Abstract
Melatonin (N-acetyl-5-methoxytryptamine) is the chief secretory product of the pineal gland and synthesized enzymatically from serotonin (5-hydroxytryptamine). These indoleamine derivatives play an important role in the prevention of oxidative damage. In the present study, DMPD radical scavenging and cupric ion (Cu2+) reducing ability of melatonin and serotonin as trolox equivalent antioxidant activity (TEAC) was investigated. Melatonin and serotonin demonstrated 73.5 and 127.4 μg/mL trolox equivalent DMPD√+ scavenging activity at the concentration of 100 μg/mL. Also, at the same concentration, melatonin and serotonin showed 14.41 and 116.09 μg/mL trolox equivalent cupric ion (Cu2+) reducing ability. These results showed that melatonin and serotonin had marked DMPD√+ radical scavenging and cupric ions (Cu2+) reducing ability. Especially, serotonin had higher DMPD radical scavenging and cupric ions (Cu2+) reducing activity than melatonin because of its phenolic group.
Introduction
The pineal hormone melatonin (N-acetyl-5-methoxytryptamine), an indoleamine, is the chief secretory product of the pineal gland. It is synthesized enzymatically from serotonin (5-hydroxytryptamine) by the sequential action of serotonin N-acetyltransferase and hydroxyindole-O-methyltransferase [Citation1]. The chemical structures of melatonin and serotonin are given in . It is also produced in other organs and found in all body fluids after its release from the pineal. It is known that melatonin influences a variety of biological processes including circadian rhythms, neuroendocrine, cardiovascular and immune functions as well as thermoregulation [Citation2–4]. Additionally, this molecule functions in protecting cell components such as nuclear DNA, membrane lipids, and cytosolic proteins from free radical damage [Citation5–7].
Serotonin (5-hydroxytryptamine) has been implicated in the control of many physiological (cardiovascular, respiratory and thermoregulatory) and behavioural (feeding and sexual behaviour, circadian rhythm, sleep-wake cycle, aggression, learning and pain sensitivity) functions that could be disturbed by depression [Citation8]. It is a biogenic amine belonging to the most common neurotransmitters in nature. Serotonin is a well-established neurotransmitter produced and activated in nervous tissues and digestive tract [Citation9]. In humans, serotonin modifies body temperature, blood pressure, sexual behaviour and mood. It is also involved in several interactions of the immune system [Citation10]. It was reported that serotonin has been implicated in many brain functions, including those related to pleasure and the intake of food, depression, suicide, anxiety, and Parkinson's and Alzheimer's diseases [Citation11].
The importance of free radicals and reactive oxygen species (ROS) has attracted increasing attention over the past decade. ROS are various forms of activated oxygen which include free radicals such as superoxide anion radicals ![]() , hydroxyl radicals (OH·) and non free-radical species such as H2O2 and singlet oxygen
, hydroxyl radicals (OH·) and non free-radical species such as H2O2 and singlet oxygen ![]() . These molecules are exacerbating factors in cellular injury and aging process [Citation12–14]. Antioxidants can protect the human body from free radicals and ROS effects and retard the progress of many chronic diseases as well as lipid oxidative rancidity in foods [Citation15–17.
. These molecules are exacerbating factors in cellular injury and aging process [Citation12–14]. Antioxidants can protect the human body from free radicals and ROS effects and retard the progress of many chronic diseases as well as lipid oxidative rancidity in foods [Citation15–17.
Antioxidant and radical scavenging activities of melatonin, and its precursor serotonin, have been evaluated by different methodologies previously. In previous studies, we determined the total antioxidant activity, reducing power, DPPH radical scavenging, superoxide anion radical ![]() scavenging activity, ferrous ion (Fe2+) chelating and hydrogen peroxide decomposition activities of melatonin [Citation3,4]. In addition, we demonstrated in vitro effects of melatonin on human erythrocyte carbonic anhydrase, in vivo effects on rat erythrocyte carbonic anhydrase [Citation1] and both in vitro and in vivo effects on rainbow trout (Oncorhynchus mykiss) erythrocyte carbonic anhydrase [Citation18]. Recently we determined DPPH radical scavenging activity, ABTS radical scavenging activity, total antioxidant activity by ferric thiocyanate method, total reducing ability by Fe3 + − Fe2+ transformation method, superoxide anion radical scavenging activity by riboflavin/methionine/illuminate system, hydrogen peroxide scavenging and ferrous ions (Fe2+) chelating activities of serotonin [Citation19]. According to our knowledge, there is no information about DMPD√+ radical scavenging and cupric ion (Cu2+) reducing ability of melatonin or serotonin obtained by Cuprac methods. It was aimed in this study to investigate the DMPD√+ radical scavenging and cupric ion (Cu2+) reducing ability of melatonin and serotonin as trolox equivalent antioxidant activity.
scavenging activity, ferrous ion (Fe2+) chelating and hydrogen peroxide decomposition activities of melatonin [Citation3,4]. In addition, we demonstrated in vitro effects of melatonin on human erythrocyte carbonic anhydrase, in vivo effects on rat erythrocyte carbonic anhydrase [Citation1] and both in vitro and in vivo effects on rainbow trout (Oncorhynchus mykiss) erythrocyte carbonic anhydrase [Citation18]. Recently we determined DPPH radical scavenging activity, ABTS radical scavenging activity, total antioxidant activity by ferric thiocyanate method, total reducing ability by Fe3 + − Fe2+ transformation method, superoxide anion radical scavenging activity by riboflavin/methionine/illuminate system, hydrogen peroxide scavenging and ferrous ions (Fe2+) chelating activities of serotonin [Citation19]. According to our knowledge, there is no information about DMPD√+ radical scavenging and cupric ion (Cu2+) reducing ability of melatonin or serotonin obtained by Cuprac methods. It was aimed in this study to investigate the DMPD√+ radical scavenging and cupric ion (Cu2+) reducing ability of melatonin and serotonin as trolox equivalent antioxidant activity.
Material and methods
Chemicals
N,N-Dimethyl-p-phenylenediamine dihydrochloride (DMPD), neocuproine (2,9-dimethyl-1,10-phenanthroline), melatonin (N-acetyl-5-methoxytryptamine), serotonin (5-hydroxytryptamine), trolox (( ± )-6-Hydroxy-2,5,7,8-tetra-methylchromane-2-carboxylic acid) and CuCl2 were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). All other chemicals used were analytical grade and obtained from either Sigma-Aldrich or Merck.
Preparation of DMPD√+ radical cation
DMPD, 100 mM, was prepared by dissolving 105 mg of DMPD in 5 mL of deionized water; 1 mL of this solution was added to 100 mL of acetate buffer (0.1 M, pH 5.25), and the coloured radical cation (DMPD√+) was obtained by adding 0.2 mL of a solution of 0.05 M ferric chloride (final concentration 0.1 mM). One mililiter of DMPD√+ radical solution was directly placed in a 1 mL quartz cuvette and then its absorbance was measured at 505 nm in spectrophotometer (CHEBIOS s.r.l. UV-VIS spectrophotometer). An optical density of 0.900 unit of absorbance was obtained and it represents the uninhibited signal. The optical density of this solution, which is daily prepared freshly, is constant up to 12 h at room temperature.
Measurement of DMPD√+ radical scavenging
DMPD√+ radical scavenging activity of melatonin and serotonin was performed according to the method described by Fogliano and co-workers [Citation20]. A standard solution of trolox was prepared as follows: 1 mg/mL of trolox was prepared by dissolving 25 mg of trolox in 25 mL of deionized water. Different concentrations of melatonin and serotonin samples (5–15 μg/mL) were added in the spectrometric cuvette and total volume of these samples were adjusted to 1 mL with distilled water. Then 1 mL of DMPD√+ radical solution was directly transferred to the quartz cuvette. After ten min incubation, absorbances of test samples were measured at 505 nm. The DMPD√+ scavenging capacity for each tested sample was expressed as trolox equivalent (μg/mL) and calculated from the calibration curve determined by linear regression (r2: 0.9831):
The percentage of DMPD√+ scavenging capability of melatonin and serotonin was calculated using the following equation:
where AC is the absorbance of the control which contains 1 mL control reaction (containing DMPD√+ solution without the melatonin or serotonin), and AS is the absorbance in the presence of serotonin and melatonin. DMPD√+ decreases significantly upon exposure to radical scavengers [Citation20].
Cupric ion (Cu2+) reducing ability-CUPRAC assay
For determination of reducing ability of melatonin and serotonin, the cupric ions (Cu2+) reducing power method was also used [Citation21] with slight modification. To a test tube were added 0.25 mL CuCl2 solution (0.01 M), 0.25 mL ethanolic neocuproine solution (7.5 × 10− 3 M) and 0.25 mL NH4Ac buffer solution (1 m), followed by mixing; different concentration of melatonin and serotonin (5–15 μg/mL). Then, total volume adjusted 2 mL with distillated water and mixed well. Absorbance against a blank reagent was measured at 450 nm after 30 min. The cupric ions (Cu2+) reducing capacity for either melatonin or serotonin was expressed as trolox equivalent (μg/mL) and calculated from the calibration curve determined by linear regression (r2: 0.995):
Results and discussion
Melatonin is a potent, endogenously produced and diet-derived free radical scavenger and broad-spectrum antioxidant [Citation22–24]. Melatonin is quenching OH√ [Citation22,25] and possibly also peroxyl radicals (LOO√) [Citation26] and peroxynitrite anion (ONOO − ) [Citation27], a reaction product of O2√ − [Citation3].
It was reported that radical scavenging by indolic compounds is not solely a question of the presence of an indole moiety, but demonstrate that the reactivity of these molecules is profoundly modulated by their functional residues. All indoleamines share a heteroaromatic ring system with high electroreactivity and they only differ in carrying functional groups in their side chains. These substituents show a great extent reactivity, potency and efficiency of radical scavenging. Variations in redox behaviour of the particular indoles could be of fundamental significance [Citation28]. This was already indicated by initial findings of Tan and co-workers [Citation23] revealing pro-oxidative properties of serotonin. The role of the methoxy group in melatonin might therefore be seen as a means of avoiding formation of o-centred indolyl radicals. But it is still unclear if the methoxy residue also contributes to melatonin's reactivity. The importance of the N-acetyl group became particularly obvious when Tan and co-workers [Citation25] demonstrated the formation of 3-hydroxymelatonin, a reaction requiring such terminal residue at the side chain. Moreover, it was indicated that 5-methoxytryptamine differed from melatonin in its capacity for scavenging OH√, despite its similar affinity, a finding difficult to interpret at that time [Citation28]. It was expected that serotonin had higher DMPD radical scavenging activity than melatonin because of its phenolic hydroxyl group.
The principle of DMPD assay is that; at an acidic pH and in the presence of a suitable oxidant solution, DMPD can form a stable and coloured radical cation (DMPD√+). DMPD√+ has a maximum absorbance at 505 nm. Antioxidant compounds which are able to transfer a hydrogen atom to DMPD√+ quench the colour and produce a decolorization in the solution which is proportional to their amount. This reaction is rapid (less than 10 min) and the end point, which is stable, is taken as a measure of the antioxidative efficiency. Therefore, this assay reflects the ability of radical hydrogen-donor to scavenge the single electron from DMPD√+. Preliminary experiments show that the choice of oxidant solution and the ratio between the concentration of DMPD and oxidative compound are crucial for the effectiveness of the method. It should be outlined that having high sensitive measurements and sufficient inhibition range, a starting point between 0.80 and 1.00 of absorbance at 505 nm are necessary.
Inhibition of the absorbance at 505 nm is linear between 20 and 100 μg/mL of trolox. This assay is based on the extent of radical cation reduction at a fixed time point but not on the rate of reduction. This feature ruled out the complications due to the monitoring of the time course of colour inhibition which are present in other methods [Citation29,30] and allows the simultaneous analysis of many samples [Citation31]. On the other hand, in contrast to the ABTS procedure, the DMPD method guarantees a very stable end point of the measure. In this way I get free of the variable “time of the measure” which is crucial for the ABTS decolorization assay. This is particularly important when a large-scale screening is required. The main drawback of the DMPD method is that its sensitivity and reproducibility dramatically decreased when hydrophobic antioxidants such as α-tocopherol or BHT were used. The same behaviour is observed when methanol is used as DMPD solvent.
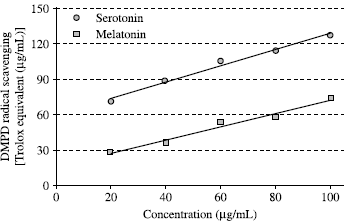
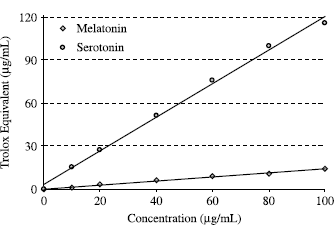
DMPD√+ scavenging efficiency was expressed in trolox equivalent antioxidant activity (TEAC) according to Miller et al. [Citation32], using the curve of standard trolox reported in . There is a correlation between the DMPD√+ scavenging and trolox concentrations. The correlation between these two parameters is more than 0.983. DMPD√+ scavenging activity of melatonin and serotonin were evaluated as trolox equivalent antioxidant activity (TEAC). As can be seen in , DMPD√+ scavenging activity of melatonin and serotonin was found as concentration dependent (20–100 μg/mL). Melatonin and serotonin were demonstrated 73.5 and 127.4 μg/mL trolox equivalent DMPD√+ scavenging activity, at the concentration of 100 μg/mL, respectively. In previous studies, we investigated the antioxidant activities of melatonin and serotonin with other in vitro models including metal chelating, hydrogen peroxide scavenging, Fe3 + − Fe2+ reducing power, DPPH radical scavenging, superoxide anion radical scavenging and ABTS√+ scavenging activity [Citation3,4,19]. In these methods, it was found that serotonin had higher antioxidant activity than melatonin. These results confirmed the results obtained from present study.
Figure 2. DMPD√+ scavenging values of trolox at different concentrations (20–100 μg/mL) for determination of calibration curve (r2:0.9831).
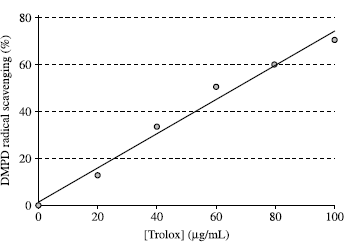
FIG 3 DMPD√+ scavenging capacity of different concentrations (10–100 μg/mL) of melatonin (r2:0.9823) and serotonin (r2:0.9860) expressed as trolox equivalent antioxidant activity (TEAC).
It was indicated that the electron donating capacity, reflecting the reducing power of bioactive compounds, is associated with antioxidant activity [Citation33]. Antioxidants can be reductants, and inactivation of oxidants by reductants can be described as redox reactions in which one reaction species is reduced at the expense of the oxidation of the other. The Cuprac method was developed for an antioxidant capacity assay. This method is simultaneously cost-effective, rapid, stable, selective and suitable for a variety of antioxidants regardless of chemical type or hydrophilicity. Moreover, it was reported that the results obtained from in vitro cupric ions (Cu2+) reducing measurements may be more efficiently extended to the possible in vivo reactions of antioxidants. Cuprac chromogenic redox reaction is carried out at a pH (7.0) close to the physiological pH [Citation34]. These method is capable of measuring thiol-type antioxidants such as glutathione and non-protein thiol unlike the widely applied FRAP test, which is non-responsive to -SH group antioxidants [Citation35]. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity [Citation36,37]. In the other studies, the reducing capacity of a melatonin and serotonin was measured by the direct reduction of Fe[(CN)6]3 to Fe[(CN)6]2. In this assay we found that serotonin had better ferric ion reducing antioxidant power than melatonin [Citation3,19].
Cupric ion (Cu2+) reducing ability (Cuprac method) of melatonin and serotonin was expressed in TEAC, using the curve of standard trolox reported in . It was found a correlation between the cupric ion (Cu2+) reducing ability and trolox concentrations (r2:0.9913). Cupric ion (Cu2+) reducing capability of melatonin and serotonin by Cuprac method was found as concentration dependent (10–100 μg/mL). At the same concentration (100 μg/mL), melatonin and serotonin showed 14.41 and 116.09 μg/mL trolox equivalent cupric ion (Cu2+) reducing power ().
Figure 4. Cupric ion (Cu2+) reducing ability of the trolox at 450 nm and different concentrations (5–50 μg/mL; r2:0.9913) for determination of calibration curve.
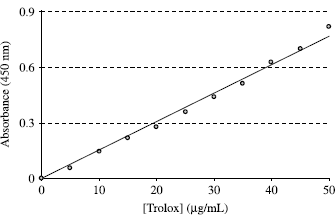
Figure 5. Cupric ion (Cu2+) reducing values of different concentrations (10–100 μg/mL) of melatonin (r2:0.9951) and serotonin (r2:0.9961).
As a conclusion, melatonin and serotonin have effective DMPD radical scavenging activity and cupric ions (Cu2+) reducing ability. Serotonin has phenolic hydroxyl group so that serotonin has higher radical scavenging and reducing activity than melatonin. Serotonin can give a hydrogen atom easily and is able to transfer this hydrogen atom to DMPD√+ quench the colour and produce a decolorization in the solution which is proportional to their amount. Also, serotonin with the phenolic group can easily reduce cupric ions (Cu2+) to cuprous ions (Cu+).
References
- Ş Beydemir, and İ Gülçin. (2004). Effect of melatonin on carbonic anhydrase from human erythrocyte in vitro and from rat erythrocyte in vivo. J Enz Inhib Med Chem 19:193–197.
- S Saarela, and RJ Reiter. (1993). Function of melatonin in thermoregulatory processes. Life Sci 54:295–311.
- İ Gülçin, ME Büyükokuroğlu, M Oktay, and Öİ Küfrevioğlu. (2002). On the in vitro antioxidant properties of melatonin. J Pineal Res 33:167–171.
- İ Gülçin, ME Büyükokuroğlu, and Öİ Küfrevioğlu. (2003). Metal chelating and hydrogen peroxide scavenging effects of melatonin. J Pineal Res 34:278–281.
- RJ Reiter, L Tang, JJ Garcia, and A Muñoz-Hoyos. (1997). Pharmacological action of melatonin in oxygen radical pathophysiology. Life Sci 60:2255–2271.
- P Montilla, A Cruz, FJ Padillo, I Túnez, F Gascon, MC Muñoz, M Gómez, and C Pera. (2001). Melatonin versus vitamin E as protective treatment against oxidative stress after extrahepatic bile duct ligation in rats. J Pineal Res 31:138–144.
- SJ Kim, RJ Reiter, W Qi, DX Tan, and J Cabrera. (2000). Melatonin prevents oxidative damage to protein and lipid induced by ascorbate-Fe3+-EDTA: Comparison with glutathione and a-tocopherol. Neuroendocrinol Lett 21:269–276.
- SM Stahl. (1998). Mechanism of action of serotonin selective re-uptake inhibitors: Serotonin receptors and path ways mediate therapeutic effects and side effects. J Affect Disord 51:215–235.
- GA Bubenik, RO Ball, and SF Pang. (1992). The effect of rood deprivation on brain and gastrointestinal tissue levels of tryptophan, serotonin, 5-hydroxyindoleacetic acid and melatonin. J Pineal Res 12:7–16.
- K Hellstrand, and S Hermodsson. (1993). Serotonergic 5-HT1A receptors regulate a cell contact-media interaction between natural killer cells and monocytes. Scand J Immunol 37:7–18.
- S Hasegawa, A Watanabe, K Nishi, KQ Nguyen, and M Diksic. (2005). Selective 5-HT1B receptor agonist reduces serotonin synthesis following acute, and not chronic, drug administration: Results of an autoradiographic study. Neurochem Int 46:261–272.
- İ Gülçin, M Oktay, Öİ Küfrevioglu, and A Aslan. (2002). Determinations of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol 79:325–329.
- İ Gülçin, Ş Beydemir, HA Alici, M Elmastaş, and ME Büyükokuroğlu. (2004). In vitro antioxidant properties of morphine. Pharmacol Res 49:59–66.
- İ Gülçin, M Elmastas, and HY Aboul-Enein. (2007). Determination of antioxidant and radical scavenging activity of basil (Ocimum basilicum) assayed by different methodologies. Phytother Res 21:354–361.
- İ Gülçin. (2007). Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 32:431–438.
- İ Gülçin, D Berashvili, and A Gepdiremen. (2005). Antiradical and antioxidant activity of total anthocyanins from Perilla pankinensis decne. J Ethnopharmacol 101:287–293.
- İ Gülçin, HA Alici, and M Cesur. (2005). Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull 53:281–285.
- O Hisar, Ş Beydemir, İ Gülçin, Ş ArasHisar, T Yanık, and Öİ Küfrevioğlu. (2005). The effect of melatonin hormone on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Turk J Vet Anim Sci 29:841–845.
- İ Gülçin, and RJ Reiter. Determination of in vitro antioxidant and radical scavenging activity of serotonin. (Unpublished data)
- V Fogliano, V Verde, G Randazzo, and A Ritieni. (1999). Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agric Food Chem 47:1035–1040.
- R Apak, K Güçlü, M Özyürek, SE Karademir, and E Erça. (2006). The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nut 57:292–304.
- DX Tan, LD Chen, B Poeggeler, LC Manchester, and RJ Reiter. (1993). Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr J 1:57–60.
- S Suzen, P Bozkaya, T Coban, and D Nebioğlu. (2006). Investigation of the in vitro antioxidant behaviour of some 2-phenylindole derivatives: Discussion on possible antioxidant mechanisms and comparison with melatonin. J Enz Inhib Med Chem 21:405–411.
- DX Tan, LC Manchester, MP Terron, LJ Flores, and RJ Reiter. (2007). One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species?. J Pineal Res 42:28–42.
- DX Tan, LC Manchester, RJ Reiter, BF Plummer, LJ Hardies, ST Weintraub, and AMM Shepherd. (1998). A novel melatonin metabolite, cyclic 3-hydroxymelatonin: A biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun 253:614–620.
- CM Pieri, F Marra, R Moroni, R Recchioni, and F Marcheselli. (1994). Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci 55:271–276.
- SB Cuzzocrea, E Zingarelli, P Gilad, P Hake, AL Salzman, and C Szabó. (1997). Protective effect of melatonin in carrageenan-induced models of local inflammation: Relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. J Pineal Res 23:106–116.
- B Poeggeler, S Thuermann, A Dose, M Schoenke, S Burkhardt, and R Hardeland. (2002). Melatonin's unique radical scavenging properties-roles of its functional substituents as revealed by a comparison with its structural analogs. J Pineal Res 33:20–30.
- WA Pryor, JA Cornicelli, LJ Devall, B Tait, BK Trivedi, DT Witiak, and M Wut. (1993). A rapid screening test to determine the antioxidant potencies of natural and synthetic antioxidants. J Org Chem 58:3521–3522.
- F Tubaro, E Micossi, and F Ursini. (1996). The antioxidant capacity of complex mixtures by kinetic analysis of crocin bleaching inhibition. JAOCS 73:173–179.
- JN Miller, CA Rice-Evans, and MJ Davies. (1993). A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 84:407–412.
- JN Miller, J Sampson, and LP Candeias. (1996). Antioxidant activities of carotenes and xanthophylls. FEBS Lett 384:240–242.
- S Arabshahi-Delouee, and A Urooj. (2007). Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem 102:1233–1240.
- R Apak, K Güçlü, M Özyürek, and SE Karademir. (2004). A novel total antioxidant capacity index for dietary polyphenols, vitamin C and E, using their cupric ion reducing capability in the presence of neocuproine: The CUPRAC method. J Agric Food Chem 52:7970–7981.
- D Huang, B Ou, and RL Prior. (2005). The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856.
- İ Gülçin. (2006). Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 217:213–220.
- İ Gülçin. (2006). Antioxidant and antiradical activities of L-carnitine. Life Sci 78:803–811.