mutagenicity and carcinogenicity has been previously studied and these properties have been attributed to the phenolic hydroxyl group [Citation6].
An important class of endogenous compounds called isoprenoids, which have significant biological functions in the areas of cell cycle regulation [Citation7], cholesterol metabolism [Citation8], and gene regulation, are produced in various eukaryotes including mammals. Isoprenoids have been shown to modulate cell growth, induce cell cycle arrest, initiate apoptosis, and suppress cellular signaling activities [Citation9,10]. Farnesol (FL,), with 15 carbon atoms in a head-to-tail isoprenoid
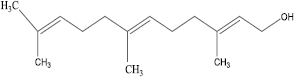
series, may also be an important regulator of hepatic metabolism since it has been shown to significantly inhibit rabbit liver microsomal cytochrome P450 enzymes. [Citation11]. It has been implicated in gene regulation and cell differentiation [Citation12], via the nuclear receptors FXR and PPARα, control of cholesterol synthesis via its interaction with HMG-CoA reductase [Citation8], and apoptosis [Citation13]. It is obtained from the essential oils of ambrette seeds, citronella, present in many aromatic plants [Citation14]. The effects of FL on drug metabolism may be mediated by the nuclear farnesoid X receptor (FXR); this receptor is expressed in several tissues including the liver, kidney, gut, and adrenal gland [Citation15]. Exposure with genotoxic agents has been widely associated with cancer and embryo toxicity [Citation16]. Genotoxic agents may alter normal DNA integrity leading to various pathological conditions [Citation17]. Early reports strongly support that soya isoflavones protect against B(a)P induced toxicity [Citation4]. In our recent findings we have shown the role of pluchea lanceolata in attenuating genotoxicity of cadmium chloride [Citation18].
In the present study, we have evaluated the effects of FL on B(a)P induced genotoxicity in an experimental mouse model.
Materials and methods
Chemicals
EDTA, Tris, Reduced glutathione (GSH), oxidized glutathione (GSSG), nicotinamide adenine dinucleotide phosphate reduced (NADPH), bovine serum albumin (BSA), 1,2-dithio-bisnitrobenzoic acid (DTNB), 1-chloro-2, 4-dinitro benzene (CDNB), [G-3H] B[a]P, sodium dithionate, bisbenzamide, EDTA, SDS, Trisma, phenol, chloroform, isoamyl alcohol and RNase were from Sigma St. Louis. and other reagents and solvents were of a high analytical grade.
Animals
Eight week old adult male Swiss albino mice (20–25 g) were obtained from the Central Animal House Facility of Hamdard University, New Delhi and were housed in a ventilated room at 25 ± 2°C under a 12-h light/ dark cycle. The animals were acclimatized for one week before the study and had free access to standard laboratory feed (Hindustan Lever Ltd., Bombay, India) and water ad libitum.
Experimental design
For the study of biochemical parameters by the alkaline unwinding assay, eight-week-old adult male Swiss albino mice (20–25 g) were divided into five groups, each group consisted of five animals. Animals were treated in accordance with Institutional Guidelines. B[a]P and FL was administered orally by gavage. B[a]P was administered in corn oil. In group I (vehicle control) animals were given corn oil orally. The animals of group II served as treated control and were administered a single oral dose of B[a]P(125 mg kg− 1.b.wt). Animals of group III were pretreated orally with 1% kg− 1 b.wt of FL while groups IV and V were given 2% kg− 1 b.wt of FL for seven consecutive days. The modulator group (V) was only for DNA adducts study. The above-mentioned doses of FL were selected based on preliminary studies carried out in our laboratory (data not shown). On day eight, the animals of group II, III and IV were administered a single oral dose of B[a]P.
The treatment protocol was the same for the DNA adduct study except that the animals were given a single i.p injection [G-3H] B(a)P (12.5mCi/mol) before sacrifice at 24 hrs.
Gel electrophoresis and DNA fragmentation
The sample was mixed with 10mL of loading solution (10 mM EDTA (pH 8.0),1%(w/v) bromophenol blue and 40%(w/v) sucrose) preheated to 70°C.The DNA samples were loaded onto a 1.8%(w/v) agarose gel and sealed with 0.8%(w/v) low melting point agarose. The DNA fragments were separated by electrophoresis at 25V for 12 h at 4°C in TBE buffer. The DNA was visualized using ethidium bromide and photographed by digital camera.
DNA Isolation
DNA was extracted from approximately 500 mg of liver tissue by homogenizing the tissue in 5 mL TNE buffer (50 mM Trisma, 100 mM EDTA, 0.5% SDS, pH 8.0) in a 2 mL ground glass homogenizer. Each sample was homogenized with 10 standardized strokes of the pestle to minimize any potential effect on DNA integrity introduced by the homogenization procedure. An equal volume of buffered phenol/chloroform/isoamyl alcohol (PCI) (25:24:1, v/v/v, pH 8.0) was then added to the sample. The sample was gently mixed and allowed to settle for 5 min and then centrifuged for 5 min at 13000 rpm at 4°C.The aqueous layer was transferred to a new micro centrifuge tube and PCI extraction was repeated. The aqueous layer was then digested by 5 μL of RNase (10 mg mL− 1) for 30 min at 37°C and the digest was extracted once by PCI and once by 500 μL of chloroform. DNA was precipitated from the resulting aqueous layer by adding 2 volumes of absolute ethanol and 1/10 volume of 3 M sodium acetate, pH 5.2. The sample was then centrifuged (13000 rpm, 15 min), and the resulting pellet rinsed with 500 μL of 70% ethanol and air-dried. The amount of DNA was quantitated spectrophotometrically at 260 and 280 nm [Citation19,4]. 2 μg/μL of DNA sample was dissolved in 1mL of TE buffer (10 mM Trisma, 1 mM EDTA) and subsequently used in the DNA alkaline unwinding assay.
Alkaline unwinding assay
The procedure used for alkaline unwinding was essentially the same as that outlined by Shugart [Citation17] with slight modifications. In the alkaline unwinding assay, the rate of transition of double stranded DNA (dsDNA) to single stranded DNA (ssDNA) under pre-defined alkaline denaturing condition was proportional to the number of breaks in the phosphodiester backbone and thus was used as a measure of DNA integrity. Bisbenzamide was used as a DNA –binding dye from the fluorescence of which various types of DNA were quantitated. For the fluorescence determination of dsDNA, ssDNA and partially unwound DNA (au-DNA), three equal portions of diluted DNA sample was prepared. The amount of dsDNA was obtained from the fluorescence of a sample without any treatment; while ssDNA was determined from the sample that had been boiled for 30 min. Fluorescence of the DNA sample which had been subjected to alkaline treatment (pH 12.2) on ice for 30 min provided an estimate of the amount of au DNA.
The fluorescence of initial or double stranded DNA was determined by placing 100 μmol DNA sample, 100 μL NaCl (25 mM) and 2 μL SDS (0.5%) in a pre-chilled test tube, followed by the addition of 3 mL 0.2 M potassium phosphate pH 9, and 3 μL bisbenzamide (1 mgmL− 1). The contents were mixed and allowed to react in the darkn for 15 min to allow the fluorescence to stabilize. The fluorescence of the sample was the measured using a spectrofluorimeter (Ex: 360 nm, Em: 450 nm). The fluorescence of single stranded DNA was determined as above but using the DNA sample that had already been boiled for 30 min to completely unwind the DNA.
50 μL NaOH (0.05 N) was rapidly mixed with 100 μL of DNA sample in a pre-chilled test tube. The mixture was incubated on ice in the darkn for 30 min [Citation20] followed by rapid addition and mixing of 50 μL HCl (0.05N). This was followed immediately by an addition of 2 μL SDS (0.5%) and the mixture was forcefully passed through a 21 G needle six times. Fluorescence of alkaline unwound DNA sample was measured as described above. Measurement of the alkaline unwound sample was performed in triplicate and the average was reported.
The ratio between double stranded DNA to total DNA (F value) was determined as follows:
where auDNA, ssDNA and dsDNA were the degrees of fluorescence from the partially unwound, single stranded and double stranded determinations, respectively. The F value was inversely proportion to the number of strand breaks present and thus could be used as an indicator of DNA integrity.
Determination of aryl hydrocarbon hydroxylase (AHH) activity
Aryl hydrocarbon hydroxylase activity (AHH) was determined as described by Weibel and Gelboin [Citation21]. The reaction mixture, in a total volume of 1 mL, contained 50 mmol Tris–chloride buffer (pH 7.4), 0.36 mmol NADPH, 3 mmol MgCl2 and 0.1 mL microsomal fraction suspended in 0.1 M potassium phosphate buffer pH 7.4 (1 mg protein/mL). One hundred nmoles of the substrate, B(a)P, was added in 0.05 mL methanol to start the reaction. After 10 min of incubation at 37°C, the reaction was terminated by the addition of 1.0 mL cold acetone and the mixture was shaken with 3.0 mL hexane for 10 min to extract the derivatives of B (a) P. A 1.0 mL of the organic layer was extracted with 2 mL 1 N NaOH. The fluorescence of the NaOH extract was measured immediately at 396 nm excitation and 522 nm emission using a spectro- fluorometer. The amount of enzyme activity is defined as the picomoles of 3-hydroxy-B(a)p formed during the incubation per mg protein per min.
Assay of Cytochrome P450 content
10% (w/v) homogenate was prepared from the liver of mice and processed for the preparation of post mitochondrial supernatant (PMS) and microsomes for CYP content as described by Omura and Sato [Citation22]. A pinch of sodium dithionate was added to 2 mL of sample which was then divided equally between two matched cuvettes. The contents of the test cuvette were gently bubbled with carbon monoxide for about 1 min and then the absorbance was taken at 450 nm and 490 nm simultaneously.
DNA adducts
Eight-week-old adult male Swiss albino mice (20–25 g) were administered a single i.p. injection of B[a]P (12.5 mCi/mol). After 24 h the mice were sacrificed, livers were excised and DNA was isolated as described above. The DNA concentration was determined fluorimetrically and the extent of radiolabeled B[a]P-DNA modification was established by scintillation counting in triplicate[Citation19]with slight modifications.
Assay for glutathione-S-transferase activity
Glutathione-S-transferase activity was assayed by the method of Habig [Citation23] The reaction mixture consisted of 1.475 mL phosphate buffer (0.1 M, pH 6.5), 0.2 mL reduced glutathione (1 mM), 0.025 mL CDNB (1 mM) and 0.3 mL PMS (10% w/v) in a total volume of 2.0 mL. The changes in the absorbance were recorded at 340 nm and enzyme activity was calculated as nmol CDNB conjugate formed per minute per mg protein using a molar extinction coefficient of 9.6 × 103 M− 1 cm− 1.
Assay for quinone reductase activity
The activity of quinone reductase was determined by the method of Benson [Citation24]. The 3 mL reaction mixture consisted of 2.13 mL Tris–HCl buffer (25 mM, pH 7.4), 0.7 mL BSA, 0.1 mL FAD, 0.02 mL NADPH (0.1 mM), and 50 μL (10%) PMS. The reduction of dichlorophenol indophenol (DCPIP) was recorded calorimetrically at 600 nm and enzyme activity was calculated as n moles of DCPIP reduced per minute per mg protein using a molar extinction coefficient of 2.1 × 104 M− 1 cm− 1.
Estimation of reduced glutathione
Reduced glutathione was determined by the method of Jollow [Citation25]. One-milliliter sample of PMS was precipitated with 1.0 mL of sulfosalicylic acid (4%). The samples were kept at 4°C for 1 h and then centrifuged at 1200 × g for 20 min at 4 °C. The assay mixture contained 0.1 mL filtered aliquot, 2.7 mL phosphate buffer (0.1 M, pH 7.4) and 0.2 mL DTNB (100 mM) in a total volume of 3.0 mL. The yellow color developed was measured at 412 nm on a spectrophotometer.
Estimation of protein concentration
The protein concentration in all samples was determined by the method of Lowry [Citation26].
Statistical analysis
The level of significance between different groups was based on ANOVA test, followed by the Dunnett's t-test
Results
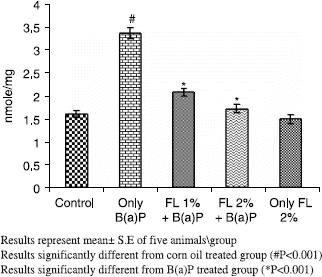
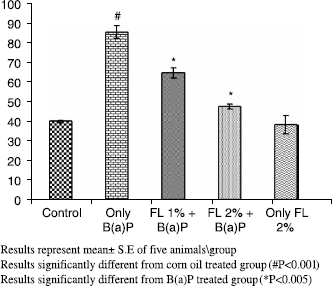
Oral treatment of mice with B(a)P caused induction of its metabolizing enzymes,loss of DNA integrity and modulation of antioxidant defense system significantly. As observed during the study, B(a)P alone (125 mg kg− 1.b.wt oral) led to modulation of several parameters of oxidative stress, relative to control animals receiving corn oil only, with concomitant enhancement of its metabolizing enzymes. B (a)P alone treatment decreased the hepatic GSH content, GST and QR activities significantly (p < 0.001) with a concomitant and significant increase in CYP activity and AHH (p < 0.001) respectively. Of the corn oil- treated control pretreatment of animals with FL at 1% and 2% kg− 1 body weight (p < 0.001) restored GSH content, GST and QR activities as shown in . There was concomitant amelioration of CYP (p < 0.005) and AHH activity (p < 0.001) at both the doses as shown in and .
Table I. Effect of pretreatment of Farnesol on B(a)P induced toxicity regarding glutathione content, glutathione S-transferase and Quinone reductase in mouse liver.
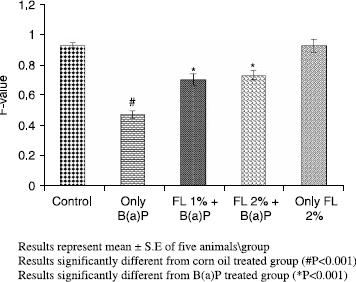
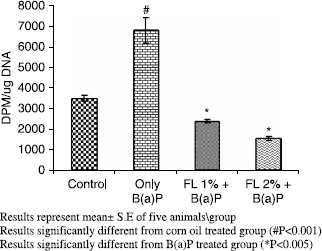
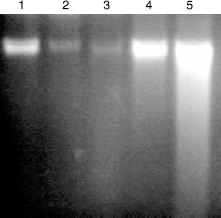
A simultaneous decrease in F-value was noted in the DNA alkaline unwinding assay (), which is a marker for alteration in DNA integrity as evident from agarose gel electrophoresis (), where results indicate that there was significant DNA fragmentation only in the toxicant group as compared to the control group while there was less fragmentation in FL-pretreated groups. During DNA fragmentation DNA damage is estimated in terms of smearing and lack of intact band control; only the FL group showed less smearing and an intact band was also observed. DNA adduct formation (p < 0.001) was observed in toxicant group. Inhibition (p < 0.005) in DNA adduct formation was recorded in FL pretreated groups as shown in .
Figure 2. Effect of pretreatment of Farnesol on B(a)P induced cytochrome P450 enzyme in mouse liver.
Discussion:
B[a]P is the most commonly studied carcinogen with sources of exposure including occupation, diet and tobacco smoke [Citation27]. The covalent binding of carcinogens to DNA is an important step in the cancer initiation process, with B[a]P requiring metabolic activation for DNA adduct formation to occur [Citation28]. The activation of carcinogens is primarily catalyzed by phase I enzymes and protection may be accomplished by inhibition of activating enzymes and/or by induction of phase II enzymes [Citation29], which leads to detoxification, and accelerated excretion of carcinogens. Considerable emphasis has been placed on the use of natural plants and plant products to combat oxidative stress and toxicity [Citation30]. In the present study FL pretreatment in modulator groups significantly repressed CYP content and AHH activity when compared only with the toxicant group. On the basis of several experimental studies which demonstrated that indoles and glucosinolates inhibit B(a)P and DMBA-induced neoplasia in rats[Citation31], it was postulated that the protective effects were due to induction of GST, which is one of the most important detoxification enzymes [Citation31]. FL has a number of activities that suggest it may be active in cancer chemoprevention. FL is an apoptosis inducer, and has been demonstrated to suppress the in vitro growth of several tumor-derived cell lines through the induction of apoptosis [Citation32,13]. In vivo, dietary administration of FL inhibits the growth of transplanted pancreatic adenocarcinoma cells in hamsters [Citation33]. FL-pretreated groups showed significant induction of GST and QR activities at both the doses. The intracellular thiol, glutathione, can reduce free radicals by hydrogen donation. For example, reduced glutathione can neutralize a hydroxyl radical and hence plays an important protective role against oxidative and inflammatory stress and is often considered as first line of defence [Citation34]. FL- pretreatment has been shown to restore the B(a)P depleted GSH levels in modulator groups. Reactive species can cause DNA damage, loss in DNA integrity and play important roles in mutagenesis, carcinogenesis and aging [Citation35]. Genomic instability is often considered as the hallmark of cancer and it has been shown earlier that B(a)P administration leads to genotoxicity and DNA strand breaks in terms of the unwinding alkaline assay [Citation4,19]. It is evident from the present study that FL not only was able to reduce cellular damage but also suppressed the formation of DNA adducts in- vivo in pretreated groups in comparison only to the B(a)P group. Present results provide direct evidence that oxidative damage can be a major contributor to DNA damage which leads to reduction in the F-value caused by B(a)P and simultaneously demonstrates the role of FL as potent protective agent against B(a)P toxicity.
Conclusion:
Our study shows that FL plays a role in inhibiting Phase I xenobiotic -metabolizing enzymes and simultaneous induction of detoxification Phase-II enzymes which supports its effectiveness in maintaining DNA integrity. Concisely, this study provides ample evidence for the protective efficacy of FL and further work at molecular targets is underway viz; estimation of isoforms to establish FL as a strong preventive tool.
Acknowledgements
The authors are thankful to the Hamdard National Foundation, New Delhi, India for providing the funds to carry out this work.
References
- D Warshawsky. (1999). Polycyclic aromatic hydrocarbons in carcinogenesis. Environ mental Health Perspect 107:317–319.
- JM Arif, WA Smith, and RC Gupta. (1999). DNA adduct formation and persistence in rat tissues following exposure to the mammary carcinogen dibenzo[a,l]pyrene. Carcinogenesis 20:1147–1150.
- TJ Lightfoot, JM Coxhead, BC Cupid, S Nicholson, and RC Garner. (2000). Analysis of DNA adducts by accelerator mass spectrometry in human breast tissue after administration of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and benzo[a]pyrene. Mutation Res 472 (1–2):119–127.
- TH Khan, L Prasad, and Sultana S Anuradha. (2005). Soy isoflavones inhibits the genotoxicity of benzo(a)pyrene in Swiss albino mice. Hum Exp Toxicol 24 (3):149–155.
- FP Guengerich. (1988). Roles of cytochrome P450 enzymes in chemical carcinogenesis and cancer chemotherapy. Cancer Res 48:2946–2954.
- H Szaefer, M Cichocki, D Brauze, and W Baer-Dubowska. (2004). Alteration in phase I and II enzyme activities and polycyclic aromatic hydrocarbons-DNA adduct formation by plant phenolics in mouse epidermis. Nutrition Cancer 48 (1):70–77.
- JC Sacchettini, and CD Poulter. (1997). Creating isoprenoid diversity. Science 277:1788–1789.
- CC Correll, L Ng, and PA Edwards. (1994). Identification of farnesol as the non-sterol derivative of mevalonic acid required for the accelerated degradation of 3-hydrixy-3-methylglutaryl-coenzyme A reductase. J Biol Chem 269:17390–17393.
- H Mo, and CE Elson. (1999). Apoptosis and cell-cycle arrest in human and murine tumor cells are initiated by isoprenoids. J Nutrit 129:804–813.
- CE Elson, and SG Yu. (1994). The chemoprevention of cancer by mevalonate-derived constituents of fruits and vegetables. J Nutrit 124:607–614.
- L. Horn Thomas, Lina Long, J. Cwik Michael, L. Morrissey Robert, M. Kapetanovic Izet, and L. McCormick David. (2005). Modulation of hepatic and renal drug metabolizing enzyme activities in rats by subchronic administration of farnesol. Chem-Biol Interac 152:79–99.
- K Hanley, LG Komuves, DC Ng, KS Schoonjans, SS He, P Lau, DD Bikle, ML Williams, PM Elias, J Auwerx, and KR Feingold. (2000). Farnesol stimulates differentiation in epidermal keratinocytes via PPARα. J Biol Chem 275:11484–11491.
- I Adany, EM Yazlovitskaya, JS Haug, PA Voziyan, and Melnykovych. (1994). Differences in sensitivity to farnesol toxicity between neoplastically- and non-neoplastically-derived cells in culture. Cancer Lett 79:175–179.
- J Zhao, P Nan, and Y Zhong. (2004). Chemical composition of the essential oils of Clausena lansium from Hainan Island, China. Z Naturforsch [C] 59:153–156.
- BM Forman, E Goode, J Chen, AE Oro, DJ Bradley, T Perlmann, DJ Noonan, LT Burka, T McMorris, WW Lamph, RM Evans, and C Weinberger. (1995). Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81:687–693.
- MF Salamone. Toxicity of 41 carcinogens and noncarcinogenic analogs. FJ deSerres, and j Ashby. Evaluation of short term tests for carcinogens. Report of the International Corroborative Program. Elsevier North-Holland: Progress in Mutation Research; (1981), 682–685.
- LR Shugart. (1988). Quantitation of chemically induced damage to DNA of aquatic organisms by alkaline unwinding assay. Aquat Toxicol 13:43–52.
- T Jahangir, TH Khan, L Prasad, and S Sultana. (2005). Pluchea lanceolata attenuates cadmium chloride induced oxidative stress and genotoxicity in Swiss albino mice. J Pharm Pharmacol 57 (9):1199–1204.
- L Xu, GJ Zheng, PKS Lam, and B Richardson. (1999). Relationship between tissue concentrations of polycyclic aromatic hydrocarbons and DNA adducts in green-lipped mussels(Perna viridis). Ecotoxicol 8:73–82.
- SS Rao, TA Neheli, and JH Carey. (1996). DNA alkaline unwinding assay for monitoring the impact of environmental genotoxins. Environ Toxicol Water Qual 11:351–354.
- FL Weibel, and HV Gelboin. (1975). Arylhydrocarbon benzo(a)pyrene hydroxylase in liver from rat of different age, sex and nutritional status. Biochem Pharmacol 24:1511–1515.
- T Omura, and R Sato. (1964). The carbon monoxide binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378.
- WH Habig, MJ Pubst, and WB Jokoby. (1974). GST the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139.
- AM Benson, MJ Hunkeler, and P Talalay. (1980). Increase of NADPH; Quinone reductase activity by dietary antioxidant. Possible role in protection against carcinogenesis and toxicity. Proc Nat Acad Sci USA 77:5216–5220.
- DJ Jollow, JR Mitchell, N Zampaglione, and JR Gillette. (1974). Bromobenzene induced liver necrosis: Protective role of glutathione and evidence for 3,4-bromobezene oxide as the hepatotoxic metabolite. Pharmacol 11:151.
- OH Lowry, NJ Rosebrough, AL Farr, and RJ Randall. (1964). Protein measurement with the phenol reagent. J Biol Chem 193:2370–2378.
- DH Phillips. (1999). Polycyclic aromatic hydrocarbons in the diet. Mutation Res 443:139–147.
- RC Garner. (1998). The role of DNA adducts in chemical carcinogenesis. Mutation Res 402:67–75.
- HJ Prochaska, and P Talalay. (1988). Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res 48:4776–4782.
- LW Wattenberg. (1997). An overview of chemoprevention: Current status and future prospects. Proc Soc Exper Biol Med 216:133–141.
- VL Sparnins, PL Venegas, and LW Wattenberg. (1982). Glutathione-S-transferase activity: Enhancement by compounds inhibiting chemical carcinogenesis and by dietary constituents. J Nat Cancer Inst 68:493–496.
- JS Haug, CM Goldner, EM Yazlovitskaya, PA Voziyan, and G Melnykovych. (1994). Directed cell killing (apoptosis) in human lymphoblastoid cells incubated in the presence of farnesol: effect of phosphatidylcholine. Biochem Biophys Acta 1223:133–140.
- YD Burke, MJ Stark, SL Roach, SE Sen, and PL Crowell. (1997). Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids 32:151–156.
- F Zunino, G Pratesi, A Micheloni, E Cavalletti, F Sala, and O Tofanetti. (1989). Protective effect of reduced glutathione against cisplatin-induced renal and systemic toxicity and its influence on the therapeutic activity of the antitumor drug. Chem-Biol Interact 70 (1–2):89–101.
- NA Doudican, B Song, GS Shadel, PW Doetsch, and DNA Oxidative. (2005). damage causes mitochondrial genomic instability in Saccharomyces cerevisiae. Mol Cell Biol 25:5196–5204.