Abstract
Studies on the seeds of Annona squamosa yielded a novel lipoxygenase inhibitor fatty acid ester, (+) - annonlipoxy (1). Compound 1 was screened for its enzyme inhibitory activity against lipoxygenase (E.C.1.14.18.1), exhibiting activity with IC50 69.05 ± 5.06 μm. Baicalein (IC50 22.6 ± 0.5 μm) was used as a positive control. Crude extracts of Annona squamosa fruit pulp and seeds were screened for its enzyme inhibitory activity against lipoxygenase and acetylcholinesterase. The crude ethanolic extract of fruit pulp and seeds of Annona squamosa also exhibited lipoxygenase activity with 22.2 and 26.7% inhibition, while the pet.ether extract of seeds of A. squamosa exhibited 52.7% inhibition at a concentration of 40 μg/200 ml. The crude ethanolic extract of seeds of Annona squamosa was also bioassayed for acetylcholinesterase inhibition and it was found inactive.
Introduction
Annonna squamosa (Family:Annonaceae) is a fruit bearing plant, and is abundantly available throughout Pakistan. It is native to the new world and naturalized throughout the tropics [Citation1]. Phytochemical studies on this plant have been reported in the literature. The roots of this plant are a powerful purgative and are useful in mental depression. The seeds are abortifacient and insecticidal and are useful in destroying lice in the hair, and it has been found that 27 different acetogenin [Citation2–4] are present in the plant, bark is reported to have only four acetogenins [Citation5]. phytochemical analysis on the other species of Annona i-e A. montana Macf, A. muricata L, A. purpurea Moc., A. Reticulate L. has also been reported [Citation6–9]. Pharmacological properties of seeds and fruit pulp of A. Squamosa are reported in literature, to little extent [Citation6,10,11] and it, thus tempted us to further evaluate the bioactivities of the plant and to isolate bioactive components from those crude extracts which possess significant bioactivity. In the context of our previous communication on bioactivities of Annona squamosa plant [Citation12], now we report the evaluation of in-vitro enzyme inhibitory activity against lipoxygenase and acetylcholinesterase of pet. ether and alcoholic extracts of fruit pulp and seeds of Annona squamosa.
Materials and methods
General experimental procedures
The mass spectra were recorded on a Jeol HX-110 instrument. The 1H and 13C NMR spectra were recorded in CDCl3 at 500 and 400 and 125 and 75 MHz, respectively, on a Bruker AM-500, 400 NMR spectrometer. The UV and IR spectra were recorded on Shimadzu UV-240 and JASCO A-320 spectrophotometers, respectively. Optical rotations were measured on a polatronic D Polarimeter. The purity of the compounds was checked on TLC (Si-gel, Merck PF254, 0.25 mm thickness). Melting points were determined in glass capillary tubes using a Buchi 535 and a Gallenkamp 30/MF-370 melting point apparatus.
Plant material
Annona squamosa fruit and seeds were collected from the suburbs of Karachi and identified by a taxonomist, Mr. Abid Askari, PSO at the Botany Section of PCSIR Laboratories Complex, Karachi. The plant specimen Annona squamosa KL/903/02 have been deposited at the Botany Section of Applied Biology Centre of PCSIR Laboratories Complex, Karachi.
Extraction and isolation
Pulp and seeds were separated from the fruits (50.52 Kg). Pulp (157.1431 gm) was soaked in ethanol for a week. The ethanol extract of fruit pulp filtered, dried (30.11 gm). seeds (2.5 kg) were dried, ground and soaked in pet. ether (60–65°C) for a week. Pet. ether extract was filtered evaporated under vacuum (51.1 gm), and subjected to lipoxygenase and acetylcholinesterase bioactivities. The remaining seeds were soaked again in ethanol for three days, filtered dried under vacuum and subjected to the same bioactivities.
The ETOH extract of seeds of A. Squamosa was concentrated to a gum (822 g), dissolved in distilled water and extracted thoroughly with chloroform (45 L). The chloroform soluble portion was evaporated under reduced pressure to yield a gum (66.92 g), which was chromatographed on a Si–gel column (Merck, 70–230 mesh, 2025 g). The elution of the column was initiated with petroleum ether (60–65°C). The combined column sub-fractions 1–8 (5.91 g) obtained by elution with 1: 9 chloroform-petroleum ether, which showed similar TLC behaviour upon spraying with ceric sulphate reagent, were combined and again subjected to CC using Silica gel (type 60, 70–230 mesh, 200 g) and the column was eluted with petroleum ether: chloroform (8:2). The sub-fractions obtained on elution of the column with n-hexane: chloroform (20:80) were compared on TLC. Fractions 7-18 showing similar behaviour on TLC were combined and further purified by preparative TLC (Merck PF254, 0.2 mm) using CHCl3 as the eluent to afford pure ceric sulphate active (1) (28 mg, 1.4x10− 4% yield). The remaining aqueous layer was acidified with acetic acid to pH 3, then extracted with CHCl3. The acidic layer was basified with NH4OH to pH 12 and extracted with CHCl3 (40 L). The CHCl3 soluble portion was dried as a crude alkaloidal mixture (74.96 g). The remaining aqueous layer was freeze dried (89 g) and chromatographed on a Si-gel column (70–230 mesh size, 2930 g). Fractions 30–35 (500 mL each) obtained on elution with 1:3 MeOH-CHCl3 showed similar behaviour on TLC using ceric sulphate reagent, were combined (1 g) and subjected to CC (70–230 mesh, 32 g) eluting with 73:27 CHCl3-MeOH. This afforded semi-pure fractions (29–45, 1 g), which were further purified by preparative TLC (Merck, PF254, 0.2 mm) using 72:28 CHCl3-MeOH to afford pure 1 (12.52 mg, 6.2 × 10− 5% yield, Rf = 0.6).
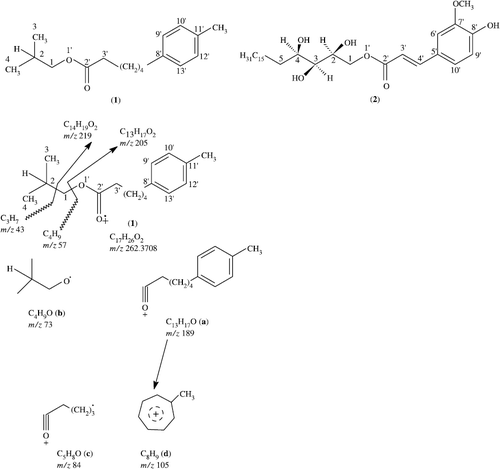
(+) - Annonlipoxy (1): White powder, [α]D+0 (c 1, CHCl3); UV/vis (MeOH): λmax(log ε) = 234 (3.01), 212 (3.01), 199 (3.84) nm, IR (CHCl3): νmax = 1136 and 1090 (C–O), 2850 (C-H), 1750 (C = O) cm− 1, 1H NMR (500 MHz, CDCl3): δ: see , 13C NMR (75 MHz, CD3OD): δ: see Table I, HREIMS: m/z 57.0630 (C4H9), 219 (C14H19O2), 205 (C13H17O2).
Table I. 1H (500 MHz) and 13C NMR (75 MHz) chemical shift assignments for compound 1.
In vitro lipoxygenase inhibition assay
Lipoxygenase inhibiting activity was conveniently measured by slightly modifying the spectrometric method developed by A. L. Tappel [29]. Lipoxygenase (1.13.11.12) type I-B and linoleic acid were purchased from sigma (St. Loius, MO, USA). All other chemicals were of analytical grade. The reaction mixture contained 160μL (100 mM) sodium phosphate buffer (pH 8.0), 10μL of test- compound solution and 20μL of lipoxygenase solution were mixed and incubated for 10 minutes at 25°C. The reaction was then initiated by the addition of 10μL linoleic acid solution, with the formation of (9Z,11E)-(13S)-13-hydroperoxyoctadeca-9,11-dienoate, the change of absorbance at 234 nm was followed for 6 min. Test compounds and the control were dissolved in methanol. All the reactions were performed in triplicate in 96-well micro-plate in Spectramax 384 plus (Molecular Devices, USA). The IC50 values were then calculated using the EZ-Fit Enzyme kinetics program (Perrella Scientific Inc., Amherst, USA). The percentage inhibition was calculated as: (E–S)/E × 100, where E is the activity of the enzyme without test compound and S is the activity of enzyme with test compound.
Acetylcholinesterase inhibition assay
The dilution range used for AChE was (100-200-300-400-500-600-800-1000) μm. Electric eel acetylcholinesterase (EC.3.1.1.7), horse butyrylcholinesterase (E.C.3.1.1.8) acetylthiocholine iodide, butyrylthiocholine chloride, 5,5′-dithiobis-2-nitrobenzoic acid and eserine were purchased from Sigma (St. Louis, MO). Buffers and other chemicals were of extra pure analytical grade. Acetylcholinesterase inhibition was determined spectrophotometrically using acetylthiocholine as the substrate by modifying the method of Ellman. The reaction was carried in 100 mM. Sodium phosphate buffer pH 8.0 at 25°C. 140 μL buffer, 20 μL enzyme preparation and 20 μL test compound solution were mixed and incubated for 30 min. 10 μL of 5-5′ dithiobis-2-nitrobenzoic acid was added and the reaction then started by adding 10 μL of acetylthiocholine. Butyrylthiocholine chloride was used as a substrate to assay the enzyme butyrylcholinesterase, all other reagents and conditions being identical. Hydrolysis of acetylthiocholine butyrylthiocholine was determined by monitoring the formation of the yellow 5-thio-2-nitrobenzoate anion (as a result of the reaction of 5,5′ dithiobis-2-nitrobenzoic acid with thiocholine, released by the enzymatic hydrolysis of acetylthiocholine or butyrylthiocholine) at 412 nm. A continuous kinetic assay was used to assay the enzymatic activity. Test compounds were dissolved in 5% ethanol: controls received the same volume of solvent. All reactions were triplicated and initial rates were measured as the rate of change in OD/min and used in subsequent calculations. According to Ellman et al [Citation13], since the extinction coefficient of the yellow anion is known, the rate of the enzymatic reaction can be calculated based upon this equation. Rate (mols/L/min) = change in absorbance per min/13600.
The IC50 values (concentrations that inhibited hydrolysis of substrates ATCh or BTCh by 50%), were determined by spectrophotometric measurement of the effect of increasing concentrations of test compounds on enzyme activity. The IC50 values were calculated using EZ-Fit Enzyme Kinetics Program (Perrella Scientific Inc., Amherst, U.S.A.).
Results and discussion
(+)-Annonlipoxy (1) was isolated as a white powder from the alcoholic extract of seeds of A. Squamosa by column and thin-layer chromatography. The UV spectrum of 1 showed absorptions at 212, 234, 199 and 192 nm, characteristic for the benzene ring bearing ester compounds. The IR spectrum of 1 showed intense absorptions at 2850 (C–H), 1630 (C = C), 1136 and 1090 (C–O) cm− 1. The high resolution electron-impact mass spectrum of 1 gave a molecular ion at m/z 262.3708, corresponding to the molecular formula C17H26O2 and indicated the presence of five rings/double bonds (). The ion at m/z 219 is due to the loss of C3H7 from the moleculer ion (262-43), while the ion at m/z 205 is due to the loss of CH2 from the ion at m/z 219 (219-14), indicating the presence of a butyl moiety. The linked scan mass spectrum of the molecular ion showed that the ion at m/z 189 (fragment a) and 73 (fragment b) arose directly from the molecular ion, thereby confirming the presence of methyl group on fragment a (). the ion at m/z 105, (fragment d) corresponding to the formula C8H9, may arise by the loss of (C5H8O) from the ion at m/z 189 due to the formation of a hydroxytropylium ion, [Citation2] confirming the presence of one CH3 group attached to the benzene ring.
The 1H NMR spectrum (CDCl3, 500 MHz) of 1 showed 3H doublets at δ = 0.82 (J3,2 = 7.0 Hz) and 0.85 (J4,2 = 7.0 Hz), which were assigned to the C-3 and C-4 methyl protons, respectively (). Two protons with overlapping chemical shifts (δ = 7.68, J9′,10′ = 6 Hz, J9′,13′ = 3 Hz) were assigned to H-9′ and H-13′. The other two aromatic protons have overlapping chemical shifts (δ = 7.51, J10′,9′ = 6 Hz, J10′,12′ = 4 Hz) were assigned to H-10′ and H-12′, respectively. Two double doublets at δ = 4.21 (J1a,2 = 8 Hz, J1b,2 = 6 Hz) and δ = 1.66 (J2,1a = 8 Hz, J2,1b = 6 Hz) were due to the C-1 and C-2 aliphatic protons. Three multiplets, resonating at δ = 1.60, 1.24 and 1.35, were assigned to H-3′, H-4′ and H-7′. While a broad multiplet resonated between δ = 1.31–1.35 integrating for 4 protons (H-5′ to H-6′) indicating the presence of an aliphatic methylene chain in the molecule. A comparison of 1H-NMR chemical shifts of 1 with commiphotetrol (2) was also carried out [Citation2,4]. compound 1 showed zero optical rotation indicating to be achiral compound.
The 13C NMR broad-band decoupled spectrum [Citation12] (CDCl3, 75 MHz) of 1 showed the resonances of seventeen carbon atoms. Two carbons C-10′/C-12′ have overlapping chemical shift (δ = 129.86). Similerly other two carbons C-9′/C-13′ also have overlapping chemical shift (δ = 132.38). The DEPT spectra [Citation7,8] showed the presence of three methyl carbons, five methine and six methylene carbons and hence, three quaternary carbons. The C-1 resonated at δ = 69.18 ppm, and reflected its attachment to oxygen. The downfield carbon resonating at δ = 169.36 was assigned to the C-2′ carbonyl carbon. Two methyl carbons resonating at δ = 11.39 and 14.37 were assigned to C-3 and C-4 carbons, respectively. Complete 1H/13C one-bond shift correlations of every protonated carbon is presented in Table I.
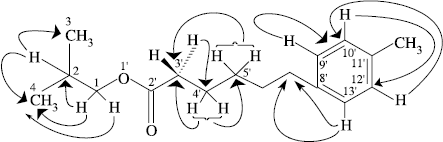
Two-dimensional NMR techniques such as COSY-45°, HMBC and HMQC [Citation6,9] were used to provide further structural information. The one-bond 13C/1H couplings were established on the basis of COSY-45° and HOHAHA (20, 60, 100 ms) experiments. The assignment for H-2 (δ 1.66) could be confirmed by the cross-peak with H-3 (δ = 0.82), H-4 (δ = 0.85) and H-1 (δ = 4.21) in the COSY-45° spectrum. Similarly the H-9′ (δ = 7.68) showed cross-peak with H-10′ (δ = 7.51) thereby, confirming the assignment of these vicinal protons to a benzene ring. The HOHAHA experiments together with the COSY results led to the existence of two main spin systems in 1. The first spin system comprises the four carbon protons i.e. H-1 (δ = 4.21), H-2 (δ = 1.66), H-3 (δ = 0.82) and H-4 (δ = 0.85). The second spin system consisted of ten protons C-3′ to C-7′.
The Heteronuclear Multiple Bond Connectivity (HMBC) [Citation9] spectrum of 1 helped to determine the point of attachment of the alkyl chain on the benzene ring. In the HMBC spectrum, C-9′ and C-13′ (δ = 132.38) showed long-range shift correlations with the methylene protons resonating at δ = 1.35 (H 7′). The protons of the C-7′ methylene carbon exhibited interactions with the quaternary carbon (δ = 133.59). Other important HMBC interactions are presented in . These studies led to structure 1 for the compound. The results suggested that the compound under study, being composed of a 10 carbon chain having a benzene ring at C-7′.
The crude ethanolic extract of seeds of Annona squamosa was bioassayed for acetylcholinesterase inhibition [Citation13], it exhibited 0.5% inhibition and found inert. Eserine [(-)- physostigmine, IC50 = 61 nM) was used as a positive control.
Compound 1 was bioassayed for lipoxygenase inhibition. Compound 1 exhibited in vitro lipoxygenase (E. C. 1. 14. 18. 1) activity with IC50 69.05 ± 5.06 μm. Baicalein (IC50 22.6 ± 0.5 μm) was used as a positive control [10,11,13].
The crude alcoholic extract of Annona squamosa exhibited lipoxygenase activity [Citation14,15,Citation16]. The crude ethanolic extract of fruit pulp and seeds of Annona squamosa exhibited lipoxygenase activity with 22.2 and 26.7% inhibition, respectively at a concentration of 40 μg/200 mL, while the pet.ether extract of seeds of A. Squamosa exhibited 52.7% inhibition at the same concentration ().
Table II. In vitro inhibition of Lipoxygenase by compound 1 and extracts of Annona squamosa.
References
- Atta-ur-Rahman, N Sultana, S Jahan, and MI Choudhary. (2005). Z Naturforsch 60b:1–5.
- Atta-ur-Rahman, N Sultana, S Jahan, and MI Choudhary. (1998). Nat Prod Lett 12 (3):223.
- KM Nadkarni. Indian Materia Medica. Bombay: Popular Prakashan Press; vol. 1. 1976. p 1142.
- N Sultana. Phytochemical and Structural Studies on the Chemical Constituents of Adhatoda vasica, Sarcococca saligna and Skimmia laureola, Ph.D. Dissertation, H.E.J., Karachi, 2000.
- K Fatima, and NJ Sultana. (2003). Chem Soc Pak. 25:328.
- Atta-ur-Rahman, N Sultana, MI Choudhary, PM Shah, and MR Khan. (1998). J Nat Prod 61 (6):713.
- MA Bendal, and DT Pegg. (1983). J Mag Reson 53:272.
- Atta-ur-Rahman. Nuclear Magnetic Resonance Spectroscopy, Basic Principles. New York: Springer-Verlag; (1986). p 227.
- Atta-ur-Rahman. One-and Two-Dimentional NMR Spectroscopy. Amsterdam: Elsevier Science Publishers; (1989). p 406.
- VJ Hearing. “Methods in Enzymology”. New York: Academic Press; (1987). p 142–154.
- K Lida, K Hase, K Shimomura, S Sudo, S Kadota, and T Amba. (1995). Planta Med 61:425.
- Atta-ur-Rahman, N Sultana, and MI Choudhary. (2002). Nat Prod Lett 16 (5):305.
- M Shiino, Y Watanabe, and K Umezawa. (2001). Bioorg Med Chem 9:1233.
- N Sultana, Atta-ur-Rahman, and A Khalid. (2006). J Enz Inhib Med Chem 21 (6):703.
- N Sultana, Atta-ur-Rahman, and A Khalid. (2007). A new fatty ester and a new triterpene from skimmia laureola. Nat Prod Research (in press)
- N Sultana. (2005). Atta-ur-Rahman. Khan TH. Z. Naturforsch. B. 60:1186.