Abstract
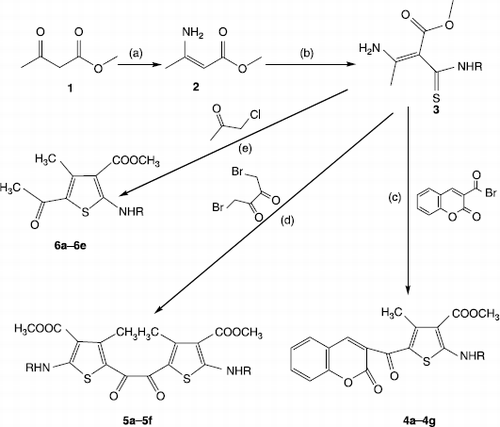
Sets of tetrasubstituted thiophene esters 4a-4g, 5a-5f and 6a-6e were synthesized by reaction of 1-(α-Carbomethoxy-β-aminothiocrotonoyl)-aryl/aroyl amines (3) with 3-(bromoacetyl)coumarin, 1,4-dibromodiacetyl and chloroacetone respectively. The compound 3 were synthesized by nucleophilic addition of aryl/aroylisothiocyanate and enamine (2). The synthesized targeted compounds (4a-4g, 5a-5f and 6a-6e) were evaluated for their in vivo anti-inflammatory activity in carrageenin-induced rat hind paw oedema model at three graded doses employed at 10, 20 and 40 mg/kg body weight using mefanamic acid, ibuprofen and in vivo analgesic activity in acetic acid induced writhing response model at 10 mg/kg dose using ibuprofen as standard drug. The compounds 4a-4f, 5c, 5f, 6c and 6e were evaluated for their in vitro antioxidant nitric oxide radical scavenging assay at the concentrations of 5, 10, 15, 20, 25, 30 and 35 μg/mL using ascorbic acid as standard drug. Among all the targeted compounds 4c showed maximum anti-inflammatory activity of 71% protection at 10 mg/kg and 77% protection at 20 mg/kg to inflamed paw and analgesic activity of 56% inhibition and also maximum in vitro nitric oxide radical scavenging activity having IC50 value 31.59 μg/mL.
Introduction
Inflammation occurs as a defensive response which induces physiological adaptations to limit tissue damage and remove the pathogenic infections. Diseases caused by inflammation are an important factor of morbidity and mortality in humans. Inflammatory disorders include rheumatoid arthritis, osteoarthritis, inflammatory bowl diseases, retinitis, multiple sclerosis, psoriasis and atherosclerosis [Citation1]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed for the treatment of acute and chronic inflammation, pain and fever. Most of NSAIDs that are available in market are known to inhibit isoforms, a constitutive form, COX-1 and an inducible form COX-2, to offer therapeutic effect. However, long-term clinical usage of NSAIDs is associated with significant side effects of gastrointestinal lesions, bleeding and nephrotoxicity [Citation2–4]. Therefore, the discovery of new safer anti-inflammatory drugs represents a challenging goal for such a research area.
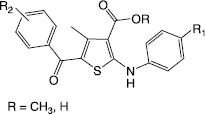
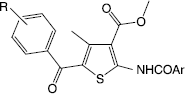
Thiophene derivatives represent an important class of compounds with diverse biological activities. Substituted thiophenes are also present in natural products. Various tri and tetrasubstituted thiophene derivatives and their anti-inflammatory activity are well documented in literature [Citation5,6]. According to our previous reports [Citation7–9], the anti-inflammatory activity of tetrasubstituted thiophene ester/acid molecules having the features of (a) COX-1 inhibitor and 5-LOX inhibitor (acid/ester) of the anthranilic acid type (fenamates), (b) p38 MAP kinase inhibitor, can be significantly modified by using substituents at R1 (both electron releasing and electron withdrawing) in anilino moiety and R2 (electron releasing and electron withdrawing) in benzoyl moiety (Structure ). The pharmacological evaluation of tetrasubstituted thiophene esters having carbonyl spacer as aroylamino at the second position of the thiophene ring which has a proton acceptor (–C = O) and a proton donor (–NH) features in adjacent position were also reported (Structure ). In continuation to our previous efforts in designing and synthesizing new tetrasubstituted thiophenes with good anti-inflammatory/antioxidant activity and selectivity, we report here synthesis, in vivo anti-inflammatory, analgesic and in vitro antioxidant nitric oxide radical scavenging activity of a new series of designed tetrasubstituted thiophene ester molecules (Scheme ).
Materials and methods
Chemistry
All reagents and solvents were used as obtained from the supplier or recrystallized/redistilled as necessary. Thin-layer chromatography was performed using glass plates coated with silica gel G and toluene:acetonitrile as a mobile phase. The spots were developed using iodine. Melting points were recorded on capillary melting point apparatus and are uncorrected. Infrared spectra (KBr discs) were recorded with a Buck Scientific M-500 Infrared spectrophotometer.1H-NMR spectra were recorded in CDCl3 and DMSO-d6 with 300/200 MHz Bruker FT-NMR (Advance DPX200) spectrometer using tetramethylsilane as internal standard and the chemical shifts (δ) are reported in ppm, coupling constants (J) are given in Hz. Mass spectra of the compounds were recorded on Perkin-Elmer Sciex atmospheric pressure ionization liquid chromatography mass instrument (LCMS) and Electron impact (EI) mass spectra were recorded on a Jeol JMS-D-300 spectrometer with the ionization potential of 70 eV. Elemental analysis data were determined using a Carlo-Erba 1108 instrument or Elementar's Vario EL III micro-analyzer. UV spectra were recorded in Shimadzu 1601 UV-Visible spectrophotometer.
General method for synthesis of compounds (4a)–(4g)
As shown in Scheme , enamine (2) was obtained by reacting ammonia (25%) with methyl acetoacetate in equimolar quantity in diethylether at 0–15°C (1). 1-(α-Carbomethoxy-β-aminothiocrotonoyl)-aryl/aroyl amines (3) were synthesized by nucleophilic addition of aryl/aroylisothiocyanate and enamine (2) as per reported procedure [Citation6]. Arylisothiocyanates were synthesized using modified Kaluza method [Citation10] whereas aroylisothiocyanate by previously reported procedure [Citation11]. The compounds 4a-4g were synthesized by adding 0.001 mol of the 3-(bromoacetyl)coumarin [Citation12] to a solution of (3) (0.001 mol) in 2 mL of acetonitrile without adding base at room temperature [Citation6]. The solution was stirred until the solid was separated from the reaction mixture or until no more of the starting materials could be detected on TLC. The solid was filtered off, washed with chilled acetonitrile, dried, recrystalized with methanol yielding coloured product corresponding to the (4a-4g) characterized as per the analytical data.
General method for synthesis of compounds (5a) - (5f)
The compounds 5a-5f were synthesized by adding 0.001 mol of 1,4-dibromodiacetyl to a solution of (3) (0.002 mol) in 5 mL of acetonitrile without adding base at room temperature. The solution was stirred until the solid was separated from the reaction mixture or until no more of the starting materials could be detected on TLC. The solid that separated was filtered off, washed with chilled acetonitrile, dried, recrystalized with DMSO yielding coloured product corresponding to the (5a-5f) characterized as per the analytical data.
General method for synthesis of compounds (6a)–(6e)
The compounds 6a-6e were synthesized by adding 0.001 mol of chloroacetone to a solution of (3) (0.001 mol) in 4 mL of acetonitrile without adding base at room temperature. The solution was stirred until the solid was separated from the reaction mixture or until no more of the starting materials could be detected on TLC. The solid that separated was filtered off, washed with chilled acetonitrile, dried, recrystalized with methanol yielding coloured product corresponding to the (6a-6e) characterized as per the analytical data.
Methyl 2-anilino-5-(3-coumarinoyl)-4-methylthiophene-3-carboxylate (4a)
Yield: 85%; m.p.: 238°C; Rf : 0.72 (7:3, toluene/acetonitrile); IR (KBr, cm-1): 1692 (C = O stretching of ester), 1606 (C = O stretching of ketone), 772, 674; 1H-NMR: (200 MHz, CDCl3) δ (ppm): 2.65 (s, 3H, CH3-4), 3.90 (s, 3H, CH3 of ester), 7.30-7.44 (m, 6H, aromatic), 7.58 (t, 2H, J = 6.70 Hz aromatic), 7.65 (d, 1H, aromatic), 7.93 (s, 1H, aromatic), 10.70 (s 1H, NH-2); MS: m/z 419 (M+); Anal. calcd. for C23H17NO5S: C, 65.88; H, 4.05; N, 3.33; Found: C, 66.16; H, 4.01; N, 3.47%.
Methyl 2-(4-methylanilino)-5-(3-coumarinoyl)-4-methylthiophene-3-carboxylate (4b)
Yield: 45%; m.p.: 162°C; Rf : 0.69 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 1678 (C = O stretching of ester), 1615 (C = O stretching of ketone), 745, 685; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.27 (s, 3H, CH3-2), 2.56 (s, 3H, CH3-4), 3.92 (s, 3H, CH3 of ester), 7.09 (d, 2H, J = 5.45 Hz aromatic), 7.19-7.22 (m, 3H, aromatic), 7.40 (t, 2H, aromatic), 7.72 (d, 1H, aromatic), 8.76 (s, 1H, aromatic), 10.09 (s, 1H, NH-2); MS: m/z 433 (M+); Anal. calcd. for C24H19NO5S: C, 66.52; H, 4.38; N, 3.23; Found: C, 66.70; H, 4.54; N, 3.65%.
Methyl 2-(4-chloroanilino)-5-(3-coumarinoyl)-4-methylthiophene-3-carboxylate (4c)
Yield: 84%; m.p.: 250°C; Rf : 0.84 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 1710 (C = O stretching of ester), 1660 (C = O stretching of ketone), 797, 690; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.65 (s, 3H, CH3-4), 3.91 (s, 3H, CH3 of ester), 7.31 (d, 2H, J = 5.12 Hz aromatic), 7.37-7.41 (m, 3H, aromatic), 7.61 (t, 2H, J = 4.10 Hz aromatic), 7.64 (d, 1H, aromatic), 7.94 (s, 1H, aromatic), 10.69 (s, 1H, NH-2); MS: m/z 455 (M++2); Anal. calcd. for C23H16ClNO5S: C, 60.88; H, 3.52; N, 3.08; Found: C, 60.89; H, 3.84; N, 3.61%.
Methyl 2-(4-bromoanilino)-5-(3-coumarinoyl)-4-methylthiophene-3-carboxylate (4d)
Yield: 38%; m.p.: 187°C; Rf : 0.73 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 1693 (C = O stretching of ester), 1648 (C = O stretching of ketone), 785, 656; 1H-NMR: (200 MHz, CDCl3) δ (ppm): 2.66 (s, 3H, CH3-4), 3.90 (s, 3H, CH3 of ester), 7.42 (d, 2H, aromatic), 7.55-7.62 (m, 3H, aromatic), 7.64 (d, 1H, aromatic), 7.93 (d, 2H, aromatic), 7.97 (s, 1H, aromatic), 10.64 (s, 1H, NH-2); MS: m/z 498 (M+); Anal. calcd. for C23H16BrNO5S: C, 55.43; H, 3.21; N, 2.80; Found: C, 55.91; H, 3.64; N, 2.58%.
Methyl 2-benzoylamino-5-(3-coumarinoyl)-4-methylthiophene-3-carboxylate (4e)
Yield: 67%; m.p.: 182°C; Rf : 0.76 (7:3, toluene/acetonitrile); IR (KBr, cm-1): 1710 (C = O stretching of ester), 1589 (C = O stretching of ketone), 781, 680; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.64 (s, 3H, CH3-4), 3.96 (s, 3H, CH3 of ester), 7.47-7.53 (m, 6H, aromatic), 8.01 (t, 2H, J = 6.70 Hz aromatic), 7.36 (d, 1H, J = 5.80 Hz aromatic), 8.75 (s, 1H, aromatic), 12.66 (s, 1H, NH-2); MS: m/z 447 (M+); Anal. calcd. for C24H17NO6S: C, 64.42; H, 3.79; N, 3.12; Found: C, 63.98; H, 3.75; N, 3.02%.
Methyl 2-(2-furoylamino)-5-(3-coumarinoyl)-4-methylthiophene-3-carboxylate (4f)
Yield: 60%; m.p.: 188°C; Rf : 0.72 (7:3, toluene/acetonitrile); IR (KBr, cm-1): 1724 (C = O stretching of ester), 1582 (C = O stretching of ketone), 779, 676; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.66 (s, 3H, CH3-4), 3.88 (s, 3H, CH3 of ester), 6.46 (q, 1H, aromatic), 7.39-7.43 (m, 3H, aromatic), 7.59 (t, 2H, J = 5.80 Hz aromatic), 7.70 (d, 1H, aromatic), 8.06 (s, 1H, aromatic); MS: m/z 437 (M+); Anal. calcd. for C22H15NO7S: C, 60.42; H, 3.43; N, 3.20; Found: C, 60.67; H, 3.61; N, 3.32%.
Methyl 2-ethylamino-5-(3-coumarinoyl)-4-methylthiophene-3-carboxylate (4g)
Yield: 35%; m.p.: 205°C; Rf : 0.68 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 3330 (alkyl NH stretching), 1717 (C = O stretching of ester), 1605 (C = O stretching of ketone), 780, 680; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 1.35 (t, 3H, J = 3.15 Hz CH3-2), 2.61 (s, 3H, CH3-4), 3.31 (q, 2H, J = 3.54 Hz CH3-2), 3.82 (s, 3H, CH3 of ester), 7.30-7.37 (m, 2H, aromatic), 7.57 (t, 2H, aromatic), 7.90 (s, 1H, aromatic), 10.65 (s, 1H, NH-2); MS: m/z 371 (M+); Anal. calcd. for C19H17NO5S: C, 61.45; H, 4.57; N, 3.77; Found: C, 61.78; H, 4.78; N, 4.01%.
Bis-(2-anilino-3-methoxycarbonyl-4-methyl-5-thienyl)ethane-1,2-dione (5a)
Yield: 80%; m.p.: 220°C; Rf : 0.68 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 3320 (aryl NH stretching), 1648 (C = O stretching of ester), 1600 (C = O stretching of ketone), 757, 693; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.05 (s, 6H, CH3-4), 3.78 (s, 6H, CH3 of ester), 7.22-7.61 (m, 10H, aromatic), 10.72 (s, 2H, NH-2); MS: m/z 548 (M+); Anal. calcd. for C28H24N2O6S2: C, 61.30; H, 4.37; N, 5.10; Found: C, 61.63; H, 4.80; N, 5.45%.
Bis-[2-(4-methylanilino)-3-methoxycarbonyl-4-methyl-5-thienyl]ethane-1,2-dione (5b)
Yield: 35%; m.p.: 245°C; Rf : 0.75 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 3368 (aryl NH stretching), 1656 (C = O stretching of ester), 1612 (C = O stretching of ketone), 760, 689; MS: m/z 576 (M+); Anal. calcd. for C30H28N2O6S2: C, 62.51; H, 4.85; N, 4.85; Found: C, 62.65; H, 4.65; N, 5.11%.
Bis-[2-(4-chloroanilino)-3-methoxycarbonyl-4-methyl-5-thienyl]ethane-1,2-dione (5c)
Yield: 90%; m.p.: 252°C; Rf : 0.75 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 3383 (aryl NH stretching), 1666 (C = O stretching of ester), 1614 (C = O stretching of ketone), 785, 698; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.68 (s, 6H, CH3-4), 4.00 (s, 6H, CH3 of ester), 7.98 (d, 4H, aromatic), 8.13 (d, 4H, aromatic), 10.90 (s, 2H, NH-2); MS: m/z (rel. abund. %) 617 (M+, 40), 307 (52), 232 (48), 157 (100), 137 (45), 107 (19); Anal. calcd. for C28H22Cl2N2O6S2: C, 54.47; H, 3.56; N, 4.53; Found: C, 54.44; H, 3.94; N, 4.47%.
Bis-[2-(4-bromoanilino)-3-methoxycarbonyl-4-methyl-5-thienyl]ethane-1,2-dione (5d)
Yield: 56%; m.p.: 262°C; Rf : 0.67 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 3390 (aryl NH stretching), 1673 (C = O stretching of ester), 1620 (C = O stretching of ketone), 786, 686; MS: m/z 706 (M+); Anal. calcd. For C28H22Br2N2O6S2: C, 47.61; H, 3.21; N, 3.96; Found: C, 47.53; H, 3.48; N, 4.08%.
Bis-(2-benzoylamino-3-methoxycarbonyl-4-methyl-5-thienyl)ethane-1,2-dione (5e)
Yield: 85%; m.p.: 239°C; Rf : 0.70 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 3416 (aryl NH stretching), 1711 (C = O stretching of ester), 1594 (C = O stretching of ketone), 779, 687; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.77 (s, 6H, CH3-4), 4.01 (s, 6H, CH3 of ester), 7.18-7.61 (m, 10H, aromatic), 12.25 (s, 2H, NH-2); MS: m/z (rel. abund. %) 604 (M+, 20), 301 (45), 207 (18), 149 (60); Anal. calcd. for C30H24N2O8S2: C, 59.59; H, 3.96; N, 4.63; Found: C, 59.37; H, 4.22; N, 4.92%.
Bis-[2-(2-furoylamino)-3-methoxycarbonyl-4-methyl-5-thienyl]ethane-1,2-dione (5f)
Yield: 68%; m.p.: 208°C; Rf : 0. 80 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 3446 (aryl NH stretching), 1724 (C = O stretching of ester), 1687 (C = O stretching of ketone), 799, 699; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.76 (s, 6H, CH3-4), 4.07 (s, 6H, CH3 of ester), 7.19-7.38 (m, 6H, aromatic), 12.13 (s, 2H, NH-2); MS: m/z (rel. abund. %) 584 (M+, 22), 289 (38), 232 (16), 154 (100), 136 (85), 107 (38); Anal. calcd. for C26H20N2O10S2: C, 53.42; H, 3.42; N, 4.79; Found: C, 53.12; H, 3.29; N, 4.67%.
Methyl 2-anilino-5-acetyl-4-methylthiophene-3-carboxylate (6a)
Yield: 48%; m.p.: 125°C; Rf : 0.65 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 1665 (C = O stretching of ester), 1621 (C = O stretching of ketone), 754, 690; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.47 (s, 3H, CH3-4), 2.74 (s, 3H, CH3-5), 3.91 (s, 3H, CH3 of ester), 7.17-7.44 (m, 5H, aromatic), 10.57 (s 1H, NH-2); MS: m/z 290 (M++1); Anal. calcd. for C15H15NO3S: C, 62.29; H, 5.18; N, 4.84; Found: C, 62.54; H, 5.31; N, 4.67%.
Methyl 2-(4-methylanilino)-5-acetyl-4-methylthiophene-3-carboxylate (6b)
Yield: 38%; m.p.: 175°C; Rf : 0.88 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 1665 (C = O stretching of ester), 1614 (C = O stretching of ketone), 750, 699; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.28 (s, 3H, CH3-2), 2.35 (s, 3H, CH3-4), 2.56 (s, 3H, CH3-5), 3.92 (s, 3H, CH3 of ester), 7.13 (d, 2H, J = 7.11 Hz aromatic), 7.21 (d, 2H, aromatic), 10.09 (s 1H, NH-2); MS: m/z 323 (M++23), 303 (M+); Anal. calcd. for C16H17NO3S: C, 63.37; H, 5.27; N, 4.61; Found: C, 63.19; H, 5.58; N, 4.37%.
Methyl 2-(4-chloroanilino)-5-acetyl-4-methylthiophene-3-carboxylate (6c)
Yield: 78%; m.p.: 162°C; Rf : 0.80 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 1720 (C = O stretching of ester), 1661 (C = O stretching of ketone), 785, 690; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.47 (s, 3H, CH3-4), 2.74 (s, 3H, CH3-5), 3.91 (s, 3H, CH3 of ester), 7.29 (d, 2H, J = 5.33 Hz aromatic), 7.36 (d, 2H, aromatic), 10.58 (s 1H, NH-2); MS: m/z 346 (M++23), 324 (M++1); Anal. calcd. for C15H14ClNO3S: C, 55.64; H, 4.32; N, 4.32; Found: C, 55.87; H, 4.48; N, 4.31%.
Methyl 2-(4-bromoanilino)-5-acetyl-4-methylthiophene-3-carboxylate (6d)
Yield: 35%; m.p.: 129°C; Rf : 0.75 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 1700 (C = O stretching of ester), 1645 (C = O stretching of ketone), 770, 658; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.57 (s, 3H, CH3-4), 2.61 (s, 3H, CH3-5), 3.93 (s, 3H, CH3 of ester), 7.07 (d, 2H, J = 4.80 Hz aromatic), 7.51 (d, 2H, aromatic), 10.28 (s 1H, NH-2); MS: m/z 369 (M++1); Anal. calcd. for C15H14BrNO3S: C, 48.92; H, 3.80; N, 3.80; Found: C, 48.73; H, 4.22; N, 3.68%.
Methyl 2-(2-furoylamino)-5-acetyl-4-methylthiophene-3-carboxylate (6e)
Yield: 56%; m.p.: 238°C; Rf : 0.72 (7:3, toluene/acetonitrile); IR (KBr, cm− 1): 3251 (amide NH stretching), 1729 (C = O stretching of ester), 1600 (C = O stretching of ketone), 764, 670; 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.36 (s, 3H, CH3-4), 2.72 (s, 3H, CH3-5), 3.96 (s, 3H, CH3 of ester), 6.34 (d, 1H, aromatic), 6.58 (t, 1H, aromatic), 7.35 (d, 1H, aromatic); MS: m/z 307 (M+); Anal. calcd. for C14H13NO5S: C, 54.72; H, 4.23; N, 5.55; Found: C, 54.59; H, 4.12; N, 4.32%.
Pharmacological screening
Animals. Albino rats (150–250 g) of either sex were provided with pellet diet (Lipton, India) and water ad libitum and kept under standard laboratory condition at 25 ± 2°C. The experimental protocol was approved by the Institutional Ethics Committee constituted by the Ministry of Social Justice and Empowerment, (Government of India).
Anti-inflammatory activity
We have used the method previously described by Winter et al [Citation13]. The animals were studied for toxicity of DMSO up to 10% v/v in saline, and 5% DMSO was selected as a vehicle to suspend the standard drugs and the test compounds. Albino rats of either sex weighing between 150–250 g were starved for 18 h prior to the experiment. The animals were weighed, marked for identification and divided into groups of six. The standard drug ibuprofen (20 mg/kg body weight) and mefanamic acid (100 mg/kg body weight) and the test compounds were given orally (10, 20 and 40 mg/kg body weight) as a suspension using 5% DMSO as a vehicle. One hour later foot paw oedema was induced by injecting 0.1 mL of 1% carrageenin subcutaneously into the planter portion of the right hind paw of each rat. Initial foot paw volume was measured immediately by mercury plethysmometer. Oedema was measured three hours after carrageenin administration. The swelling in test group animals was used to calculate the percent inhibition ± SEM of oedema achieved by the compound at the test dose compared with the vehicle control group. The percentage protection of oedema was calculated according to the formula, % anti-inflammatory activity = 100 × (1 − Vt/Vc) where Vt and Vc are the volume of oedema in test compounds and control groups respectively.
Analgesic activity: Acetic acid induced writhing response model
All the targeted compounds were investigated for their analgesic activity in acetic acid induced writhing response in albino mice (20–25 g) at 10 mg/kg body weight dose following the method of Siegmund et al. [Citation14]. 10 mg/kg of the selected compounds was administered intra-peritoneally to groups of mice (6 in each group) starved for 16 h. The first group received the test compounds while the groups which served as positive and negative controls received 10 mg/kg ibuprofen and 0.5 mL/100 g body weight of 1% DMSO solution respectively. One hour after treatment, the animals in each group received 0.1 mL of 3% acetic acid to induce the characteristic writhing response. The number of writhing occurring within 30 min was recorded and the mean was compared with that of the control and converted into % inhibition.
Antioxidant activity: Nitric oxide radical scavenging assay
Nitric oxide generated from sodium nitroprusside in aqueous solution at physiological pH interacts with oxygen to produce nitrite ions, which can be measured by Griess reagent [Citation15]. The reaction mixture (3 mL) containing sodium nitroprusside (10 mmol) in phosphate buffered saline (PBS) and test compounds (4a-4f, 5c, 5f, 6c and 6e) at different concentrations (5, 10, 15, 20, 25, 30, and 35 μg/mL) were incubated at 25°C for 150 minutes. Each 30 min, 0.5 mL of the incubated sample was removed. 0.5 mL of Griess reagent (1% sulphanilamide, 0.1% naphthylethylene diamine dihydrochloride in 2% H3PO4) was added to the 0.5 mL aliquot of the sample removed. The absorbance of the chromophore formed was measured at 546 nm. The experiment was performed (in triplicate) and % scavenging activity was calculated using the formula 100 − [100/blank absorbance × sample absorbance]. The activity was compared with ascorbic acid at concentration 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 μg/mL, which was used as a standard antioxidant.
Result and discussion
The synthesized compounds (4a-4g, 5a-5f and 6a-6e) were screened by in vivo assay for their anti-inflammatory activity using carrageenin-induced rat hind paw oedema model at three graded doses employed at 10, 20 and 40 mg/kg body weight using mefanamic acid, ibuprofen and analgesic activity using acetic acid-induced writhing response in albino mice at dose of 10 mg/kg using ibuprofen and the results are shown in . In order to arrive at possible mechanism of the anti-inflammatory activity of the compounds (4a-4f, 5c, 5f, 6c and 6e) which gave more than 50% protection to the inflamed paw, were selected for investigating their in vitro antioxidant nitric oxide radical scavenging assay at the concentrations of 5, 10, 15, 20, 25, 30 and 35 μg/mL using standard drug ascorbic acid and the results are shown in .
Table I. Chemical structures, anti-inflammatory and analgesic activity of tetrasubstituted thiophenes.
Table II. Antioxidant activity of tetrasubstituted thiophenes.
Taking into account the diverse biological activities coumarin derivatives namely anticoagulant and anti-inflammatory activities [Citation16–18] the compounds 4a-4d were synthesized keeping coumarin-3-yl constant at fifth position and introducing both electron releasing (–CH3) and electron withdrawing groups (–Cl, Br) at fourth position in arylamino moiety at second position of thiophene nucleus. In order to examine the effect of introduction of carbonyl spacer attached to –NH group in the form of benzoyl and furoyl at the second position of thiophene moiety, keeping coumarin–3-yl constant substituted at fifth position of thiophene moiety 4e and 4f were synthesized. The compound 4g was synthesized having ethyl group at second position to explore the effect of presence of aliphatic chain on inflammatory activity of profile of the candidate.
Among the compounds 4a-4g, 4c showed maximum anti-inflammatory activity. It displayed 71% protection at 10 mg/kg and 77% protection at 20 mg/kg to inflamed paw, however % protection decreased to 67% at 40 mg/kg as compared to the reference drugs ibuprofen which showed 36% protection at 20 mg/kg and mefanamic acid which displayed 42% protection at 100 mg/kg. The compounds 4a, 4b, 4d, 4e and 4f showed % protection of 57%, 51%, 59%, 64%, 68% at 10 mg/kg and 70%, 65%, 67%, 71%, 76% at 20 mg/kg to inflamed paw which were comparable to anti-inflammatory activity of both ibuprofen and mefanamic acid. The compounds 4a, 4b, 4d, 4e and 4f also showed decrease in % protection to inflamed paw at 40 mg/kg dose which was similar pattern as observed for 4c the most potent candidate among 4a-4g. The compound 4g showed poorer anti-inflammatory activity at all the three graded doses employed. On the basis of structure-activity relationship studies of 4a-4g it can be concluded that in this series of compounds the presence of –Cl group in anilino moiety at second position of the thiophene nucleus contribute in enhancing the anti-inflammatory activity profile of the candidate (4c). The presence of –CH3 in anilino moiety at second position of the thiophene nucleus seems to reduce the inflammatory activity profile of the candidate (4b). Also the presence of benzoyl (4e) and 2-furoyl moiety (4f) attached to –NH at the second position of the thiophene also contributes significantly to anti-inflammatory activity profile of the candidates.
There are several reports in literature which indicate that incorporation of more than one minimum structural feature essential for activity in a single molecule can lead to significant enhancement in the activity profile of the targeted compounds [Citation19,20]. The compounds 5a-5f were synthesized incorporating two minimum structural features (as in fig I) essential for the anti-inflammatory activity on the basis of structure-activity relationship studies of tetrasubstituted thiophenes and their pharmacological profile reported in our earlier work [Citation8–11] in targeted compounds in the assumption that an additional = CO in (5a-5f) will perhaps provides one more hydrogen bond acceptor feature to facilitates better binding and superior signal transduction on the macromolecule target or targets.
In case of 5a-5f, the difference of anti-inflammatory activity of the compounds was observed too small at the three-graded doses employed at 10, 20 and 40 mg/kg. The compounds 5c, 5e and 5f showed % protection of 62%, 44% and 61% respectively at 10 mg/kg dose and nearly similar % protection at both 20 and 40 mg/kg dose. The compounds 5a, 5b and 5d showed poorer anti-inflammatory activity at all the employed three-graded dose. On the basis of structure-activity relationship studies of 5a-5f it is evident that in their cases also the presence of –Cl group in anilino moiety at second position of the thiophene nucleus contribute in enhancing the anti-inflammatory activity profile of the candidate. The presence of 2-furoyl moiety attached to –NH at the second position of the thiophene contribute to anti-inflammatory activity profile of the candidate in same scale.
The compounds 6a-6d were synthesized in which keeping acetyl at fifth position, substituents at R1 in anilino moiety were modified by using both electron releasing and electron withdrawing groups. The compound 6e was synthesized in which keeping acetyl at fifth position, 2-furoylamino moiety were introduced at second position which has a proton acceptor (–C = O) and a proton donor (–NH) features in adjacent position. In case of 6a-6e compounds 6c, 6e and 6b showed comparable anti-inflammatory activity of 51%, 58% and 49% protection at 10 mg/kg dose. The anti-inflammatory activity of 6a-6e compounds significantly decreases at 20 and 40 mg/kg doses. For 6a-6e our attempt to correlate biological result with variation of substituents attached to –NH at second position of thiophene was unsuccessful.
Among the 4a, 5a and 6a the substituents attached to carbonyl group at the fifth position of thiophene nucleus are coumarin-3-yl, tetra substituted thiophene and methyl respectively. Similar is the case with 4b, 5b and 6b; 4c, 5c and 6c and 4f, 5f and 6e. On the basis of variation of substituents at fifth position of thiophene nucleus keeping other substituents (at second, third and fourth) constant; we attempted to find out structure-activity relationship of trio like 4a, 5a and 6a and similar compound groups. On the basis of these structure-activity relationship studies of trio 4a, 5a, 6a and similar groups it was found that the presence of coumarin-3-yl (4a) significantly contribute to anti-inflammatory activity of the candidate as compare to both tetrasubstituted thiophene (5a) derivatives and methyl (6a) group attached to carbonyl function at fifth position of thiophene.
All the synthesized compounds were evaluated for their analgesic activity by in vivo assay using acetic acid induced writhing response test in albino mice at 10 mg/kg dose. Among 4a-4g only 4c and 4f showed comparable analgesic activity of 56% and 55% inhibition as compared to reference drug ibuprofen which displayed 62% inhibition at 10 mg/kg dose. The compounds 4a and 4e showed moderate analgesic activity of 40% and 38% inhibition at 10 mg/kg dose. Among 5a-5f, all the compounds displayed very poor analgesic activity. Among 6a-6e, only 6c and 6e showed moderate analgesic activity of 38% and 32% inhibition. The results of analgesic activity of compounds 4a-4g showed that presence of 4-chlorophenyl and 2-furoyl attached to –NH at second position of thiophenes and coumarin-3-yl attached to –CO group at fifth position of thiophene ring contribute significantly to analgesic activity profile of the candidates 4c and 4f. In case of 6a-6e, for 6c and 6e also the presence of 4-chlorophenyl and 2-furoyl attached to –NH at second position of thiophenes contributes moderately to analgesic activity profile of the candidates 6c and 6e.
Compounds (4a-4f, 5c, 5f, 6c and 6e) which gave more than 50% protection to the inflamed paw were selected for investigating their in vitro antioxidant nitric oxide radical scavenging assay at the concentrations of 5, 10, 15, 20, 25, 30 and 35 μg/mL using standard drug ascorbic acid. Among these compounds 4c showed maximum in vitro nitric oxide radical scavenging activity having IC50 value 31.59 μg/mL. The compounds 4f and 5f showed IC50 value of 31.12 and 34.18 μg/mL respectively. The compounds 4a, 4b, 4e and 5c showed in vitro nitric oxide radical scavenging activity of 34.01 ± 0.001, 41.24 ± 0.003, 34.25 ± 0.001 and 44.84 ± 0.001 respectively at 35 μg/mL. The compound 6e was found to have poor in vitro antioxidant nitric oxide radical scavenging activity whereas 4d and 6c have showed no scavenging activity. The best candidate among whole series was 4c however it was found to have poor in vitro antioxidant nitric oxide radical scavenging activity as compared to standard drug ascorbic acid which showed IC50 value 0.88 μg/mL at concentration 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 μg/mL. From the IC50 values of 4a-4f, 5c, 5f, 6c and 6e, it can be concluded that the presence of 2-furoyl attached to –NH at second position of thiophene contribute moderately to antioxidant nitric oxide radical scavenging activity profile of the candidates 4f and 5f. When 4-chlorophenyl is attached to –NH at second position of thiophene and coumarin-3-yl attached to –CO group at fifth position of thiophene ring 4c showed maximum in vitro nitric oxide radical scavenging activity having IC50 value 31.59 μg/mL; however when 4-chlorophenyl is attached to –NH at position 2 of thiophene and methyl group is attached to –CO group at position 5 of thiophene ring 6c showed no scavenging activity. This result shows that the presence of coumarine-3-yl attached to –CO group at fifth position of thiophene ring contribute in enhancing in vitro nitric oxide radical scavenging activity of 4c and in 4a, 4b and 4e.
Conclusion
The synthesized targeted compounds (4a-4g, 5a-5f and 6a-6e) were evaluated for their in vivo anti-inflammatory, analgesic activities and in vitro antioxidant activity. On the basis of structure-activity relationship studies of 4a-4g it can be concluded that presence of –Cl group in anilino moiety (4c) and benzoyl (4e), 2-furoyl moiety (4f) attached to –NH at the second position of the thiophene contributes significantly to anti-inflammatory and analgesic activity profile of the candidates. In case of structure-activity relationship of 5a-5f it is evident that in these cases the presence of –Cl group in anilino moiety and 2-furoyl moiety attached to –NH at the second position of the thiophene contribute to anti-inflammatory activity profile of the candidate in same scale. For 6a-6e our attempt to correlate our biological result with variation of substituents attached to –NH at second position of thiophene was unsuccessful. On the basis of these structure-activity relationship studies of trio 4a, 5a, 6a and similar groups it was found that the presence of coumarin-3-yl (4a) significantly contribute to anti-inflammatory activity of the candidate as compare to both tetrasubstituted thiophene (5a) derivatives and methyl (6a) group attached to carbonyl function at fifth position of thiophene. Among the compounds (4a-4f, 5c, 5f, 6c and 6e) only 4c, 4f and 5f showed IC50 value of 31.59 μg/mL, 31.12 and 34.18 μg/mL respectively suggesting that the mechanism of anti-inflammatory activity of potent candidates could be mediated through inhibition of nitric oxide burst in inflammatory situation. Further studies are needed to explore the efficacy and safety of the most potent candidate 4c.
Acknowledgements
We gratefully acknowledge the management, Prashant and Mittal Kansara Pharmacy College, Pipaliya, Waghodia, Vadodara, India and Prof. Gaurang B. Shah, Head, Department of Pharmacology, K.B.I.P.E.R., Gandhinagar, India for the facilities and encouragement. We also wish to thank RSIC of CDRI Lucknow, Bombay Tablets Mfg. Co. Pvt. Ltd. Gandhinagar and Cadilla Pharmaceuticals Ahmadabad, India for providing the 1H-NMR, C, H, N analysis, I.R. data and animals respectively.
References
- PJ Delves, and IM Riott. (2000). The immune system. N Eng J Med 343 (37-49):108–117.
- JR Vane, YS Bakhle, and RM Botting. (1998). Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120.
- JV Ryn, G Trummlitz, and M Pairet. (2000). COX-2 selectivity and inflammatory processes. Curr Med Chem 7:1145–1161.
- M Beuck. (1999). Nonsteroidal anti-inflammatory drugs: A new generation of cyclooxygenase inhibitors. Angew Chem Int Ed 38:631–633.
- KR Gans, W Galbraith, RJ Roman, SB Haber, JS Kerr, WK Schmidt, C Smith, WE Hewes, and NR Ackerman. (1990). Anti-inflammatory and safety profile of DuP 697, a novel orally effective prostaglandin synthesis inhibitor. J Pharmacol Exp Ther 254:180–187.
- AD Pillai, PD Rathod, FP Xavier, M Patel, M Nivsarkar, KK Vasu, H Padh, and V Sudarsanam. (2003). Novel drug designing approach for dual inhibitors as anti-inflammatory agents: Implication of pyridine template. J Biochem Biophys Res Commun 301:183–186.
- KI Molvi, KK Vasu, MM Patel, V Sudarsanam, and N Haque. (2006). Design and synthesis of bioisosteric tetrasubstituted thiophenes as novel anti-inflammatory agents: Part-I. Ethiop Pharm J 24:1–11.
- KI Molvi, KK Vasu, SG Yerande, V Sudarsanam, N Haque. Syntheses of new tetrasubstituted thiophenes as novel anti- inflammatory agents. Eur J Med Chem, 2007;42:1049–1058.
- KI Molvi, MM Patel, V Sudarsanam, N Haque. Design, synthesis and pharmacological evaluation of novel tetrasubstituted thiophene analogues as anti-inflammatory agents. J Enz Inhib Med Chem, 2008; e pub ahead of print.
- JE Hodgkins, and WP Reeves. (1964). The modified Kaluza synthesis III. The synthesis of some aromatic isothiocyanates. J Org Chem 29:3098–3099.
- WP Reeves, A SimmonsJr, JA Rudis, and TC Bothwell. (1981). Phase transfer catalysis preparation of acyl isothiocyanates. Synth Commun 11:781–785.
- CF Koelsch. (1950). Bromination of 3-acetocoumarin. J Am Chem Soc 72:2993–2995.
- CA Winter, EA Risley, and GW Nuss. (1962). Carrageenan-induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111:544–547.
- EA Siegmund, RA Cadmus, and G Lu. (1957). A method for evaluating both nonnarcotic and narcotic analgesics. Proc Soc Exp Biol 95:729–731.
- N Sreejayan, and MNA Rao. (1997). Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol 49:105–107.
- M Ghate, RA Kusanur, and MV Kulkarni. (2005). Synthesis and in vivo analgesic and anti-inflammatory activity of some bi heterocyclic coumarin derivatives. Eur J Med Chem 40:882–887.
- C Kontogiorgis, and D Hadjipavlou-Litina. (2003). Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidant agents. J Enz. Inhib Med Chem 18:63–69.
- M Ghate, D Manohar, V Kulkarni, R Shobha, and SY Kattimani. (2003). Synthesis of vanillin ethers from 4-(bromomethyl) coumarins as anti-inflammatory agents. Eur J Med Chem 38:297–302.
- TO Johnson, J Ermolieff, and MR Jirousek. (2002). Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discovery 1:696–709.
- DC Rees, M Congreve, CW Murray, and R Carr. (2004). Fragment-based lead discovery. Nat Rev Drug Discovery 3:660–673.